Abstract
Objective(s):
This study was undertaken to investigate the protective effects of vitamin B6, cofactor for cystathionine-γ lyase and cystathionine-β synthase (producers of H2S), alone and in combination with L-cysteine, H2S precursor, on indomethacin-, and ethanol-induced gastric lesions in male NMRI mice.
Materials and Methods:
Fasted male NMRI mice were randomly assigned into 12 groups (7 in each). The gastroprotective activity of vitamin B6 alone and in combination with L-cysteine and sodium hydrosulfate (NaHS) was evaluated against ethanol-, and indomethacin-induced gastric lesions. The animals were received vehicle, vitamin B6, L-cysteine, L-cysteine+vitamin B6, NaHS or NaHS+B6 before the induction of gastric lesions by ethanol (50%, 0.5 ml/25 g of body weight, orally) or indomethacin (40 mg/kg, orally). One and five hours after the administration of ethanol and indomethacin, respectively, the animals were sacrificed using anesthetics. The stomachs were removed, rinsed with normal saline and assessed for gastric wall mucus changes.
Results:
Pretreatment with L-cysteine, sodium hydrosulfate, and vitamin B6 significantly decreased the total area of gastric lesions (P<0.01). The mucus production in L-cysteine-, sodium hydrosulfate-, and vitamin B6-treated animals were significantly higher than in control rats P<0.05). The gastroprotective activity of L-cysteine and sodium hydrosulfate in combination with vitamin B6 were higher than when administered alone (P<0.05).
Conclusion:
The result of this survey showed that the protective activity of L-cysteine and sodium hydrosulfate enhances in the presence of vitamin B6.
Keywords: Ethanol, Indomethacin, L-cysteine, Mice, NaHS, Vitamin B6
Introduction
Hydrogen sulfide (H2S) is a colorless gas with a strong odor, produced in different environments, but it is also found in mammalian tissues, where it is generated during cysteine metabolism (1). It is formed in mammalian cells by the activity of two pyridoxal phosphate-dependent enzymes including cystathionine-γ lyase (CSE) and cystathionine-β synthase (CBS), which convert L-cysteine to sulfide sulfide (2).
Beneficial effect of H2S, its precursors and donors have been reported on different experimentally gastric ulcer in rat and mouse (3-5). Recently, H2S is introduced as a rescue molecule for mucosal defense and repair (6). The underlying gastro-protective mechanisms of hydrogen sulfide were attributed to maintenance and elevation of gastric mucosal blood flow (3, 7), stimulation of bicarbonate secretion (8), reduction of pro-inflammatory cytokine expression/release (5), increase of prostaglandin synthesis (9), decrease of reactive oxygen metabolite production (10) and enhancement of tissue repair (11).
To our knowledge, there is no study about the effect of vitamin B6, an essential cofactor for endogenous production of H2S, on indomethacin-, and ethanol-induced gastric mucosal lesions in mice. Therefore, this study aimed to evaluate the effect of vitamin B6 alone and in combination with L-cysteine and sodium hydrosulfate on two models (indomethacin and ethanol) of experimentally-induced gastric ulcer in mice.
Materials and Methods
Chemicals
Ethanol was purchased from Merck (Germany). L-cysteine and sodium hydrosulfate were purchased from Sigma (USA). Indomethacin and vitamin B6 were purchased from Tolid Daru (Iran). L- cysteine and indomethacin were dissolved in distilled water and prepared freshly.
Animals
NMRI male mice (25 to 30 g) were supplied from the animal house of Ahvaz Jundi Shapur University of Medical Sciences, Ahvaz, Iran. Animals were fed on conventional diets and tap water. They were maintained under standard conditions of humidity, temperature (22±2°C) and light/dark cycle (12 hr: 12 hr). All experiments were carried out in accordance with ethics committee of Ahvaz Jundishapur University of Medical Sciences (RDC-9202). Animals were fasted 24 hr before the experiments. Animals were randomly divided into 12 groups (7 in each).
