Abstract
Objective(s):
To investigate the effect of topical administration of alpha-lipoic acid into chitosan conduit on peripheral nerve regeneration using a rat sciatic nerve transection model.
Materials and Methods:
Forty five Wistar rats were divided into three experimental groups randomly. A 10-mm gap of sciatic nerve was bridged with a chitosan conduit following surgical preparation and anesthesia. In treatment group, the conduit was filled with 30 µl alpha-lipoic acid (10 mg/kg/bw).It was filled with 30 µl phosphate buffered saline solution in control group. In Sham group sciatic nerve was just exposed.
Results:
The recovery of nerve function was faster in treatment group than in control, at 4 and 8 weeks after surgery (P-value<0.05). Conduction velocity was better in treatment group than in control group at 4 and 12 weeks (P-value<0.05). Recovery index was higher in treatment group than the control group, 8 weeks after surgery (P-value <0.05). Greater nerve fiber diameter, axon diameter, and myelin sheath thickness were observed in treatment group compared to control group at 8 and 12 weeks after surgery (P-value<0.05). The immunoreactivity of regenerated axons and myelin sheath in treatment group were far more similar to sham group.
Conclusion:
Alpha-lipoic acid when loaded in a chitosan conduit could improve transected sciatic nerve regeneration in rat.
Keywords: Alpha-lipoic acid, Chitosan conduit, Rat, Sciatic nerve regeneration
Introduction
Return to normal function of peripheral nerve injuries is one of the most important aims in neurosurgery. Numerous surgical methods such as nerve graft and conduits filled with neurotrophic substances and cells have been successfully used for improvement of peripheral nerve regeneration (1). Trauma to peripheral nerve leads to acute myelinoaxonal degeneration in the distal area of the damaged nerve, macrophage infiltration, Schwann cell proliferation, and axonal regrowth (2).
It is known that nerve injury is associated with enhanced oxidative stress. Increase of the free oxygen radical levels and reduced activities of antioxidant enzymes are observed after nerve trauma (2-4). The positive effects of local administration of vitamin E and pyrroloquinoline quinone on peripheral nerve regeneration in rat sciatic nerve transection model have been reported (5). Also, the neuroprotective and neurotrophic effects of intraperitoneal injection of ubiquinone (CoQ10) and Crocin on nerve regeneration in rat sciatic crush model have been shown (6, 7).
Antioxidants such as acetyl-L-carnitine, vitamin E, and alpha-lipoic acid (LA) are used successfully in treatment of experimentally nerve crush injuries (4, 8, 9).
LA (1,2-dithiolane-3-pentanoic acid), a disulphide derivative of octanoic acid, is known to act as an efficient anti-oxidant (10). Several studies demons-trated that LA can decrease ischemia-reperfusion injuries in the cerebral cortex (11), heart (12), and peripheral nerve (13). Also, Senoglu et al showed the positive effects of intraperitoneal LA administration on sciatic nerve crush by measuring superoxide dismutase and catalase activities (4). However, the effect of LA on peripheral nerve regeneration in transection model of injury has not been clarified.
The purpose of this study was to investigate the effect of topical administration of LA into chitosan conduit on peripheral nerve regeneration using a rat sciatic nerve transection model. Assessment of nerve regeneration was based on functional (walking track analysis), electrophysiological measurement, muscle mass, histomorphometric, and immunohistochemistry (Schwann-cell detection by S100 expression) criteria at 4, 8, and12 weeks after surgery.
Materials and Methods
Animals
Forty five healthy adult male Wistar rats, weighing 220-250 g were randomized into three groups of 15 animals each. Each group was further subdivided into three subgroups of five animals each. Two weeks before and during the entire experiments, rats were maintained in groups of 5 per cage in a natural day/night cycle in a controlled ambient temperature (23±2°C) with ad libitum food and water. All procedures were carried out in accordance with the guidelines of the Ethics Committee (14) and were approved by the Urmia University Research Council, Urmia, Iran.
