Abstract
Objective(s):
Oxytetracycline (OTC) is a broad spectrum antibiotic widely used for treatment of a wide range of infections. However, its improper human and animal use leads to toxic effects, including hepatonephrotoxicity. Our objective was to evaluate protective effects of Nigella sativa oil (NSO) and/or ascorbic acid (AA), against OTC-induced hepatonephrotoxicity in rabbits.
Materials and Methods:
Forty male white New Zealand rabbits were divided into 5 groups of eight each. The 1st group (control) was given saline. The 2nd group was given OTC (200 mg/kg, orally). The 3rd and 4th groups were orally administered NSO and AA (2 ml/kg and 200 mg/kg respectively) 1 hr before OTC administration at the same dose regimen used for the 2nd group. Both NSO and AA were given in combination for the 5th group along with OTC administration. Serum biochemical parameters related to liver and kidney injury were evaluated, and lipid peroxidation as well as antioxidant markers in hepatic and renal tissues were examined.
Results:
OTC-treated animals revealed significant alterations in serum biochemical hepato-renal injury markers, and showed a markedly increase in hepato-renal lipid peroxidation and inhibition in tissue antioxidant biomarkers. NSO and AA protect against OTC-induced serum and tissue biochemical alterations when each of them is used alone or in combination along with OTC treatment. Furthermore, both NSO and AA produced synergetic hepatoprotective and antioxidant properties.
Conclusion:
The present study revealed the preventive role of NSO and/or AA against the toxic effects of OTC through their free radical-scavenging and potent antioxidant activities.
Keywords: Antioxidant, Ascorbic acid, Hepato-Renal, Nigella sativa, Oxytetracycline, Rabbit
Introduction
Oxytetracycline (OTC) is one of the tetracycline antibiotics, which are widely used for treatment of infectious bacterial diseases in human and animals caused by susceptible Gram-positive and Gram-negative microorganisms. It has a broad spectrum of activity against many intracellular microorganisms such as Mycoplasma, Rickettsiae, Haemofelis, Chlamydia, Malaria, and Theileria (1). Toxic doses of OTC have life threatening adverse effects, including hepato-renal toxicity (2-3). OTC leads to severe microvesicular steatosis of the liver and even hepatic injury when given in high doses in human (4). Oxidative stress, mitochondrial damage, and intracellular glutathione reduction, are the most important factors contributing to the prediction of hepato-renal toxicity (5-6). Mammalian cells overcome oxidative stress by directly scavenging oxygen radicals via endogenous non-enzymatic and enzymatic antioxidants or indirectly through removing the damaged nucleotides and lipid peroxidation products (7-9).
Nigella sativa belonging to family Ranunculaceae, also known as black seed or black cumin, possesses a wide range of pharmacological activities, including, carminative, antidiabetic, stimulant, analgesic, anti-pyretic and anti-inflammatory and diuretic (10). N. sativa oil (NSO) is a complex mixture that includes fatty acids, vitamins, pigments, and volatile compo-nents. It contains thymoquinone and its related compounds; thymol and dithymoquinone as major active constituents (10), which have antioxidant activities (11-14), scavenging oxygen free radicals, generated during many degenerative diseases (10).
Ascorbic acid (AA) is a low molecular mass antioxidant that quenches free radicals. It is highly water soluble, and acts as an effective reducing agent. It is one of the most effective antioxidants and free radical scavengers, inhibiting lipid peroxidation induced by peroxyl radicals (15-16). It may also be essential for maintenance of other antioxidants such as vitamin E (17). Vitamin C ameliorated oxidative stress associated with a wide variety of toxicants such as lead, cadmium and arsenic (18-20).
As far the authors know, no previous studies evaluated the hepatic and renal toxicity of OTC or tried to ameliorate such toxicity in rabbits. Therefore, the present study was designed to investigate the protective effects of NSO and/or AA supplementation on ameliorating the detrimental effects related to liver and kidney injuries as well as hepatic and renal lipid peroxidation and oxidative stress induced by OCT in rabbits.