Animal grouping and procedures
In the first set of experiments, to evaluate the protective effect of L-cysteine, sodium hydrosulfate and vitamin B6 alone and in combination on ethanol-induced gastric lesions, six groups of mice were recruited. They were positive control-, L-cysteine-, vitamin B6-, L-cysteine+vitamin B6-, NaHS-, and NaHS+vitamin B6-treated groups. The positive control rats were given normal saline (0.1 ml, IP) 30 min before the induction of gastric lesions by ethanol (50%, 0.5 ml/25g of body weight, orally) (12); L-cysteine-treated animals received a single dose of L-cysteine (100 mg/kg, IP) 60 min before the induction of gastric lesion by ethanol (5); vitamin B6-treated animals administered a single dose of vitamin B6 (10 mg/kg, IP) (13) 30 min before the induction of gastric lesion by ethanol; L-cysteine+vitamin B6-treated rats received a single intraperitoneally administration of L-cysteine (100 mg/kg, IP) and vitamin B6 (10 mg/kg, IP), 60 min and 30 min, respectively before the induction of gastric lesion by ethanol; animals in the fifth group given a single administration of NaHS (80 µg/kg, IP) 30 min prior to intervention and the sixth group of animals were received NaHS (80 µg/kg, IP)(14)+vitamin B6 (10 mg/kg, IP) 30 min before the induction of mucosal lesions by ethanol. One hour after the administration of ethanol, animals were sacrificed under a high dose of diethyl ether, and their stomachs were rapidly removed, opened along the greater curvature, rinsed with physiologic saline and pinned out on ice-cooled saline. Immediately after taking photo of the stomachs for measurement of the surface area of gastric lesions by Image J software, the gastric wall mucus was scrapped for determining the gastric wall mucus.
In the second set of experiments, the gastro-protective activity of L-cysteine, NaHS and vitamin B6 alone and in combination on indomethacin-induced gastric lesions were tested on six groups of mice. They were control-, L-cysteine-, vitamin B6-, L-cysteine+vitamin B6-, NaHS-, and NaHS+vitaminB6-treated groups. The control rats were given normal saline (0.1 ml) 30 min before the induction of gastric lesion by indomethacin (40 mg/kg, orally)(15). L-cysteine-treated animals received a single dose of L-cysteine (100 mg/kg, IP) 60 min before the induction of gastric lesion by indomethacin. Vitamin B6-treated animals administered a single dose of vitamin B6 (10 mg/kg, IP) 30 min before the induction of gastric lesion by indomethacin, L-cysteine+vitamin B6-treated group given a single intraperitoneally administration of L-cysteine (100 mg/kg, IP) and vitamin B6 (10 mg/kg, IP) 60 min and 30 min, respectively before the induction of gastric lesion by indomethacin; animals in the fifth group received a single administration of NaHS (80 µg/kg, IP) 30 min prior to intervention and rats in the sixth groups administered NaHS (80 µg/kg, IP) + vitamin B6 (10 mg/kg, IP) 30 min before the induction of mucosal lesions by indomethacin. Five hours after the administration of indomethacin, animals were sacrificed under a high dose of diethyl ether. Calculating the total area of gastric lesions and determining the gastric wall mucus were carried out similar to the first protocol.
Determination of gastric wall mucus
To measure the gastric wall mucus, Perera et al method was used (16). Briefly, the stomachs were washed by normal saline, and then the gastric wall mucus was scraped, and homogenized in 1 ml of distilled water. Difference between the weight of obtained homogenate and the original 1 ml of water considered as the weight of mucus (mg).
Histological evaluation
For histological evaluation, stomachs from control and treated animals were fixed in 10% formalin, dehydrated in grade ethanol, and embedded in paraffin. Thereafter, sections of tissue were cut at 5 µm using a microtome, stained with hematoxylin and eosin, and assessed under an Olympus microscope (IX50).
