Abstract
Objective(s):
The neuroprotective effect of lithium has been attributed to its therapeutic action. However, the role of glial cells particularly astrocytes, and the possible interactions between neurons and astrocytes in neuroprotective effects of lithium have been disregarded. Thus, the aim of this study was to evaluate the direct effects of lithium on brain derived neurotrophic factor (BDNF) and glial cell line derived neurotrophic factor (GDNF) in rat primary neuronal, astrocytes, and mixed neuro-astroglial cultures to assess the possible effects of lithium on astrocytes and neuro-astroglia interactions.
Materials and Methods:
Rat primary astrocyte, neuronal and mixed neuro-astrocyte cultures were prepared from cortices of 18-day embryos. Cell cultures were exposed to lithium (1 mM) or vehicle for 1 day (acute) or 7 days (chronic). BDNF and GDNF mRNA and protein levels were determined by RT-PCR and ELISA, respectively.
Results:
Chronic but not acute lithium treatment increased intracellular BDNF and GDNF protein levels in rat primary neuronal and astrocyte cultures, respectively (P<0.05). However, chronic lithium treatment had no significant effect on intracellular BDNF protein level in astrocyte and mixed neuron-astrocyte cultures or GDNF protein levels in mixed neuron-astrocyte culture. Furthermore, acute and chronic lithium treatment had no significant effect on mRNA and extracellular BDNF and GDNF protein levels in three studied cultures.
Conclusion:
Present study showed that chronic lithium treatment affected neurotrophins both in neurons and astrocytes in a cell-type specific manner with no effect on neuron-astrocyte interactions. The findings of this study also highlighted the importance of astrocytes as drug targets involved in the neuroprotective action of lithium.
Keywords: Astrocytes, BDNF, Culture, GDNF, Lithium, Neuro-astroglial, Neurons
Introduction
Bipolar disorder (BD) is a prevalent and highly debilitating chronic disease (1). Despite extensive investigation, the pathophysiology of this disorder is still poorly understood. Growing evidence indicated regional reductions in brain volume accompanied by cellular atrophy/loss in BD (2, 3). Persistent reduction of neurotrophic factors have been proposed to be involved in the underlying processes of cell loss in BD patients (4, 5). In this regard, reduced brain derived neurotrophic factor (BDNF) in serum and cortical area of patients suffering from BD has been shown (5-7). Moreover, an association between BDNF genetic polymorphism and BD onset and incidence has been demonstrated (8, 9). Also, it was proposed that dysregulation of astrocytic neurotrophic factors might play important roles in neuroanatomical changes in brain in BD (10, 11). There are evidences that glial cell line-derived neurotrophic factor (GDNF), as the main neurotrophic factor synthesized and released by astrocytes may be involved in the pathophysiology of BD (12, 13). Taken together, neurotrophic factors BDNF and GDNF may play important roles in the pathogenesis of BD.
Chronic treatment with a lithium salt is the classical treatment for BD. The critical site of action of lithium is still vague. However, most recent cellular, animal and human studies focused on the effects of lithium on neuroplasticity and neuronal resilience, suggest a neuroprotective effect for lithium (14-16). Increasing evidence indicated that lithium can produce neuroprotective effects via increasing neurotrophic factors levels in CNS (17, 18) which may be relevant to its efficacy in the treatment of BD (3). However, the exact mechanism is still elusive. Neuroprotective effect of lithium may be mediated through its direct effect on neurons or indirectly through its effect on glial cells (mainly astrocytes) which provide trophic support for neurons by releasing neurotrophic factors (19-21). Most of the previous studies investigating the mechanism of neuroprotective effects of lithium emphasize its direct neuronal effect and thus might have excluded the role of glial cells. Therefore, the principle objective of this study is to determine to what extent glial cells contribute to neuroprotective effect of lithium. In this regard, considering the involvement of neurotrophic factors BDNF and GDNF in the pathophysiology of BD as described above and regarding the studies implicating the effect of lithium on neurotrophic factors (17, 18), this study was designed to simultaneously evaluate the direct effect of lithium on GDNF and BDNF in neurons, astrocytes and also a mixed neuron-astrocyte culture to determine the possible effect of lithium on neuron-astrocytes interaction.