Materials and Methods
Chemicals
Oxytetracycline HCl and ascorbic acid were purchased from Adwia Pharmaceuticals, Cairo, Egypt. N. sativa oil (Baraka® 450 mg capsules) was purchased from Pharco pharmaceuticals, Alexandria, Egypt. All kits were purchased from Biodiagnostics Co., Cairo, Egypt. LDH kit was purchased from Randox Laboratories Ltd, U.K. Other chemicals used in this study were analytical grade.
Animals and experimental design
In the current study, a total of 40 healthy male white New Zealand rabbits weighing 1800±200 g and aged 75±15 days were used, and housed in metal batteries. The basal diet and water were offered ad libitum. After one week, all animals were randomly divided into five groups (of eight each). Group I (control) received saline and served as negative control, and group II (OTC) orally received oxytetracycline at a dose of 200 mg/kg body weight (21), and served as positive control. Groups III (OTC-NSO) and IV (OTC-AA) were administered with NSO and AA at doses of 2 ml/kg and 200 mg/kg body weight, respectively, one hour prior to OTC intoxication; these doses were chosen according to Saleem et al, 2012 (22). The animals in the group V (OTC-NSO-AA) were administered both NSO and AA in combination at the same dose and regimen used for the group III and IV, 1 hr prior to OTC administration at the dose used for group II. All treatments were daily given orally, using intra-gastric intubation for 15 successive days (Table 1).
Table 1.
Summary of different rabbit groups and their treatment
| Group | Oxytetracycline | Nigella sativa oil | Ascorbic acid |
|---|---|---|---|
| Control | - | - | - |
| OTC | + | - | - |
| OTC-NSO | + | + | - |
| OTC-AA | + | - | + |
| OTC-NSO-AA | + | + | + |
Oxytetracycline 200 mg/kg body weight, daily for 15 days (OTC), Nigella sativa oil 2 ml/kg body weight, 1 hr before OTC, daily for 15 days (NSO), Ascorbic acid; 200 mg/Kg body weight, daily for 15 days, 1 hr before OTC administration (AA)
At the end of the experiment, blood samples were collected from the ear vein and centrifuged at 3000 rpm for 15 min to obtain clear sera. All animals were slaughtered, and livers and kidneys were excised for further preparation for lipid peroxidation and antioxidant parameters evaluation. The experimental design and animal handling conform to the National Institutes of Health (NIH) guidelines and were approved by an Egyptian local committee at Suez Canal University, Faculty of Veterinary Medicine.
Serum biochemical analysis
Freshly separated sera were used for estimation of serum hepatic and renal injury markers according to manufacturer’s protocol. The kits were used for evaluation of the activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) according to Reitman and Frankel, 1957 (23). Alkaline phosphatase (ALP) was determined according to Tietz et al, 1983 (24). The enzyme activity was expressed as units/liter computed directly from the absorbance values. Serum cholesterol was measured according to Allain et al, 1974; Richmond, 1973 (25-26). The serum level of total protein was evaluated according to Lowry et al, 1951 (27), While total bilirubin was determined according to Schattmann, 1952 (28). Serum LDH activity was determined enzymatically according to Buhl and Jackson, 1978 (29). The renal products; urea was evaluated according to Coulombe and Favreau, 1963 (30), uric acid according to Whitehead et al, 1991(31), and creatinine according to Larsen, 1972 (32).
Evaluation of lipid peroxidation and antioxidant biomarkers
The hepatic and renal tissues collected from each rabbit group were excised, cleaned, and immediately perfused with cold saline. The tissues were homo-genized in cold phosphate buffer saline (pH 7.4, 0.1 M). Then, homogenate was filtered and centrifuged (at 2000 g for 20 min). The supernatant was then stored at -80°C until use for further biochemical analysis of lipid peroxidation and antioxidant biomarkers.
Lipid peroxidation was evaluated through measurement of MDA content in the tissues according to Mihara and Uchiyama, 1978 (33). Oxidative status was assessed by evaluation of the enzymatic antioxidant biomarker; catalase (CAT) according to
Aebi, 1984 (34), superoxide dismutase (SOD) according to Nishikimi et al, 1972 (35), and the non-enzymatic antioxidant marker; reduced glutathione (GSH) according to Beutler et al, 1963 (36). Moreover, total antioxidant capacity (TAC) was determined according to Koracevic et al, 2001 (37).