Statistical analysis
Data are shown as mean±SEM. Statistical analysis was performed by one-way ANOVA and followed by post hoc Tukey’s test. Significance was set at a P<0.05 level.
Results
Effect of L-cysteine, NaHS and vitamin B6 alone and in combination on gastric mucosa lesions induced by ethanol and indomethacin
Histological examination showed gastric lesions such as multiple erosions, exfoliation and necrosis of superficial cells, hemorrhages in the mucosal layer, and severe alterations in the architecture of glandular parts of the gastric mucosa one hour after ethanol (50%, 0.5 ml/25 gr of body weight) administration and five hours after indomethacin (40 mg/kg, orally) treatment in the corresponding control groups. Pretreatment with L-cysteine, vitamin B6, NaHS, L-cysteine+vitamin B6, NaHS+Vitamin B6 attenuated the gastric lesions induced by ethanol and indomethacin (Figure 1 and 3).
Figure 1.
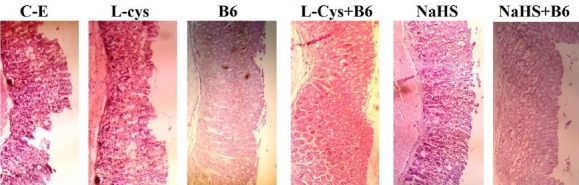
Histological evaluation of gastric mucosa. Representative gastric sections were obtained 1 hr after ethanol administration. C-E: Control group indicate severe disruption to the upper half of mucosal thickness and necrotic lesions penetrating deeply into mucosa; L-Cys, B6, NaHS, L-Cys+B6, NaHS+B6: animals pretreated with L-cysteine (100 mg/kg, IP), vitamin B6 (10 mg/kg, IP), NaHS (80 µg/kg, IP), L-cysteine (100 mg/kg, IP)+vitamin B6 and NaHS (80 µg/kg, IP) +vitamin B6, demonstrate moderate disruption of the surface epithelium. All of the sections stained with hematoxylin and eosin; ×100 magnification
Figure 3.
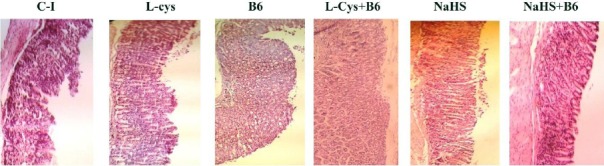
Histological evaluation of gastric mucosa. Representative gastric sections were obtained 5 hr after indomethacin administration. C-I: Control group indicate severe disruption to the upper half of mucosal thickness and necrotic lesions penetrating deeply into mucosa; L-Cys, B6, NaHS, L-Cys+B6, NaHS+B6: animals pretreated with L-cysteine (100 mg/kg, IP), vitamin B6 (10 mg/kg, IP), NaHS (80 µg/kg, IP), L-cysteine (100 mg/kg, IP)+vitamin B6 and NaHS (80 µg/kg, IP) +vitamin B6, demonstrate moderate to mild disruption of the surface epithelium. All of the sections stained with hematoxylin and eosin; ×100 magnification
As shown in Figure 2, a single administration of ethanol (50%, 0.5 ml/25 g of body weight) produced gastric mucosal lesions in fasted male NMRI mice. Figure 2, also shows that pretreatment with L-cysteine, vitamin B6 and NaHS protected the gastric mucosa against ethanol-induced gastric lesions. The total area of gastric lesions in pretreated groups, L-cysteine, vitamin B6 and NaHS, was significantly lower than in control rats (P<0.01, P<0.001 and P<0.001, respectively). The protective activity of combination of L-cysteine and NaHS with vitamin B6 were higher than when administered them alone (P<0.05).
Figure 2.