Materials and Methods
Preparation of rat primary neuronal, astrocyte and neuro-astroglial cultures
The experimental protocol of this study was approved by the animal ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran.
Embryonic cortices were obtained from 18-day embryos of Sprague-Dawley rats (22). Cortices were dissected and triturated in cold Hank’s Balances Salt solution followed by centrifugation at 800 g for 10 min. Precipitated cells were resuspended in Hank’s Balances Salt solution and used for different primary cultures as previously described (22). Briefly, neuronal cells (3.5×106) were seeded in 60mm polyethylene imine (PEI)-coated dishes in neurobasal media (Gibco, USA) supplemented with 2% B-27, 2 mM L-glutamine, 50 U/ml penicillin and 50 µg/ml streptomycin. To prepare astroglial culture, dissociated cells (10-20×106) were transferred to 175-cm2 un-coated culture flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) containing horse serum (10%), L-glutamine (2 mM), penicillin (50 U/ml), and streptomycin (50 μg/ml), as previously described (22). Upon reaching confluence, astrocytes were separated using standard shaking procedures for 72 hr. During shaking procedure, the media was changed every 24 hr to remove all cells, except astrocytes, that were detached from the flask. After 72 hr, the purified astrocytes were detached using trypsin–EDTA (0.05%) and seeded in 10 cm PEI-coated dishes, containing the same culture medium as above. Horse serum was replaced with 1% G5 as a serum free supplement, before exposing the cells to lithium.
Mixed Neuro-astroglia culture was prepared as previously described (22). Dissociated cells (5×106) were seeded in PEI-coated dishes in DMEM medium with 10% horse serum, 2 mM L-glutamine, 50 U/ml penicillin and 50 µg/ml streptomycin. After 72 hr, B27 (1%) was added to the culture medium. On the 4th day, 1.5 mM leucine-leucine methyl ester was added to the medium to deplete microglia from neuron-glia mixed cultures. On the 8th day, horse serum was replaced with 1% G5 supplement, before exposing the cells to lithium.
Cultures were kept at 37°C in a- 95% O2/5% CO2 humidified incubator. The cells were exposed to lithium (1mM) or vehicle for 24 hr (acute treatment) or seven days (chronic treatment) (18, 23, 24). The Cultures’ media were replenished every other day during the seven days of lithium exposure.
The purity of cell cultures and the cell type proportions in each culture was determined by immunocytochemistry as described previously (22) using specific antibodies against specific markers of neurons (MAP-2) and astrocytes (GFAP) and DAPI were used for counting cells (22). At the time of lithium treatment, the enrichment of neuronal and astrocyte cultures was more than 90%. The mixed neuro-astroglia cultures were comprised of approximately 55% astrocytes and 44% neurons.
Quantitative RT-PCR for BDNF and GDNF
Total RNA was extracted by phenol-chloroform extraction method using TriPure Isolation reagent in accordance with manufacturer instruction (25). cDNA was synthesized from 1μg total RNA using RevertAid H Minus First Starnd cDNA Synthesis Kit according to manufacturer guidelines. The relative levels of BDNF [RefSeq: NM_012513], GDNF [RefSeq: NM_019139] and GAPDH [RefSeq: NM017008.3] mRNAs were determined by quantitative real time PCR assay using ABI PRISM 7500 real-time PCR system (Applied biosystem, USA). Specific primers were designed with Allele ID software (version 6). The primers were: for BDNF, forward primer 5’-CAA AGC CAC AAT GTT CCA CCA G-3’, and reverse primer 5’- GCC CAT TCA CGC TCT CCA G -3’; for GDNF, forward primer: 5’- CGC TGA CCA GTG ACT CCA ATA TG -3’, and reverse primer: 5’- TTG ACC ATT TGC CTG AAT GTG TG-3’; and for GAPDH: forward primer 5’- CGT GAT CGAGGGCTGTTG G-3’, and reverse primer 5’-CTGCTTCAGTTG GCC TTT CG-3’. Primers were designed to span exon-exon junction in order to preclude the amplification of genomic DNA. The target amplicon sizes were 179bp, 121bp and 97 bp for BDNF, GDNF and GAPDH, respectively. The threshold cycles (Ct) of samples were used to calculate the ratio of expressions between lithium treated and untreated samples using Pfaffl method (26).