Statistical analysis
All data were expressed as means±SEM and statistically analyzed using SPSS 17.0., using one-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test to examine the difference among the experimental groups, at significance level of P≤0.05.
Results
Serum biochemical analysis
Oral administration of OCT (200 mg/kg/daily, for 15 days) significantly (P≤0.05) increased hepatic damage marker enzyme activities in rabbit serum. Transaminases; ALT and AST were significantly elevated by more than 2 folds compared to the control group. Moreover, ALP, LDH, cholesterol and total bilirubin were raised in rabbit serum after OTC administration. Meanwhile, serum total protein level was decreased in the OTC group. In the same way, the renal injury markers were significantly (P≤0.05) elevated after oral OTC administration; serum urea, uric acid and creatinine were significantly increased. Oral administration of AA (200 mg/kg bw) or NSO (2 ml/kg bw) alone or in combination 1 hr before OTC administration significantly (P ≤0.05) decreased the elevated hepatic and renal injury marker compared with OTC treated group. The results revealed that NSO and AA in combination tend to have a trend toward better protection as compared to single treated groups with either NSO or AA (Table 2).
Table 2.
Serum enzymes activity and biochemical parameters in control and different treated groups
| Enzymes | Experimental groups | ||||
|---|---|---|---|---|---|
| Control | OCT | OTC-NSO | OTC-AA | OTC-NSO-AA | |
| AST u/l | 58.66a± 2.43 | 120.04c±7.11 | 74.80b± 3.82 | 83.56b± 1.46 | 61.16a± 1.68 |
| ALT u/l | 27.21a± 1.54 | 60.77d±3.25 | 40.03b± 2.50 | 49.26c± 0.77 | 29.71a ± 1.45 |
| ALP u/l | 20.66a± 0.66 | 31.09b± 2.74 | 24.15a± 1.02 | 25.53a± 0.77 | 22.16a± 0.84 |
| LDH u/l | 45.52a± 1.76 | 77.20c± 3.42 | 52.94ab± 2.22 | 55.76b± 2.43 | 48.06a± 1.80 |
| Cholesterol mg/dl | 60.80a ± 1.89 | 91.14c ± 3.33 | 70.51b ± 1.54 | 76.52b ± 1.42 | 61.30a ± 1.70 |
| T.protein g/dl | 8.14a±0.16 | 6.02c± 0.21 | 7.59ab± 0.14 | 7.24b± 0.13 | 7.95a± 0.18 |
| T bilirubin mg/dl | 1.4a± 0.03 | 1.89c± 0.02 | 1.43a± 0.02 | 1.55b± 0.05 | 1.44a± 0.02 |
| Urea mg/dl | 26.09a± 1.40 | 42.78c± 2.29 | 34.90b ± 1.88 | 37.79b ± 1.22 | 29.08a± 1.57 |
| Uric acid mg/dl | 25.90a± 1.37 | 45.22c± 2.03 | 33.63b± 1.31 | 37.37b± 1.25 | 28.02a± 1.09 |
| Creatinine mg% | 0.60a± 0.03 | 3.13c± 0.18 | 1.77b± 0.10 | 1.93b± 0.24 | 0.95a± 0.07 |
Data are expressed as means±SEM of eight rats. Oxytetracycline (OTC), Nigella sativa oil (NSO), ascorbic acid (AA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total protein (T protein), total bilirubin (T bilirubin). One way ANOVA-test followed by Duncan’s Multiple Range Test; n=8 (P ≤0.05) Within the same row different superscript indicate statistical significance (P ≤0.05)
Hepatic lipid peroxidation and antioxidant status
Daily administration of OTC (200 mg/kg) significantly (P≤0.05) increased the hepatic lipid peroxidation; MDA compared with the control group. Moreover, OTC significantly reduced liver antioxidant capacity as indicated by declines in GSH, SOD and CAT to levels ranging from about one-third to two-thirds of those in the control group.