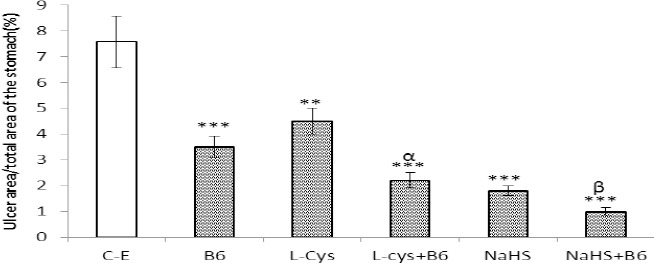
A graghic represenation of the ulcer index following ethanol adminisitration among various treatment groups. C-E (control): rats were received ethanol (0.5/25 g of body weight, orally); L-cys: animals were given L-cysteine (100 mg/kg) 60 min prior to ethanol administration; B6: animals received vitamin B6 (10 mg/kg, IP) 30 min prior to ulcer induction by ethanol; L-cys+B6: animals received L-cysteine 60 min prior to intervention and vitamin B6 30 min prior to ulcer inducting by ethanol. **P<0.01, ***P<0.001 versus the control group and αP<0.05 versus the L-cysteine-, and vitamin B6-treated groups.βP<0.05 as compared with NaHS-treated animals
As shown in Figure 4, a single administration of indomethacin at 40 mg/kg (orally) produced gastric mucosal lesions in male fasted NMRI mice. Pretreatment with L-cysteine, vitamin B6 and NaHS protected the gastric mucosa against indomethacin -induced gastric lesions.
Figure 4.
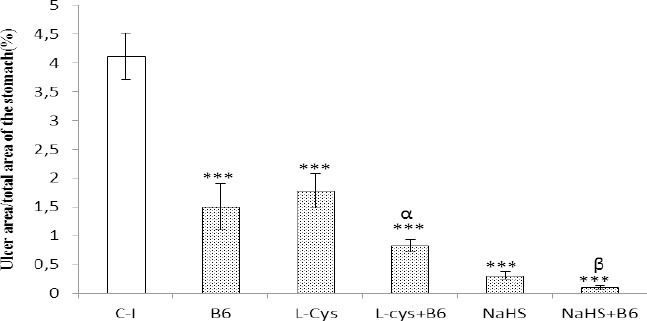
A graghic represenation of the ulcer index following indomathacin adminstration among various treatment groups. C-I:control (animals were received indomethacin at 40 mg/kg, orally); L-cys: animals were given L-cysteine (100 mg/kg, IP) 60 min prior to intervention; B6: animals received vitamin B6 (10 mg/kg) 30 min prior to intervention; L-cys+B6: animals received L-cysteine (100 mg/kg, IP) 60 min prior to intervention and vitamin B6 (10 mg/kg, IP) 30 min prior to intervention. **P<0.01, ***P<0.001 versus the control group and αP<0.05 versus the L-cysteine-, and vitamin B6-treated groups. βP<0.05 as compared with NaHS-treated animals
The total area of gastric lesions in pretreated groups, L-cysteine, vitamin B6 and NaHS, was signifycantly lower than in control rats (P<0.01, P<0.001 and P<0.001, respectively). The protective effect of vitamin B6 was pronounced when co-administered with L-cysteine and NaHS. The total area of mucosal lesions in animals received a combination of L-cysteine+vitamin B6 and NaHS+vitamin B6 was significantly lower than in L-cysteine-, and NaHS-treated rats (P<0.05). The gastro-protective activity of vitamin B6 in combination with L-cysteine was higher than when administered it alone (P<0.05) (Figure 4).
Effect of L-cysteine and vitamin B6 alone and in combination pretreatment on gastric wall mucus
The mucus content in L-cysteine-, NaHS-, and vitamin B6-treated animals was significantly higher than in control rats (P<0.05). The muco-tropic effect of L-cysteine and NaHS in combination with vitamin B6 was higher than when administered them alone (P<0.05) (Table 1).
Table 1.