ELISA for quantification of intracellular and extracellular BDNF and GDNF protein levels
The intracellular and extracellular amounts of BDNF or GDNF protein were determined. For intracellular protein measurement, cells were lysed using NP40 buffer. For determination of extracellular BDNF or GDNF, media was collected after each time that culture media was refreshed i.e. one day after acute treatment and every other day during chronic lithium treatment. Samples were stored at -70 °C until use. Total protein was measured by Bradford method (27) using 6 concentrations of BSA as standards. Intracellular and extracellular BDNF or GDNF protein levels in samples (media and cell lysates) were determined by BDNF Emax Immuno Assay or GDNF Emax Immuno Assay ELISA systems (Promega, USA) according to the manufacturer guidelines and expressed as pg per mg of total protein. In brief, acid treated samples and standards were added to wells precoated with anti-BDNF or anti-GDNF monoclonal antibody and incubated overnight. Wells were washed and horse raddish peroxide (HRP) conjugate was added and incubated with tetramethylbenzidine as the HRP substrate at room temperature for 15 min. After adding stop solution, the absorbance was measured at 450nm in a micro-plate reader (Micura, England). The BDNF and GDNF concentrations were interpolated from the standard curves using samples with known BDNF or GDNF concentrations. The intra-assay and inter-assay coefficient of variance was 6% and 10%, respectively.
Statistical analysis
Data were expressed as mean±SEM. To compare BDNF or GDNF gene expressions between lithium- and vehicle-treated cells, Pfaffl method was used by REST software (REST-384-beta) (26) due to different amplification efficiency of BDNF, GDNF and GAPDH. Differences between lithium- and vehicle-treated cells in BDNF or GDNF protein levels were assessed by paired t-test. In addition, to compare the effect of lithium on BDNF or GDNF between three studied cultures, the percent changes from control of each culture were analyzed using one way ANOVA followed by Post hoc LSD test. Statistical analyses were performed using SPSS version 18 and a P value of < 0.05 was considered statistically significant.
Results
Effects of acute and chronic lithium treatment on BDNF and GDNF mRNA levels
Chronic lithium treatment down-regulated BDNF mRNA level in mixed neuro-astroglial, neuronal and astroglial cultures by the factor of 1.40±0.36, 1.34±0.31 and 1.06±0.40, respectively, in com-parison with respective vehicle treated culture (Figure 1a). However, these changes were not statistically significant (P<0.05). In addition, acute lithium treatment up-regulated BDNF mRNA level by the factor of 1.14±0.17 in mixed neuro-astroglial culture while down-regulated BNDF mRNA by the factor of 1.31±0.43 and 1.28±0.31 in neuronal and astroglial cultures, respectively, as compared with respective vehicle treated cultures (Figure 1a). Again, the differences between lithium and vehicle treated cultures were not statistically significant (P>0.05).
Figure 1.