The combination of oral NSO (2 ml/kg/day) and AA (200 mg/kg/day) significantly (P≤0.05) reinstated the antioxidant capacity in the rabbit liver homogenate of the OTC-NSO-AA group to the normal level, and tended to return GSH, SOD and CAT comparable to the control values. In the OTC- NSO or OTC- AA group, the MDA level was significantly (P ≤0.05) decreased, while the other non-enzymatic; GSH and enzymatic; SOD and CAT antioxidant markers were significantly increased in comparison with the OTC treated group, but were still below the normal range. These results indicate the synergistic hepatoprotective and antioxidant effects of NSO and AA when they were given in combination (Figure 1).
Figure 1.
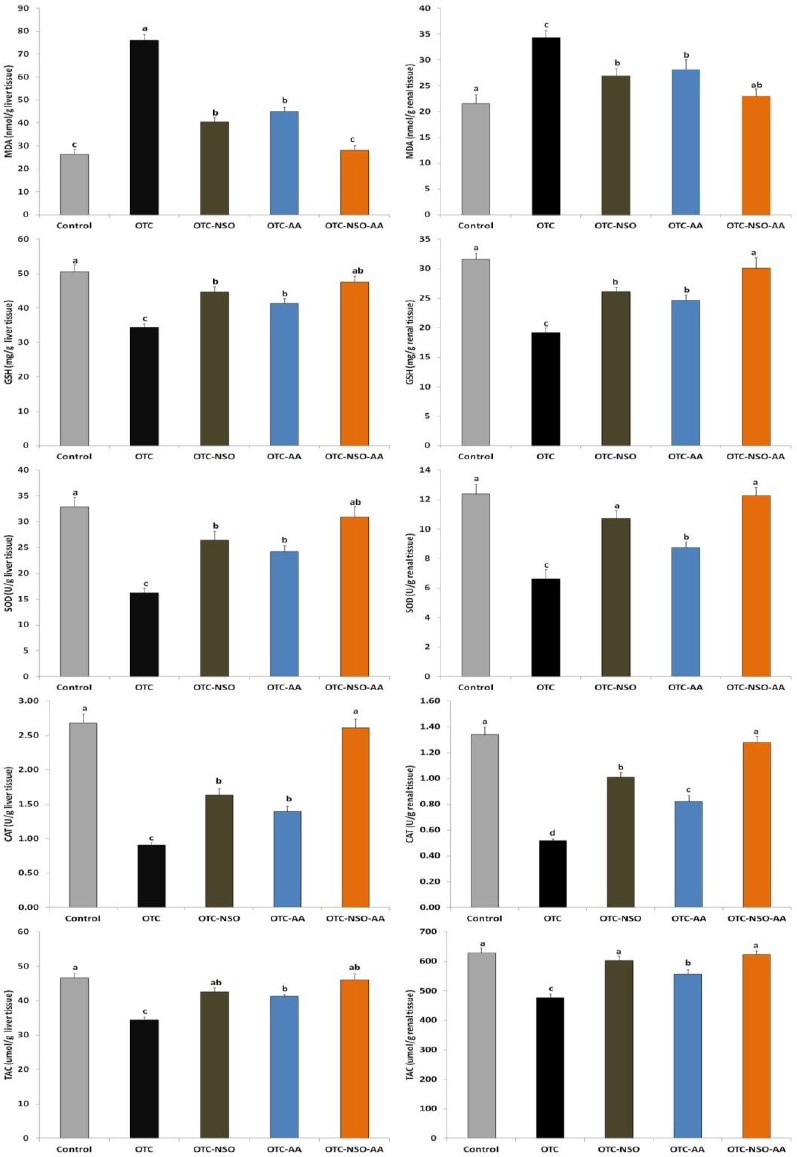
Oxytetracycline (OTC), Nigella sativa oil (NSO), ascorbic acid (AA), malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (TAC), One way ANOVA-test followed by Duncan’s Multiple Range Test; n=8 (P ≤0.05), different letters indicate statistical significance (P ≤0.05). All parameters were evaluated per g wet tissue
Renal lipid peroxidation and antioxidant status
The effects of OTC with and without NSO and/or AA supplementation on the renal lipid peroxidation and antioxidant activities were demonstrated in Figure 1. Parallel to results of hepatic MDA and oxidative status, OTC intoxication significantly (P ≤0.05) elevated the renal MDA and reduced SOD, CAT, GSH and TAC when compared to the control group. In the same manner as effects on liver, daily administration of NSO and AA in combination at the dose of 2 ml/kg and 200 mg/kg, respectively, significantly (P≤0.05) restored the antioxidant activities in the OTC-NSO-AA group to normal levels. NSO or AA, when each of them was given alone, significantly reduced MDA and increased GSH, SOD, CAT and TAC compared to the OTC group, but each one at the selected dosage was unable to restore all renal biochemical parameters to normal levels.