Effect of L-cysteine (100 mg/kg), vitamin B6 (10 mg/kg, IP), NaHS (80 μg/kg), L-cysteine+vitamin B6 and NaHS+vitamin B6 on gastric mucus content in ethanol and indomethacin-induced gastric lesions in NMRI male mice.
| Groups | Mucus (mg) |
|---|---|
| Saline+ethanol | 18±1 |
| L-cysteine+ethanol | 25±1.5* |
| Vitamin B6+ethanol | 26±1.6* |
| NaHS+ethanol | 26.3±0.8* |
| L-cysteine+vitamin B6+ethanol | 31±2.1**α |
| NaHS+vitamin B6+ethanol | 32±2.3**β |
| Saline+indomethacin | 24±1.5 |
| L-cysteine+indomethacin | 29±1.8* |
| Vitamin B6+indomethacin | 31±2.5* |
| NaHS+indomethacin | 30±1.8* |
| L-cysteine+vitamin B6+indomethacin | 36±3.1**α |
| NaHS+ vitamin B6+indomethacin | 37±2.1**β |
Discussion
The findings of the present study showed that: (1) the administration of L-cysteine, NaHS and vitamin B6 reduced the total area of the acute gastric mucosal lesions induced by ethanol and indomethacin; (2) the protective activity of L-cysteine and NaHS on ethanol-, and indomethacin-induced gastric lesions significantly increased in the presence of vitamin B6; and (3) the contents of gastric wall mucus in the control groups (animals received ethanol or indomethacin) were lower than in L-cysteine-, vitamin B6-, NaHS-, and L-cysteine+vitamin B6-treated rats.
H2S is produced in mammalian cells via both enzymatic and nonenzymatic pathways, although the nonenymatic pathway only accounts for a small portion of H2S production (17). Among enzymes involved in H2S production, CBS and CSE have been investigated extensively, both using pyridoxal 5’-phosphate (vitamin B6) as a cofactor. Vitamin B6 is a water-soluble molecule that is involved in a wide range of metabolic, physiological and developmental processes. It itself is an enzyme cofactor required for more than 140 biochemical reactions (18).
Moreover, the vitamin is a potent antioxidant, rivaling carotenoids or tocopherols in its ability to quench reactive oxygen species(19). Many studies have shown the neuroprotective activity of vitamin B6 (20). In vitro studies have shown the anti-tumor and anti-inflammatory effect of vitamin B6 (21, 22). Furthermore, it has been demonstrated that dietary vitamin B6 inhibits nitric oxide (NO) production in response to LPS administration (21).
Nonsteroidal anti-inflammatory drugs cause gastro-intestinal damage through blocking prostaglandin (PGs) synthesis [inhibition of the cyclooxygenase (COX) enzymes] and also through inhibiting a number of autacoids [NO and H2S] acting in concert with prostaglandins in maintaining the gastric mucosal barrier (23). PGs have been demonstrated to stimulate mucus and bicarbonate secretion as well as mucosal blood flow, and induce angiogenesis (24). All these factors contribute to accelerated ulcer healing.
The results of the present study showed that L-cysteine, NaHS and vitamin B6 increase mucus secretion. Therefore, it can be concluded that this protective activity could largely be due to potentiation of mucosal barrier through increasing gastric wall mucus secretion. This effect in concert with other protective effect including up-regulating the gene expression of cyclooxygenase-2 in mucosal tissue of the rat stomach as shown by our previous report (14) and also by Wallace et al report that showed endogenous and exogenous hydrogen sulfide through the up-regulation of COX-2, promotes resolution of colitis in rats (9), and explains the protective activity of vitamin B6 against indometha-cin-induced gastric lesions.
Our recent report also showed that NaHS [a H2S donor] and L-cysteine [a H2S precursor] decrease the acid output in response to gastric distention (14). Therefore, these results suggest that one of the possible mechanisms of the protective activity of L-cysteine and vitamin B6 against indomethacin and ethanol could be largely mediated by their antacid effects. Abdallah et al showed that indomethacin causes a remarkably significant increase in ulcer index, gastric juice free and total acidity (25). Taken together, these findings show that the excitatory effect of indomethacin on acid output as shown by Abdallah et al report was prevented by the anti-secretory effect of H2S as shown by previous work.