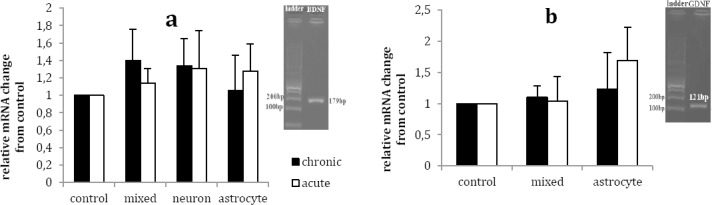
Effects of acute (1 mM for 1 day) and chronic (1 mM for 7 days) lithium treatment on BDNF (a) and GDNF (b) mRNA level in rat primary mixed neuro-astroglial, neuronal and astroglial cultures in comparison to vehicle treated cultures (control, 100 µl sterile distilled water). Data are presented as mean±SEM
Chronic lithium treatment increased the GDNF mRNA level in astrocytes and neuro-astroglial cultures by the factor of 1.10±0.19 and 1.23±0.59, respectively, as compared to vehicle-treated cultures (Figure 1b). However, these changes were not statistically significant (P>0.05). Acute lithium treatment reduced GDNF mRNA levels in mixed neuro-astroglial and astrocyte cultures by the factor of 1.69±053 and 1.04±0.39, respectively, in comparison with vehicle treated cultures (Figure 1b), but these changes did not reach statistical significance (P>0.05).
Effects of acute and chronic lithium treatment on intracellular BDNF and GDNF protein levels
Chronic lithium treatment significantly increased BDNF intracellular protein level by the factor of 3.39 (P<0.05) in neuronal, but not in mixed neuro-astroglial and astrocytes, culture as compared to vehicle (Figure 2a). In addition, acute lithium treatment exerted no significant effect on intracellular BDNF protein levels in three studied cultures in comparison with vehicle treated cultures (Figure 2a).
Figure 2.
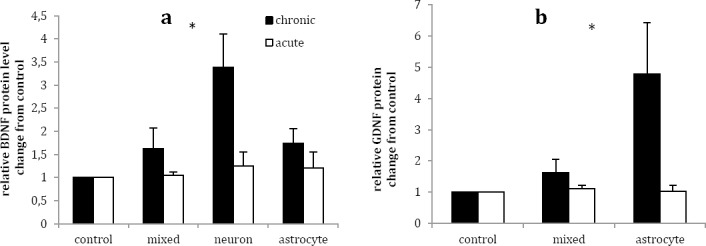
Effects of acute (1 mM for 1 day) and chronic (1 mM for 7 days) lithium treatment on BDNF (a) and GDNF (b) intracellular protein level in rat primary mixed neuro-astroglial, neuronal and astroglial cultures in comparison to vehicle treated cultures (control, 100 µl sterile distilled water). Data are presented as mean±SEM. *P< 0.05 was considered as significantly value
Chronic lithium treatment significantly increased intracellular GDNF protein levels in astrocyte culture by the factors of 4.78 ± 1.66 in comparison to vehicle (P<0.05) (Figure 2b). Although chronic lithium treatment increased intracellular GDNF in mixed neuro-astroglial culture by the factor of 1.63±0.43 compared with vehicle, but it was not statistically significant (P >0.05). Acute lithium treatment had no significant effect on intracellular GDNF protein levels in mixed neuro-astrolglia and astrocytes cultures in comparison with respective vehicle treated cultures (P >0.05) (Figure 2b).
Effects of acute and chronic lithium treatment on extracellular BDNF and GDNF protein levels
Chronic lithium treatment raised extracellular BDNF protein levels in mixed neuro-astroglial, neuronal and astrocyte cultures by the factor of 1.05±0.05, 1.06±0.05 and 1.04±0.15, respectively, but the differences were not statistically significant in comparison with vehicle (P>0.05) (Figure 3a). Furthermore, acute lithium treatment had no significant effect on extracellular BDNF protein levels in three studied cultures as compared to vehicle (P>0.05) (Figure 3a).
Figure 3.