Grafting procedure and animal grouping
Under ketamine-xylazine (intra-peritoneal, ketamine hydrochloride 5%; 90 mg/kg and xylazine hydrochloride 2%; 5 mg/kg) anesthesia, surgical technique was done according to standard procedures (15). Briefly, in sham group, after exposing of the left sciatic nerve through a gluteal muscle incision, the muscle was sutured with absorbable 4-0 vicryl sutures, and the skin with 3-0 nylon. In control and treatment groups, following sciatic exposure the nerve was transected proximal to the tibio-peroneal bifurcation where an 8 mm segment was excised, leaving a gap about 10 mm due to retraction of the nerve ends.
The transected proximal and distal stumps were each inserted 2 mm into the 12 mm chitosan conduit and two 10-0 nylon sutures were placed at each end of the cuff to fix the tube in place. In treatment group the chitosan conduit was filled with LA solution (10 mg/kg/bw; prepared up to 30 µl with PBS solution, Sigma-Aldrich Chemie, Munich, Germany) and in control group the chitosan conduit was filled with 30 µl PBS solution (4). Sterile Vaseline was used to seal the ends of the tubes to avoid leakage. The surgical incision was closed as mentioned above.
The preparation and the efficacy of chitosan conduit on peripheral nerve regeneration in rat model have been described in our pervious study (16). Briefly, chitosan solution was prepared by dissolving medium molecular weight, crab shell chitosan (~400 kDa, 85% deacetylated) (Fluka, Sigma-Aldrich St. Louis, MO, USA) in an aqueous solution (1% v/v) of glacial acetic acid (Merck, Darmstadt, Germany) to a concentration of 2% (w/v) while stirring on a magnetic stirrer-hot plate. The solution was stirred with low heat (at 50°C) for 3 hr. The resultant chitosan was filtered through a Whatman No. 3 filter paper. Again, to remove any un-dissolved particles the solution was filtrated through vacuum filtration. To overcome the undesired fragile character, glycerol (Sigma Chemical Co., St. Louis, MO, USA) was added as 30% (w/w) of the total solid weight in solution (17). Chitosan conduit was made by gentle injection of the prepared solution into a home-made mold. The conduit was 2 mm in internal diameter and 12 mm in length (16).
No drugs were administered during the postoperative period. The animals were anesthetized (described above) and euthanized by transcardial perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH 7.4) at 4 (n= 5), 8 (n= 5), and 12 weeks (n= 5) after surgery.
Sciatic functional index (SFI)
Evaluation of SFI was done on one day before surgery and on 4, 8, and 12 weeks following surgery based on the work of Bain et al (18). After painting of hind paws with water soluble blue ink, rats immediately walked along an 8 × 80 cm corridor lined with white paper. The paw-prints were collected. Paw length and toe spread were measured. SFI was calculated for each animal by the following formula:
SFI= -38.3[(EPL-NPL)/NPL]+109.5[(ETS-NTS)/NTS] + 13.3[(EIT-NIT)/NIT] - 8.8
PL is the distance from the third toe to its heel, TS is the distance from the first to the fifth toe, and IT is the distance from the second toe to the fourth toe on the experimental side (E) and the contralateral normal side (N) in each rat. SFI equal to -100 indicates significant impairment, whereas an SFI oscillating around 0 is considered to reflect normal function.
Electrophysiological measurements
At 4, 8, and 12 weeks after surgery, the electro-physiological studies were performed under general anesthesia (as described above) with Nacro bio system 320-3760 A trace 80 (Austin, Texas, USA). After exposing of sciatic nerve (both the operated side and non-operated side), single electrical pulses (at supramaximal intensity) were delivered via bipolar electrodes placed in turn at the proximal and distal trunk of the regenerated nerve cable and electromyography (EMG) was recorded by inserting an electrode into the belly of gastrocnemius muscle. After recording of EMG, differences in latency of EMG, the amplitude and the distance between the proximal and distal sites of stimulation were measured to calculate the conduction velocity (19). To remove variations between animals, the conduction velocity of the bridged nerve was expressed as a percentage of that on the intact side of each animal (% CVR) (20). The recovery index of EMG amplitude in all groups was calculated by the formula: recovery index = peak amplitude of the operated side/peak amplitude of the intact side (21).
Muscle weight measurement
Following electrophysiological assessments, the animals were euthanized and the gastrocnemius muscles were collected and weighed. The muscle wet weight ratio was determined by the following equation: experiment site muscle wet weight/contralateral normal site muscle wet weight x 100%.