Discussion
Recently, pharmaceuticals have been receiving increasing attention regarding their potential harmful effects, mainly because they are proposed to induce biological effects; they are lipophilic, persistent, and have been found in soils, water, and organisms (38). OTC is an important example of these pharmaceuticals, widely used to cure many of the human and animal diseases. In developing countries, OCT is often used for extended periods at overdose, without veterinarian supervision, which might lead to unexpected toxicological problems; some with apparent clinical symptoms; diarrhea and gastrointestinal upset, while others are with hidden symptoms such as immunosuppression, for ordinary breeders this leads to severe economic losses (39).
In the present study, hepatorenal injuries caused by OTC might be attributed to the oxidative stress resulting from excessive free radical production manifested by increased serum liver injury markers; AST, ALT, ALP, cholesterol LDH, and total bilirubin, and decreased serum total protein level. Although these biomarkers are not specific for liver injury, the increase in their activity reflects active liver dysfunction. Furthermore, OTC treatment elevated levels of serum renal injury markers such as urea, uric acid, and creatinine (Table 2). OTC treatment elevated ALT and AST enzyme activities as well as urea, uric acid and creatinine levels in rats and rabbits (3-4, 21). These alterations might differ depending on exposure time and the dose given. OTC treatment elevated lipid peroxidation through increased hepatic and renal MDA levels, decreased hepatic and renal enzymatic; SOD and CAT as well as non-enzymatic; GSH antioxidant levels. At the same time, hepatic and renal TAC was also reduced (Figure 1). All these effects are involved in OTC-induced hepato-renal oxidative damage and toxicity, as a result of excessive generation of free radicals, which have been reported to affect various biological molecules, including lipids and induce lipid peroxidation. The activities of the enzymes involved in glutathione pathways were also disrupted in the OTC treated group (Figure 1), indicating the involvement of oxidative stress in OTC-mediated hepatorenal damage. These results are in harmony with the previous findings (3-4, 22), and point to the role of ROS in OTC-mediated injury and toxicity.
The exact mechanism of OTC-induced hepato-renal toxicity is still unknown. It may result from accumulation of OTC when they are not eliminated quickly enough or by administration of frequent and/or large doses above recommended therapeutic dosages (4). Many lines of evidence show that OTC produces severe microvesicular steatosis of the liver and even hepatic injury when given in high doses in human (4). The present study revealed that hepatic and renal injuries caused by OTC might be attributed to the oxidative stress resulting from free radical production. Oxidative stress, mitochondrial damage and intracellular glutathione depletion are the most important factors contributing to the prediction of hepato-renal toxicity (7-8). Reactive oxygen species (ROS) are instantly produced in the mammalian body due to exposure to a wide range of exogenous chemicals, drugs and xenobiotics in our ecosystem (8, 40). Under normal circumstances, equilibrium between the ROS generated and the endogenous antioxidants exists, as the ROS generated is neutralized by these antioxidants (8, 41). Hazard effects induced by ROS occur as a result of an imbalance between the ROS production and inactivation, leading to irregularities in cellular function and different pathological conditions (42). ROS has been implicated in the etiology of many degenerative diseases such as cancer, cataract, stroke, coronary heart disease, diabetes, Alzheimer’s, rheumatoid arthritis, and ageing process (43-46).
In the current study, the pre-administration of NSO (2 ml/kg) reduced the serum hepatic and renal injury markers. Moreover, it reduced the lipid peroxidation in hepatic and renal tissues. In addition, there were elevations of liver and renal antioxidant enzymes and glutathione levels due to NSO administration. The antioxidant and protective effects of NSO are owed to their content of antioxidant active constituents such as thymoquinone and many related compounds, including thymol and other volatile oils. Many previous literatures showed the protective and antioxidant effects of NSO and its active constituents against drugs, chemicals and xenobiotics (11-14).