It has been indicated that the endogenous production of H2S increases in various models of tissue injury (26, 27). Wallace et al demonstrated that the expression of CSE and CBS profoundly increases after the induction of gastric ulcer, and that the administration of propargylglycine (PAG) prevents gastric ulcer healing by L-cysteine (a H2S precursor) treatment (4). Several studies have demonstrated that PAG exacerbates gastric mucosal lesions induced by acetylsalicylic acid (3). Taken together, these findings imply that under damage conditions, the body limits injury by increasing the production of protective autacoids such as H2S. Also, it can be concluded that potentiation of the involved tissue by exogenous H2S precursor, by L-cysteine pretreatment, or by increasing the activation of involved enzymes, CSE and CBS, to increase the endogenous production/release of H2S by pretreatment of essential enzyme cofactor, vitamin B6, or by a combination of them significantly protected the gastric mucosal tissue against indomethacin and ethanol as shown by the present results.
Ethanol induces severe mucosal ulcers in the stomach mainly by activation of the inflammatory reaction (28, 29). The mucosal lesions resulted from ethanol is characterized by epithelial cellular loss, mucosal edema, and sub-epithelial hemorrhage (12, 30). As shown in Figure 1, pretreatment with L-cysteine, vitamin B6 and NaHS protected the gastric mucosa against ethanol-induced mucosal lesions. Recently, we have shown that L-cysteine and NaHS protected the gastric mucosa against ischemia-reperfusion injury in rat through down-regulating the mRNA expression and plasma level of pro-inflammatory cytokines, IL-1β and TNF-α (5). These findings together show that L-cysteine, NaHS and vitamin B6 by increasing H2S production/release, and it in turn through a decrease in pro-inflammatory cytokines protect the gastric mucosa against ethanol. The interesting finding of the present study was the gastro-protective activity of pyridoxine (vitamin B6) against ethanol and indomethacin-induced mucosal lesions. As shown in Figures 1 and 2, the protective activity of both L-cysteine and NaHS increase when co-administered with vitamin B6. These results clearly show that H2S production increases in the presence of enzyme cofactor. In the present study, we did not measure the tissue levels of hydrogen sulfide but a higher gastroprotective effect of L-cysteine and NaHS after the administration of vitamin B6 represents an increase in the production of hydrogen sulfide.
Conclusion
The result of this survey for the first time showed the gastroprotective effect of vitamin B6 on ethanol and indomethacin-induced gastric lesions in rats. The findings of this study demonstrated that: a) Pretreatment with B6 decreased the total area of acute gastric mucosal lesions induced by ethanol and indomethacin. b) The protective activity of L-cysteine enhances in the presence of vitamin B6, cofactor for natural enzymatic pathways for endogenous production of H2S. c) The gastric wall mucus production in B6 and L-cysteine pretreated rats was higher than in the control.
Acknowledgment
The authors are thankful to Vice Chancellor of Research Affairs of Ahvaz Jundi Shapour University of Medical Sciences, Ahvaz, Iran, for financial support (RDC-9202) and Dr Esrafil Mansori for confirmation of the histopathological results. This paper was issued from thesis of Mr Ardeshir Ashabi (MSc student in Medical Physiology).