Histological preparation and morphometric studies
Graft middle cable of sham, treatment, and control groups was harvested and immediately fixed in 2.5% glutaraldehyde. The grafts were then embedded in paraplast paraffin, cut in 5μm, and then stained with toluidine blue. Morphometric analysis was performed using an image analyzing software (Image-Pro Express, version 6.0.0.319; Media Cybernetics, Silver Springs, MD). Equal opportunity, systematic random sampling, and two-dimensional dissector rules were followed to cope with sampling- related, fiber-location-related, and fiber-size related biases.
Immunohistochemical analysis
Anti-S-100 (1:200, DAKO, North America, Inc. 6392, Via Real, Carpinteria, CA) was used as marker for myelin sheath. Specimens prior to immunohisto-chemistry were fixed with 4% paraformaldehyde for two hr and embedded in paraffin. After blocking non-specific immunoreactions, sections were incubated in S-100 protein antibody solution for one hr at room temperature. They were washed three times with PBS and incubated in biotynilated anti-mouse rabbit IgG solution for one hr. Secondary antibody conjugated to horseradish peroxidase was developed by the diaminobenzidine method. The results of Immunohistochemistry were examined under a light microscope. The immunohistochemical results were stratified as positive, more positive, and clearly more positive terms (22).
Statistical analysis
Experimental results were expressed as means ± SD. All data were analyzed by one-way ANOVA to assess differences between experimental groups (SPSS 17.0 for Windows, Chicago, IL, USA). Dunnett’s test for pair wise comparisons was used to examine the effect of time and treatments. The differences were considered significant when P-value <0.05.
Results
Figure 1 shows the mean SFI values in the three experimental groups at 4, 8, and 12 weeks after surgery. Prior to the surgery, the SFI values in all groups were near zero. The mean SFI value decreased to -100 due to the complete loss of sciatic nerve function in control and treatment groups. All groups indicated tendencies to improve in SFI with time. Statistical analysis revealed that the recovery of nerve function was significantly faster in the treatment group than in the control group (P-value< 0.05) at 4 and 8 weeks after surgery. The mean SFI values in the treatment group were -34.4 ± -3.21, -26.8 ± -2.32, -21.3 ± -1.7 at 4, 8, and 12 weeks after surgery, respectively. The mean SFI value in the control group at 4, 8, and 12 weeks after surgery was -56.3 ± -3.39, -38.6 ± -2.18, and -23.7 ± -3.51, respectively.
Figure 1.

Diagrammatic representation of effects on sciatic functional index. Treatment with alpha-lipoic acid gave better results in functional recovery of the sciatic nerve. Data were presented as mean ± SD. *P-value <0.05, compared with control group
The percentage conduction velocity rate (CVR) and recovery index of EMG amplitude of experimental groups are given in Table 1. In all experimental groups, data showed that nerve regeneration improves with time. Electrophysiological parameters of treatment group were better than control group, particularly; there were significant difference at 4 and 12 weeks after surgery for %CVR and at 8 week after surgery for recovery index.
Table 1.
Electromyography results of the each experimental group: values are given as mean±SD
| % Conduction velocity rate (%CVR) | Recovery index | |||||||
|---|---|---|---|---|---|---|---|---|
| Weeks | 4 | 8 | 12 | 4 | 8 | 12 | ||
| Group | ||||||||
| Sham | %96±1.8 | %95 ± 1.4 | %97 ± 1.9 | 0.84±0.03 | 0.86±0.02 | 0.89±0.01 | ||
| Treatment | %16 ± 2* | %29 ± 1.4 | %39 ± 1.8* | 0.13 ± 0.03 | 0.26 ± 0.02* | 0.29 ± 0.02 | ||
| Control | %6.9 ± 1.2 | %24.6 ± 2.3 | %26.5 ± 1.5 | 0.1 ± 0.02 | 0.16 ± 0.02 | 0.25 ± 0.03 | ||
P-value <0.05 versus control group
In sciatic nerve transection, lack of neural innervation to gastrocnemius muscle resulted in decrease in the muscle mass. The mean weight of gastrocnemius muscle in left operated limb was significantly (P-value< 0.05) higher in treatment group (0.8±0.05 g) compared to control group (0.63±0.03 g). The muscle wet weight ratio was in favor of treatment group however, this difference was not statistically significant between treatment (%36.5±%2.5) and control groups (%32.8±%1.4) (P-value> 0.05). In general, these measurements showed weight loss of the gastrocnemius muscle made better using LA.