Pre-treatment with AA played a role in ameliorating OTC-induced toxicity, and its free radical scavenging abilities seem to mediate such a protective effect, indicated by the reduction of MDA as well as the elevation of GSH and SOD, CAT levels in hepatic, renal and heart tissues (14, 15). Jayanthi and Subash, 2010 revealed that, OTC elevated AST, ALT, ALP, LDH enzymes’ activity as well as bilirubin levels. In addition, MDA and hydroperoxides were increased in both plasma and liver tissues. Moreover, SOD, CAT and glutathione peroxidase (GSH-Px) were also elevated in the hepatic tissue (21). All these alterations were modulated by the pre-administration of the antioxidant caffeic acid. Gnanasoundari and Pari, 2006 studied the effect of OCT (200 mg/kg 15 days; similar to the dose and duration used in the current study) on rat kidney and found that it significantly increased serum urea and creatinine, significantly increased lipid peroxidation markers (lipid hydroperoxide and MDA) and decreased antioxidant enzymes (SOD, CAT and GSH-Px). Administration of the flavonoid and free radical scavenger; naringenin attenuated the OCT-induced nephrotoxicity (47). The protective effect of NSO and/or AA against OTC-induced oxidative stress in our rabbit model could be either direct by inhibiting lipid peroxidation and scavenging free radicals, or indirect through the enhancement of SOD and CAT activities; the enzymatic free radical scavengers in the cells. Therefore, NSO and AA could be used in combination to prevent and treat hepatic and renal diseases, especially those induced by oxidative damage.
Acknowledgment
This research received no grants from any funding.
Footnotes
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Nelson ML, Levy SB. The history of the tetracyclines. Ann N Y Acad Sci. 2011;1241:17–32. doi: 10.1111/j.1749-6632.2011.06354.x. [DOI] [PubMed] [Google Scholar]
- 2.Polec RB, Yeh SD, Shils ME. Protective effect of ascorbic acid, isoascorbic acid and mannitol against tetracycline-induced nephrotoxicity. J Pharmacol Exp Ther. 1971;178:152–158. [PubMed] [Google Scholar]
- 3.Naseer F, Alam M. The protective effect of ascorbic acid on oxytetracycline induced nephrotoxicity and hepatotoxicity. J Pak Med Assoc. 1987;37:73–75. [PubMed] [Google Scholar]
- 4.Saraswat B, Visen PK, Patnaik GK, Dhawan BN. Protective effect of picroliv, active constituent of Picrorhiza kurrooa, against oxytetracycline induced hepatic damage. Indian J Exp Biol. 1997;35:1302–1305. [PubMed] [Google Scholar]
- 5.Ibrahim AE, Abdel-Daim MM. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell Journal Cell J. 2015;17(1):137–144. doi: 10.22074/cellj.2015.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madkour FF, Abdel-Daim MM. Hepatoprotective and Antioxidant Activity of Dunaliella salina in Paracetamol-induced Acute Toxicity in Rats. Indian J Pharm Sci. 2013;75:642–648. [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol. 2014;92:679–685. doi: 10.1139/cjpp-2014-0144. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Daim MM. Pharmacodynamic interaction of Spirulina platensis with erythromycin in Egyptian Baladi bucks (Capra hircus) Small Ruminant Res. 2014;120:234–241. [Google Scholar]
- 10.Lutterodt H, Luther M, Slavin M, Yin JJ, Parry J, Gao JM, et al. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. Lwt-Food Sci Technol. 2010;43:1409–1413. [Google Scholar]
- 11.Kanter M, Coskun O, Budancamanak M. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J Gastroentero. 2005;11:6684–6688. doi: 10.3748/wjg.v11.i42.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25:218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 13.Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: Evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239–51. [PubMed] [Google Scholar]
- 14.Barakat MK, Oda NR, Bayoumy FA, Bayoumy FA. Effect of Nigella sativa on Carbon Tetrachloride and Paracetamol Induced hepatotoxicity: Role of Antioxidant Enzymes and Cytokines. Faseb J. 2010:24. [Google Scholar]