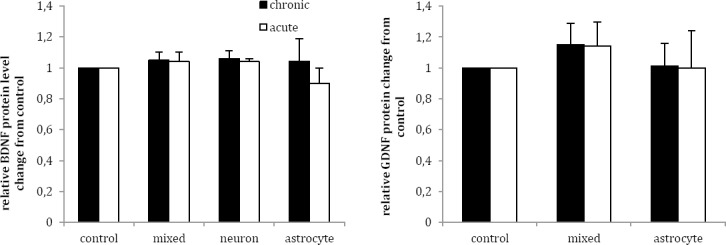
Effects of acute (1 mM for 1 day) and chronic (1 mM for 7 days) lithium treatment on BDNF (a) and GDNF (b) extracellular protein level in rat primary mixed neuro-astroglial, neuronal and astroglial cultures in comparison to vehicle treated cultures (control, 100 µl sterile distilled water). Data are presented as mean±SEM
Chronic lithium treatment non-significantly increased extracellular GDNF protein levels in mixed neuro-astroglial and astrocytes cultures by the factor of 1.15±0.14 and 1.01±0.15 in comparison with vehicle (Figure 3b). Acute lithium treatment exerted no significant effects on extracellular GDNF protein levels in mixed neuro-astroglial and astrocytes cultures as compared to vehicle (P>0.05) (Figure 3b).
Discussion
The present study showed that chronic, but not acute, lithium treatment increased intracellular BDNF protein levels in neurons and GDNF protein levels in astrocytes. These findings suggest that lithium may exert its properties through its direct effect on both neurons and astrocytes but independent of an interaction between neurons and astrocytes, at least under non-stressful condition used in this study. In addition, it can be suggested that a combination of different neurotrophic factors and a cell-type specific pattern contribute to neuroprotective effects of lithium. This may be due to the fact that many neurotrophic factors are mainly specific for a certain cell type i.e. astrocytes are the main source of GDNF synthesis (28) while BDNF is produced more specifically by neurons (29). Therefore, lithium may affect a neurotrophine factor which has a prominent function for a given cell.
In the current study, chronic but not acute lithium treatment increased intracellular BDNF protein levels in neuronal culture. Increased intracellular BDNF protein levels has been observed after 5-day (but not after 3 or 7-day) lithium treatment (1mM) in rat cortical neuronal culture (23). On the contrary, there is a report that lithium (1 mM) increased and decreased intracellular BDNF levels after 3 and 5 days, respectively, in rat neuronal cortical culture as compared to untreated culture (18). The reasons for discordant findings of the two studies after 5-day lithium treatment and the time dependent effect of lithium on intracellular BDNF protein levels in these studies are not clear. Although an increase in the secretion of BDNF was proposed to underlie the decreased BDNF level after 5-day lithium treatment, but this might be related to the unstable condition of their experiment as can be implied from the increased BDNF levels in their untreated neuronal culture after 5 days (18). In addition, our study showed that 7-day lithium treatment had no significant effect on extracellular BDNF protein level in comparison with untreated culture under normal condition.
Chronic lithium treatment had no effect on intracellular and extracellular BDNF protein levels in astrocytes culture. To our knowledge, there is no study evaluating the effects of lithium on BDNF in rat primary astrocytes culture for comparison. However, there is a report that 7-day lithium (0.1-3 mM) exposure had no significant effect on intracellular BDNF level but decreased extracellular BDNF protein levels in human astrocytoma cell line (24). These inconsistent findings may be related to the differences in the nature of cancerous cell lines as opposed to primary cells used in this study.
Chronic lithium treatment exerted no significant effect on intracellular and extracellular BDNF and GDNF protein levels in neuro-astroglia cultures. No similar study was found for comparison. However, our findings suggest that lithium had no effect on neurons-astrocytes interactions in regard to BDNF and GDNF levels, at least under normal situation.
Lithium increased intracellular neuronal BDNF and astrocytes GDNF protein levels while had it no effect on extracellular protein levels of these neurotrophic factors. The exact reason for this observation is not clear. However, activation or stimulation of astrocytes or neurons and neurons or astrocytes injury may contribute to increased extracellular neutrophic factors (30). Therefore, it is possible that the increased intracellular astrocytes GDNF and neuronal BDNF levels that were caused by lithium act as reservoir to be secreted following a stress or injury when astrocytes or neurons become activated (24). This notion can be addressed in future studies evaluating the effects of lithium on intracellular and extracellular GDNF and BDNF levels under stressful conditions such as oxidative stress.
