Abstract
Objective(s):
Mesenchymal stem cells (MSC) can be isolated from adult tissues such as adipose tissue and other sources. Among these sources, adipose tissue (because of easy access) and placenta (due to its immunomodulatory properties, in addition to other useful properties), have attracted more attention in terms of research. The isolation and comparison of MSC from these two sources provides a proper source for clinical experimentation. The aim of this study was to compare the characteristics of MSC isolated from human adipose tissue and placenta.
Materials and Methods:
Adipose and placental MSC were isolated from the subcutaneous adipose tissues of 10 healthy women (25 to 40 years) and from a fresh term placenta (n= 1), respectively. Stem cells were characterized and compared by flow cytometry using CD29, CD31, CD34, CD44, CD45, CD105, CD166 and HLA-DR markers. Osteocytes and adipocytes were differentiated from isolated human mesenchymal stem cells (HMSC).
Results:
Adipose and placenta-derived MSC exhibited the same morphological features. ADSC differentiated faster than placenta; however, both were differentiated, taking up to 21 days for osteocyte and 14 days for adipocyte differentiation. About 90% of PLC-MSC and ADSC were positive for CD29, CD44, CD105, and CD166; and negative for CD31, CD34, CD45, and HLA-DR.
Conclusion:
The two sources of stem cells showed similar surface markers, morphology and differentiation potential and because of their multipotency for differentiating to adipocytes and osteocytes, they can be applied as attractive sources of MSC for regenerative medicine.
Keywords: Adult stem cells, Differentiation, Fetal stem cells, Mesenchymal stem cells
Introduction
Stem cells have two defining properties: the ability to differentiate to different lineages and the capacity for self-renewal. These cells have two general types: embryonic stem cells (ESC) and adult stem cells (1). ESC are acquired from the inner cell mass of the blastocyst and are related to tumor genesis; therefore, their application involves ethical and safety concerns. However, applying adult MSC is less challenging. MSC are stromal cells that have the capacity for self-renewal and also exhibit multilineage differentiation. MSC can be isolated from two cell types, i.e., adult sources (bone marrow, peripheral blood, adipose, etc.) and fetal sources (placenta, amniotic fluid, umbilical cord and umbilical cord blood) (2-5). MSC have been generally used in experimental and clinical research because of their unique biological characteristics and their advantages, especially (3-8) in bone marrow transplantation (6, 9), tissue engineering (9, 10) and cell therapy (9, 11, 12).
Adipose tissue is a main source of adult stem cells, called adipose stem cells (ADSC) (13, 14). ADSCs are fairly easy to obtain from adipose tissue and unlike bone marrow tissue, can be grown in cell cultures. (15). Additionally, it has many of the same properties as bone marrow, including wide proliferation, the ability to undertake multilineage differentiation and evident plasticity both in vitro and in vivo (16-18). ADSC can represent the biochemical profile in vitro of adipocytes, chondrocytes and osteoblasts under proper culture conditions (19, 20). Therefore, human adipose-derived MSC are today viewed as potential sources for stem cell banks and in tissue engineering.
From fetal sources, placenta–due to its easy access without invasive procedures (contrary to bone marrow harvest), its pluripotency potential (as adipose tissue) (21, 22) and its immunomodulatory properties – is defined as a good source of MSC for use in medical applications (4, 23-25). Therefore, the aim of this study was to isolate MSC from adipose tissue and placenta and then to differentiate them into the adipocyte and osteocyte lineages. In addition, we compared morphological and immunophenotypic characteristics and the success rates of stem cells isolated from these two derived sources.
Materials and Methods
This study was performed at the Bu-Ali Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran in 2012. After receiving approval from the ethics committee (no 900886) and obtaining informed consent from participants, samples were obtained from adipose tissues of 10 healthy women and one placenta. For the isolation of ADSC, subcutaneous adipose tissues (50-100 g) were obtained from the abdomen region of healthy women aged 25 to 40 undergoing liposuction surgery (samples were collected by a surgeon in Qaem Hospital, Mashhad, Iran.). All samples outside the stated age parameters or those weighing less than 50 g, or samples with a particular disease–especially cancer and cardiovascular disorders– were excluded from the study. The tissues were transferred in a sterile solution of phosphate-buffered saline (PBS), a 2% fetal bovine serum (FBS; Stem Cell Technology Inc., London, UK), 100 units/ml penicillin (Gibco-Invitrogen) and 100 µg /ml streptomycin (Gibco-Invitrogen). A fresh term placenta (38 to 40 weeks gestation) was obtained from a normal delivery.