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- 1.Guidotti TL. Hydrogen sulphide. Occup Med (Lond) 1996;46:367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- 2.Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trend Pharmacol Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 4.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 5.Mard SA, Neisi N, Solgi G, Hassanpour M, Darbor M, Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia–reperfusion injury in rat. Dig Dis Sci. 2012;57:1496–1503. doi: 10.1007/s10620-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JL. Hydrogen sulfide: a rescue molecule for mucosal defence and repair. Dig Dis Sci. 2012;57:1432–1434. doi: 10.1007/s10620-012-2119-2. [DOI] [PubMed] [Google Scholar]
- 7.Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ise F, Takasuka H, Hayashi S, Takahashi K, Koyama M, Aihara E, et al. Stimulation of duodenal HCO3- secretion by hydrogen sulphide in rats: relation to prostaglandins, nitric oxide and sensory neurones. Acta Physiol. 2011;201:117–126. doi: 10.1111/j.1748-1716.2010.02152.x. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastro-enterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 11.Szabó C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011;164:853–865. doi: 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros JVR, Bezerra VH, Gomes AS, Barbosa ALR, Lima-Júnior RCP, Soares PMG, et al. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 2009;330:764–770. doi: 10.1124/jpet.109.152801. [DOI] [PubMed] [Google Scholar]
- 13.Masisi K, Suidasari S, Zhang P, Okazaki Y, Yanaka N, Kato N. Comparative study on the responses of concentrations of B(6)-vitamers in several tissues of mice to the dietary level of pyridoxine. J Nutr Sci Vitaminol. 2012;58:446–451. doi: 10.3177/jnsv.58.446. [DOI] [PubMed] [Google Scholar]
- 14.Mard SA, Askari H, Neisi N, Veisi A. Antisecretory effect of hydrogen sulfide on gastric acid secretion and the involvement of nitric oxide. Bio Med Res Int. 2014;2014 doi: 10.1155/2014/480921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plants A, Karaj I. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J Med Plants. 2009;8:30–38. [Google Scholar]
- 16.Perera LM, Ruedas D, Gómez BC. Gastric antiulcer effect of Rhizophora mangle L. J Ethnopharmacol. 2001;77:1–3. doi: 10.1016/s0378-8741(01)00277-x. [DOI] [PubMed] [Google Scholar]
- 17.Nalli AD. Virginia: Virginia Commonwealth University Richmond; 2013. Regulation of gastroin testinal smooth muscle function. [Google Scholar]
- 18.Hellmann H, Mooney S. Vitamin B6: a molecule for human health? Molecules. 2010;15:442–459. doi: 10.3390/molecules15010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahlavanzadeh F, Bidadkosh A, Derakhshanfar A, Rastegar AM, Rushanzamir M. Antioxidant protecting effects of vitamin B6 at reducing hemodynamic toxicity of gentamicin in rat model of nephrotoxicity. Comp Clin Pathol. 2013;22:637–643. [Google Scholar]
- 20.Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanaka N, Koyama T, Komatsu S, Nakamura E, Kanda M, Kato N. Vitamin B6 suppresses NF-kB activation in LPS-stimulated mouse macrophages. Int J Mol Med. 2005;16:1071–1076. [PubMed] [Google Scholar]
- 22.Komatsu S, Watanabe H, Oka T, Tsuge H, Kat N. Dietary vitamin B6 suppresses colon tumorigenesis, 8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide synthase protein in azoxymethane-treated mice. J Nutr Sci Vitaminol. 2002;48:65–68. doi: 10.3177/jnsv.48.65. [DOI] [PubMed] [Google Scholar]
- 23.Musumba C, Pritchard D, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther. 2009;30:517–531. doi: 10.1111/j.1365-2036.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 25.Inas ZA, Hala AK, Gehan HH. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 2011;8:433–445. [Google Scholar]
- 26.Yusuf M, Kwong Huat BT, Hsu A, Whiteman M, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulfide biosynthesis. Biochem Biophys Res Commun. 2005;333:1146–1152. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 28.Szabo S, Trier J, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- 29.Peskar B, Lange K, Hoppe U, Peskar B. Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins. 1986;31:283–293. doi: 10.1016/0090-6980(86)90054-7. [DOI] [PubMed] [Google Scholar]
- 30.Guslandi M. Effects of ethanol on the gastric mucosa. Dig Dis. 1987;5:21–32. doi: 10.1159/000171159. [DOI] [PubMed] [Google Scholar]