Acute and chronic lithium treatment had no significant effects on BNDF and GDNF mRNAs. In accordance with our findings, other studies have also reported that lithium had no effects on BDNF mRNA (24, 31). The discordant increase in BDNF protein level with no change in BDNF mRNA following lithium treatment suggests that lithium may regulate BDNF level via post-translational modification to improve protein stability or prevent protein degradation. However, it is difficult to explain why lithium had no effect on GDNF mRNA because the regulatory mechanism of GDNF mRNA has not been completely clarified (32).
Increased levels of BDNF in neurons and GDNF in astrocytes following chronic lithium treatment have important clinical implications. It was proposed that lack of neurotrophic factors such as GDNF could be responsible (12), at least partly, for the reduced gray matter volume and glial loss in BD brain (33). Interestingly, lithium increased gray matter volume in BD brain (34) suggestive of inhibition of glial cell loss (35). It is possible that lithium may protect astrocytes from apoptosis by increasing GDNF (36), possibly through enhanced levels of bcl-2 (22, 37), and in turn, reduction of glial loss in BD.
In addition, reductions in neuronal size and density in cortical areas and limbic systems of brain BD (38) were proposed to be indicative of diminished glial ability to support neuronal survival or plasticity (38). GDNF has been shown to have neuroprotective effects (39, 40), thus, lithium may prevent glial loss by increasing astrocytes GDNF, and possibly in turn, may restore neuronal abnormality and damage in BD.
Increased neuronal BDNF that was induced by lithium may be complementary to its ability to improve neuronal plasticity in glial cells. Impaired neuroplasticity has been indicated as an important feature of BD (3) which was attributed to a deficiency in BDNF (11). In the present study, we observed increased neuronal BDNF following chronic lithium treatment, which may be relevant to lithium effects on normalizing neuroplasticity and its beneficial effects in the treatment of BD.
Conclusion
The current findings of increased neuronal BDNF accompanied by increased astrocyte GDNF protein levels following chronic lithium treatment suggest that lithium may exert its neuroprotective effect by affecting neurotrophins in both neurons and astrocytes in an independent cell-type specific manner. These findings may be relevant to the therapeutic effects of lithium in prevention of BD brain from glial loss and restoration of neuronal abnormality and damage in BD.
Acknowledgment
This work was financially supported by a grant (#89-5193) from Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences, Shiraz, Iran. The results described in this paper were part of a PhD student thesis. The authors would like to thank Mrs. Maryam Rahmani Fard for her assistance in culture of the cells.
References
- 1.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 2.Rajkowska G. Cell pathology in bipolar disorder. Bipolar disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 3.Schloesser RJ, Huang J, Klein PS, Manji HK. Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsycho-pharmacol. 2008;33:110–133. doi: 10.1038/sj.npp.1301575. [DOI] [PubMed] [Google Scholar]
- 4.Shaltiel G, Chen G, Manji HK. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol. 2007;7:22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 7.Machado-Vieira R, Dietrich MO, Leke R, Cereser VH, Zanatto V, Kapczinski F, et al. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode. Biol Psychiatry. 2007;61:142–144. doi: 10.1016/j.biopsych.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 8.Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim YM, Tsan G, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 9.Skibinska M, Hauser J, Czerski PM, Leszczynska-rodziewicz A, Kosmowska M, Kapelski P, et al. Association analysis of brain-derived neurotrophic factor (BDNF) gene Val66Met polymorphism in schizophrenia and bipolar affective disorder. W J Biol Psychiatry. 2004;5:215–220. doi: 10.1080/15622970410029936. [DOI] [PubMed] [Google Scholar]