Isolation of ADSC
The samples were transferred to the Bu-Ali Research Institute’s tissue culture department. After settling the adipose tissue above the bloody portion of the solution, the blood was removed using a sterile pipette and the sample was washed three times by way of a sterile PBS solution containing penicillin and streptomycin. Then, the adipose tissue was cut carefully into 1 mm³ pieces to remove the connective tissue and blood vessels. In the next step, the extracellular matrix was digested by adding 0.1% collagenase Type I at 37°C, and shaken vigorously for 60 min to detach the stromal cells from primary adipocytes. Then, by adding an equivalent volume of low glucose-Dulbecco’s modified Eagle’s medium (L-DMEM) containing 10% fetal bovine serum (FBS), the collagenase was inactivated and the supernatant was centrifuged for 10 min at 1000 RPM.
The cellular pellet was re-suspended in DMEM/10% FBS and filtered through 100, 70 and 40 µm filters to remove debris. The filtrate was centrifuged at 600 g for 10 min and was incubated with a lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 10 min at 22°C to 25°C, then centrifuged at 300 g for 10 min before finally discarding the lysis buffer. By placing the cells for one hr on a glassy surface (e.g., a Petri dish), hematopoietic cells were attached to the surface and isolated; then, floating cells were transferred onto a six-well plate to culture at the final concentration of 1×106/m/in a complete medium (DMEM, 10% FBS, 100 units/ml penicillin, 100 mg /ml streptomycin). Finally, MSC, upon reaching 80% confluence, were detached using Trypsin-EDTA (0.25% trypsin+0.02%EDTA, Gibco-Invitrogen) and were cultured as the primary culture.
Isolation of placenta mesenchymal stem cells (PLC-MSC)
Initially, the placenta tissue was washed with PBS, pH 7.2; blood clots and vessels were mechanically removed and the tissue was minced into small pieces and washed again with PBS. Then, tissues were incubated with 0.01% DNase I (Roche Diagnostics Australia Pty. Ltd., Australia) and 0.25% trypsin (Gibco-Invitrogen) for one hr at 37°C for removing trophoblasts. The sample was then filtered through a 250 μm metal sieve. The remnants were collected and digested with collagenase I 0.1 % (1 hr at 37°C). Digested tissue was passed through a 250 μm metal sieve and 100 μm cell nylon membranes, to eliminate undigested fragments. Following filtrate centrifugation at 300 g for 10 min, cells were collected; red cells were lysed in a buffer containing 155 mmol/L NH4Cl and 20 mmol/l Tris for five min and cells were centrifuged at 300 g for 10 min. The cell pellet was suspended in a complete medium (low glucose content (1 g/l) DMEM/20% FBS plus 100 U/ml penicillin, 100 μg/ml streptomycin, 5 ng/ml basic fibroblast growth factor (b-FGF, Promega) and 1mM/L-glutamine. Cultures were incubated in humidified 5% CO2 incubators, at 37°C and plated into two 75 cm2 flasks. Non adherent cells were removed (after 24 hr) and replaced with fresh medium every three days, and adherent MSC were trypsinized upon reaching 80% confluence with Trypsin-EDTA (0.25% trypsin+0.02%EDTA, Gibco-Invitrogen) after 14 days of culture.
Flow cytometry analysis of ADSC and PLC-MSC
For immunophenotyping, cells were initially washed twice with ice-cold 20 mM PBS, pH 7.2 and trypsinized with 0.25% trypsin, 0.02%EDTA, then 10 µl of secondary conjugated Ab (AbD Serotec company, USA) per 200-500×103 cells (up to 106 cells) in 100 µl PBS to which CD45, CD34, CD29, CD105, CD31, CD166, CD44 and HLA-DR were added. Following on, the final volumes of all the tubes were enhanced to 1 ml with PBS. In the next step, cells suspended with specific antibodies were incubated for 45 min at 4°C. Finally, after washing the samples in PBS, they were analyzed by flow cytometer (BD FACSCalibur flow cytometer; Becton Dickinson. USA) using the CellQuest software. All markers were stained with secondary conjugated Ab (mouse anti-human Ab) with fluorescein isothiocyanate (FITC), except HLA-DR, which was conjugated with phycoerythrin (PE).
Differentiation study
Osteocyte and adipocyte differentiations were qualitatively determined using standard methods. Placenta- and adipose-derived adherent cells were trypsinized. Then, cells were transferred into replicate 24-well plates and were incubated for 24 hr to adhere cells to plates. The medium was replaced with a differentiation medium. All differentiation studies were conducted at passage three.