Quantitative morphometric analyses showed that there were regenerated axons and fibers into the chitosan conduit in both treatment and control groups (Table 2). Morphological parameters of the treatment group were significantly better than the control group (P-value< 0.05) at 4 weeks after surgery. Furthermore, treatment group showed significantly greater nerve fibers diameter, axon diameter, and myelin sheath thickness than of control group (P-value< 0.05) at 8 and 12 weeks after surgery. The number of fibers was higher in the treatment group compared to the control group, however, this difference was not significant between two groups at 8 and 12 weeks after surgery.
Table 2.
Morphometric analyses of the sciatic regenerated nerves for each of the experimental group
| Sham | Treatment (alpha-lipoic acid) | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | 4 | 8 | 12 | 4 | 8 | 12 | 4 | 8 | 12 |
| N | 8024±404 | 8379 ± 446 | 8124 ± 385 | 2511 ± 183 | 2733±231 | 3255± 189 | 1378 ± 176† | 2673 ± 215 | 3024 ± 198 |
| D | 12.01±0.01 | 11.93±0.17 | 12.06±0.23 | 7.34± 0.49 | 9.88± 0.33 | 10.54± 0.42 | 2.83 ± 0.18† | 4.51± 0.24† | 4.86 ± 0.19† |
| d | 7.03 ± 0.02 | 6.97 ± 0.39 | 7.06 ± 0.46 | 3.44 ± 0.39 | 5.37± 0.27 | 6.22 ± 0.18 | 2.12 ± 0.22† | 3.74± 0.23† | 3.77±0.21† |
| T | 2.56 ± 0.01 | 2.48 ± 0.02 | 2.53 ± 0.01 | 2.14± 0.32 | 2.21 ± 0.26 | 2.29 ± 0.29 | 0.41± 0.02† | 0.43 ± 0.03† | 0.63 ± 0.03† |
N: number of fibers, D: diameter of fibers (µm), d: diameter of axon (µm), T: thickness of myelin sheath (µm).
results were significantly different at P-value <0.05
Immunoreactivity to S-100 protein was extensively observed in the cross-sections of regenerated nerve segments. The expression of S-100 protein signal was located mainly in the myelin sheath. The axon also showed a weak expression indicating that a Schwann cell-like phenotype was existed around the myelinated axons (Figure 2). The immunoreactivity of regenerated axons and myelin sheath in treatment group was far more similar to sham group. In both treatment and control groups, the expression of S-100 corresponded with the results of the morphometric analyses.
Figure 2.
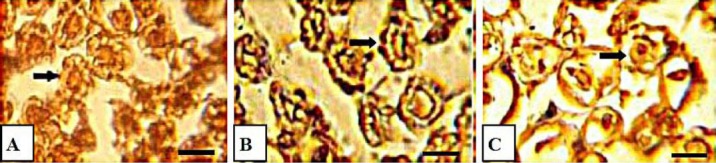
Photomicrographs showing results of immunohistochemical analysis of the regenerated nerves at 12 weeks after surgery in mid-graft sections obtained from the sham-surgery (A), treatment (B), and control (C) groups. There is clearly more positive staining of the myelin sheath–associated protein S-100 (arrow) within the nerve periphery, indicating well-organized structural nerve reconstruction, in the LA-treated nerve. Scale bar: 10 μm, LA: alpha lipoic acid
Discussion
The results of present study showed when LA loaded in a bridge of chitosan conduit is able to enhance sciatic nerve regeneration. To establish a favor technique in peripheral nerve repair, experimental and clinical trials have been implemented through entubulization and/or using trophic or tropic factors.
Severed axon may result in degeneration through Wallerian degeneration (23). After axonal injury, oxidative stress is considered to be one of the main causes of neural damage. It has been reported that antioxidant molecules including catalase, superoxide dismutase, and glutathione-S-transferase play an important role in peripheral nerve injury and regeneration (24).