- 10.Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- 11.Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Expert Rev Neurother. 2008;8:1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- 12.Michel TM, Frangou S, Camara S, Thiemeyer D, Jecel J, Tatschner T, et al. Altered glial cell line-derived neurotrophic factor (GDNF) concentrations in the brain of patients with depressive disorder: A comparative post-mortem study. Eur Psychiatry. 2008;23:413–420. doi: 10.1016/j.eurpsy.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, et al. Epigenetic status of gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann RF, Schloesser RJ, Gould TD, Manji HK. Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiol. 2005;32:173–202. doi: 10.1385/MN:32:2:173. [DOI] [PubMed] [Google Scholar]
- 15.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- 16.Machado-Vieira R, Manji HK, Zarate CA., Jr The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacol. 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: An essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacol. 2002;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H, Fu YS, Guo JW. Ability of GDNF to diminish free radical production leads to protection against kainate-induced excitotoxicity in hippocampus. Hippocampus. 2004;14:77–86. doi: 10.1002/hipo.10145. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 21.Takeshima T, Johnston J, Commissiong J. Mesen-cephalic type 1 astrocytes rescue dopaminer-gic neurons from death induced by serum deprivation. J Neurosci. 1994;14:4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshavarz M, Emamghoreishi M, Nekooeian AA, J Warsh JW, Zare HR. Increased bcl-2 protein levels in rat primary astrocyte culture following chronic lithium treatment. Iran J Med Sci. 2013;38:255–262. [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- 24.Nishino S, Ohtomo K, Numata Y, Sato T, Nakahata N, Kurita M. Divergent effects of lithium and sodium valproate on brain-derived neurotrophic factor (BDNF) production in human astrocytoma cells at therapeutic concentrations. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:17–22. doi: 10.1016/j.pnpbp.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger NJ. The Bradford Method for Protein Quantitation. In: Walker JM, editor. The Protein Protocols Handbook. New York City: Humana Press; 2002. pp. 15–21. [Google Scholar]
- 28.Saavedra A, Baltazar G, Duarte EP. Driving GDNF expression: The green and the red traffic lights. Prog Neurobiol. 2008;86:186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appel E, Kolman O, Kazimirsky G, Blumberg PM, Brodie C. Regulation of GDNF expression in cultured astrocytes by inflammatory stimuli. Neuroreport. 1997;8:3309–3312. doi: 10.1097/00001756-199710200-00023. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen JPR, Mørk A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 32.Oh-hashi K, Kaneyama M, Hirata Y, Kiuchi K. ER calcium discharge stimulates GDNF gene expression through MAPK-dependent and independent pathways in rat C6 glioblastoma cells. Neurosci Lett. 2006;405:100–105. doi: 10.1016/j.neulet.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 34.Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, et al. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- 35.Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, et al. Greater Cortical Gray Matter Density in Lithium-Treated Patients with Bipolar Disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu AC, Liu RY, Zhang Y, Sun HR, Qin LY, Lau LT, et al. Glial cell line-derived neurotrophic factor protects astrocytes from staurosporine- and ischemia- induced apoptosis. J Neurosci Res. 2007;85:3457–3464. doi: 10.1002/jnr.21345. [DOI] [PubMed] [Google Scholar]
- 37.Ghribi O, Herman MM, Forbes MS, DeWitt DA, Savory J. GDNF protects against aluminum-induced apoptosis in rabbits by upregulating Bcl-2 and Bcl-XL and inhibiting mitochondrial Bax translocation. Neurobiol Dis. 2001;8:764–773. doi: 10.1006/nbdi.2001.0429. [DOI] [PubMed] [Google Scholar]
- 38.Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 2002;4:105–116. doi: 10.1034/j.1399-5618.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 39.Nicole O, Ali C, Docagne F, Plawinski L, MacKenzie ET, Vivien D, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of nmda-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. 2001;21:3024–3033. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonde C, Kristensen BW, Blaabjerg M, Johansen TE, Zimmer J, Meyer M. GDNF and neublastin protect against NMDA-induced excitotoxicity in hippocampal slice cultures. Neuroreport. 2000;11:4069–4073. doi: 10.1097/00001756-200012180-00032. [DOI] [PubMed] [Google Scholar]