- 15.El-Demerdash FM, Yousef MI, Zoheir MA. Stannous chloride induces alterations in enzyme activities, lipid peroxidation and histopathology in male rabbit: antioxidant role of vitamin C. Food Chem Toxicol. 2005;43:1743–1752. doi: 10.1016/j.fct.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041–1064. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- 17.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 18.Grosicki A. Influence of vitamin C on cadmium absorption and distribution in rats. J Trace Elem Med Biol. 2004;18:183–187. doi: 10.1016/j.jtemb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004;17:132–139. [PubMed] [Google Scholar]
- 20.Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A. Amelioration of lead toxicity on rat liver with Vitamin C and silymarin supplements. Toxicology. 2005;206:1–15. doi: 10.1016/j.tox.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Jayanthi R, Subash P. Antioxidant effect of caffeic Acid on oxytetracycline induced lipid peroxidation in albino rats. Indian J Clin Biochem. 2010;25:371–375. doi: 10.1007/s12291-010-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleem U, Ahmad B, Rehman K, Mahmood S, Alam M, Erum A. Nephro-protective effect of vitamin C and Nigella sativa oil on gentamicin associated nephrotoxicity in rabbits. Pak J Pharm Sci. 2012;25:727–730. [PubMed] [Google Scholar]
- 23.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, et al. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. 1983;29:751–761. [PubMed] [Google Scholar]
- 25.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- 26.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Schattmann K. A spectrophotometric quantitative caffein-free method for determination of the serum bilirubin index with the Lange universal colorimeter. Arztl Wochensch. 1952;7:1154–1156. [PubMed] [Google Scholar]
- 29.Buhl SN, Jackson KY. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate-to-pyruvate and pyruvate-to-lactate reactions in human serum at 25, 30, and 37 degrees C. Clin Chem. 1978;24:828–831. [PubMed] [Google Scholar]
- 30.Coulombe JJ, Favreau L. A new simple semimicro method for colorimetric determination of urea. Clin Chem. 1963;9:102–108. [PubMed] [Google Scholar]
- 31.Whitehead TP, Bevan EA, Miano L, Leonardi A. Defects in diagnostic kits for determination of urate in serum. Clin Chem. 1991;37:879–881. [PubMed] [Google Scholar]
- 32.Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clin Chim Acta. 1972;38:475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- 33.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 35.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 36.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 37.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halling-Sorensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lutzhoft HC, Jorgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment--a review. Chemosphere. 1998;36:357–393. doi: 10.1016/s0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- 39.Southwood LL. Principles of antimicrobial therapy: what should we be using? Vet Clin North Am Equine Pract. 2006;22:279–296. doi: 10.1016/j.cveq.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 41.Sadeghnia HR, Kamkar M, Assadpour E, Boroushaki MT, Ghorbani A. Protective Effect of safranal, a constituent of Crocus sativus on quinolinic acid-induced oxidative damage in rat hippocampus. Iran J Basic Med Sci. 2013;16:73–82. [PMC free article] [PubMed] [Google Scholar]
- 42.Eldahshan OA, Abdel-Daim MM. Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux-vomica. Cytotechnology. 2014 doi: 10.1007/s10616-014-9723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Daim M, Funasaka Y, Kamo T, Ooe M, Matsunaka H, Yanagita E, Itoh T, Nishigori C. Preventive effect of chemical peeling on ultraviolet induced skin tumor formation. J Dermatol Sci. 2010;60:21–28. doi: 10.1016/j.jdermsci.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Funasaka Y, Abdel-Daim M, Kawana S, Nishigori C. Effect of chemical peeling on the skin in relation to UV irradiation. Exp Dermatol. 2012;21:31–35. doi: 10.1111/j.1600-0625.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Daim M, Funasaka Y, Kamo T, Ooe M, Matsunaka H, Yanagita E, et al. Effect of chemical peeling on photocarcinogenesis. J Dermatol. 2010;37:864–872. doi: 10.1111/j.1346-8138.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 46.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 47.Gnanasoundari M, Pari L. Impact of naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren Fail. 2006;28:599–605. doi: 10.1080/08860220600843805. [DOI] [PubMed] [Google Scholar]