In the present study, to promote sciatic nerve regeneration we applied LA topically into a chitosan conduit. The results showed beneficial effects of LA through faster and significant improvement of the nerve functional recovery in walking track analysis (SFI). This appropriate method is a coordinated activity involving sensory input, motor response, and cortical integration (25).
EMG has a determining power when evaluating nerve regeneration and electrical functionality of the regenerated nerves (26). The results of EMG assessment in the present study showed that better improvement of conduction velocity indicated the greater extent of myelin sheath formation in treatment group which is in agreement with other findings (27). Also, better recovery index in treatment group could result from better innervating of the regenerated nerve fibers to the muscle in treatment group. Meanwhile the amplitude of EMG is directly comparative to the number of nerve fibers innervating the muscle that allows the conduction velocity of the motor nerve to be calculated (28).
In the present study, both experimental groups showed reduction in gastrocnemius muscle mass in the left (injured) limb relative to the right (uninjured) limb at 12 weeks after surgery. The mean muscle weight ratios were greater in treatment group compared to control group indicating indirect evidence of successful end-organ reinnervation by topical application of LA. As the posterior tibial branch of the sciatic nerve regenerates into the muscle, it will regain its mass proportional to the amount of axonal reinnervation (29).
In the present study, alongside with the SFI and electrophysiological assessments, extensive morphological analysis of the regenerated nerves showed the distinct positive effect of LA on multiple parameters such as axonal and fiber diameters and myelinated surfaces in treatment group compared to control group.
In immunohistochemistry, the location of positive reactions to S-100 in treatment group were clearly more positive than control group further implying that those regenerated axon and Schwann cell-like cells existed and were accompanied by the process of myelination and the structural recovery of regenerated nerve fibers.
Evaluated parameters of our study showed that using of LA has positive greatest effect on sciatic nerve regeneration at 4 week after surgery and this effect is persevered until the end of the experimental period. Although, the exact mechanism of LA in peripheral nerve regeneration remains unclear, this effect may be resulted from its antioxidant property. The neuroprotective effect of LA after sciatic nerve crush injury by measuring of superoxide dismutase (SOD), catalase (CAT) activities, and malondialdehyde (MDA) levels have been reported (4). The neuroprotective and neurotrophic effects of antioxidants on peripheral nerve regeneration have been shown. Systemic administration of vitamin E in crush injury (10) and local administration of vitamin E and pyrroloquinoline quinone in transection model (5) have been demonstrated. The neuroprotective effects of LA and vitamin E on neuropathies associated with diabetes has been also reported (11, 30).
Conclusion
The results of the present study showed that LA when loaded in a chitosan conduit could improve transected sciatic nerve regeneration in rat.
Acknowledgment
This work was financially supported by the Faculty of Veterinary Medicine of Urmia University, Urmia, Iran. We thank Dr Mohammadi, and Mr Jaafary for their expert technical help. The results described in this paper were part of student thesis.
References
- 1.Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12:65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 2.Rotshenker S. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation. 2011;8:109. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coban YK, Ciralik H, Kurulas EB. Ischemic preconditioning reduces the severity of ischemia-reperfusion injury of peripheral nerve in rats. J Brachial Plex Peripher Nerve Inj. 2006;1:2. doi: 10.1186/1749-7221-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senoglu M, Nacitarhan V, Kurutas EB, Senoglu N, Altun I, Atli Y, et al. Intraperitoneal Alpha-Lipoic Acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj. 2009;4:22. doi: 10.1186/1749-7221-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizi A, Azizi S, Heshmatian B, Amini K. Improvement of functional recovery of transected peripheral nerve by means of chitosan grafts filled with vitamin E, pyrroloquinoline quinone and their combination. Int J Surg. 2014;12:76–82. doi: 10.1016/j.ijsu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Moradi Z, Azizi S, Hobbenaghi R. Ubiquinone improves functional recovery and morphometric indices of sciatic nerve regeneration. Iran J Veterinary Res. 2014;15:392–396. [PMC free article] [PubMed] [Google Scholar]
- 7.Tamaddonfard E, Farshid AA, Ahmadian E, Hamidhoseyni A. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran J Basic Med Sci. 2013;16:83–90. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson AD, Hart A, Brännström T, Wiberg M, Terenghi G. Delayed acetyl-L-carnitine administration and its effect on sensory neuronal rescue after peripheral nerve injury. J Plast Reconstr Aesthet Surg. 2007;60:114–118. doi: 10.1016/j.bjps.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Dokkaew J. Vitamin E reduces sensory neuronal loss and improves nerve regeneration after sciatic nerve injury. Asian Biomed. 2013;7:649–655. [Google Scholar]
- 10.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 12.Freisleben HJ. Lipoic acid reduces ischemia-reperfusion injury in animal models. Toxicology. 2000;148:159–171. doi: 10.1016/s0300-483x(00)00207-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Tritschler HJ, Low PA. Alpha-lipoic acid provides neuroprotection from ischemia reperfusion injury of peripheral nerve. J Neurol Sci. 1999;163:11–16. doi: 10.1016/s0022-510x(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann M. Ethical guidelines for investigations of experimental pain inconscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 15.Nicoli Aldini N, Fini M, Rocca M, Giavaresi G, Giardino R. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int Orthop. 2000;24:121–125. doi: 10.1007/s002640000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raisi A, Azizi S, Delirezh N, Heshmatian B, Amini K. Use of Chitosan Conduit for Bridging Small-Gap Peripheral Nerve Defect in Sciatic Nerve Transection Model of Rat. Iran J Veterinary Surg. 2012;5:89–100. [Google Scholar]
- 17.Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122:161–166. [Google Scholar]
- 18.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Farjah Gh, Heshmatian B, Karimipour M, Saberi A. Using eggshell membrane as nerve guide channels in peripheral nerve regeneration. Iran J Basic Med Sci. 2013;16:901–905. [PMC free article] [PubMed] [Google Scholar]
- 20.Di Benedetto G, Zura G, Mazzucchelli R, Santinelli A, Scarpelli M, Bertani A. Nerve regeneration through a combined autologous conduit (vein plus a cellular muscle grafts) Biomaterials. 1998;19:173–181. doi: 10.1016/s0142-9612(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Tanihara M, Ohnishi K, Suzuki K, Endo K, Nishimura Y. Cat peripheral nerve regeneration across 50 mm defect repaired with a novel nerve guide composed of freeze-dried alginate gel. Neurosci Lett. 1999;259:75–78. doi: 10.1016/s0304-3940(98)00924-0. [DOI] [PubMed] [Google Scholar]
- 22.Choi BH, Zhu SJ, Kim BY, Huh JH, Lee SH, Jung JH. Transplantation of cultured bone marrow stromal cells to improve peripheral nerve regeneration. Int J Oral Maxillofac Surg. 2005;34:537–542. doi: 10.1016/j.ijom.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Johnson EO, Zobous AB, Soucacos PN. Regeneration and repair of peripheral nerves. Injury. 2005;36:24–29. doi: 10.1016/j.injury.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Lanza C, Raimondo S, Verganil L, Catena L, Senes F, Tos P, et al. Expression of antioxidant molecules after peripheral nerve injury and regeneration. J Neurosci Res. 2012;90:842–848. doi: 10.1002/jnr.22778. [DOI] [PubMed] [Google Scholar]
- 25.Castañeda F, Kinne RK. Omental graft improves functional recovery of transected peripheral nerve. Muscle Nerve. 2002;26:527–532. doi: 10.1002/mus.10229. [DOI] [PubMed] [Google Scholar]
- 26.Vleggeert-Lankamp CL. The role of evaluation methods in the assessment of peripheral nerve regeneration through synthetic conduits: a systematic review. Laboratory investigation. J Neurosurg. 2007;107:1168–1189. doi: 10.3171/JNS-07/12/1168. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defectbridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- 29.Hou Z, Zhu J. An experimental study about the incorrect electrophysiological evaluation following peripheral nerve injury and repair. Electromyogr Clin Neurophysiol. 1998;38:301–304. [PubMed] [Google Scholar]
- 30.Van Dam PS, Bravenboer B, van Asbeck BS, Marx JJ, Gispen WH. High rat food vitamin E content improves nerve function in streptozotocin-diabetic rats. Eur J Pharmacol. 1999;376:217–222. doi: 10.1016/s0014-2999(99)00376-3. [DOI] [PubMed] [Google Scholar]