Osteogenic differentiation
Cells were plated in an osteocytogenic differentiation medium containing L-DMEM, 10% FBS, 0.1 μM dexamethasone (Sigma-Aldrich), 200 μM L-ascorbic acid-2-phosphate (Sigma-Aldrich) and 10 mM β-glycerol phosphate (Sigma-Aldrich) for 21 days and induction was confirmed by Alizarin Red S staining (26). Alizarin Red Stain positive cells (differentiated MSC with calcium deposits) under microscope were stained bright orange-red, while negative cells (undifferentiated MSC without calcium deposits) were stained as faintly reddish cells.
Adipogenic differentiation
Cells were seeded onto a medium consisting of DMEM/10% FBS, 50 μmol/l indomethacin, 10 μM insulin, 1 μmol/l dexamethasone and 0.5 mM 3-isobutyl-1-methyl-xanthine (all from Sigma-Aldrich) for two weeks and induction was confirmed by Oil Red O staining using a standard method (27). Observation of Oil Red droplets in microscopic evaluation proved the adipose differentiation of MSC.
Results
Adipose and placenta-Derived MSC exhibited the same morphological features. MSC from both adipose and placenta were observed as fibroblast-like adherent cells at passage 0, that at first appeared as small cells with two forms –epithelial and fibroblast-like; at later stages (third passage) the epithelial-like morphology was eliminated and the remaining cells were larger with fibroblast-like and cobblestone morphologies. With these morphologic findings, we were unable to differentiate MSC from the differently-derived sources (MSC morphologies were imaged using a Carl Zeiss Inverted Axiovert 40 microscope, equipped with a camera) (Figure 1a-1f). The isolation process for ADMSC (adipose-derived mesenchymal stem cells) were conducted with a 100% success rate for all the samples (n=10); this ratio was not calculated for PLC-MSC, because of limitations in the number of evaluated placenta samples in this study. In all passages, the culture medium was removed and replaced when MSC reached 80% confluence.
Figure 1.
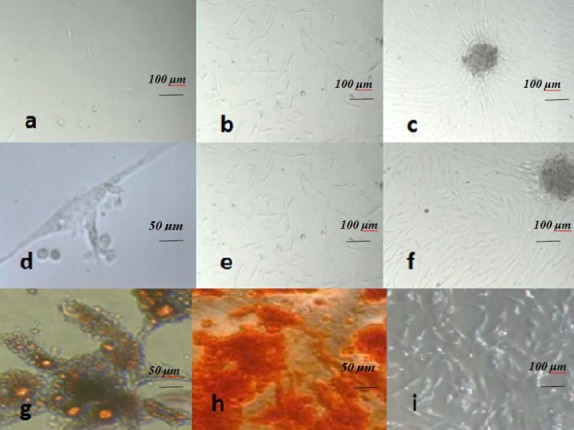
Human mesenchymal stem cells (a-i), Adipogenic and osteogenic differentiation; (g,h) morphology and growth of fibroblastoid-adherent cells or placenta-derived Mesenchymal stem cells at phase 0 on days 2, 5 and 12, respectively (a-c). (a) Small, round cells (Magnification: ×100). (b,c) Long, thin cells (Magnification: ×100) with spindle-shape, which were reached to high confluence and were formed in a colony, as seen in the picture. (c) With morphology and growth of adipose-derived Mesenchymal stem cells at passage 0 on days 2, 4 and 8 (colony), respectively (d-f). (d) A single long spindle-shaped ADSC with thin processes; (Magnification: ×1000). (e,f) ADSCs that were growing (cells were created in a colony (f)) (Magnification: ×100). Adipogenic differentiation was evidenced by the formation of lipid vacuoles (yellow) by Oil Red O staining at passage three in adipose-derived MSC (g) (Magnification: ×1000). Osteogenic differentiation was evidenced by the formation of a mineralized matrix at passage three in adipose-derived MSC (Magnification: ×400) (h) Undifferentiated adipose MSC (i) Scale bar is 50 μm d, g, h) and 100 μm (a, b, c, e, f, i)
The ability of MSC to differentiate into mesodermal cells, such as adipocytes, osteocytes and myocytes was proven. Adipose and placenta-derived MSC were similarly differentiated into mesodermal cells. These abilities were proved via Oil Red O staining and showed the presence of lipid droplets (Figure 1g), while Alizarin Red S staining showed calcium deposits (Figure 1h). These results were verified by comparing the samples with control cultures (no morphological changes were observed in control cultures). ADSC was differentiated quicker than placenta; however, both were differentiated within 21 days for osteocytes and 14 days for adipocyte differentiation (all samples of the two sources were differentiated to adipocytes and osteocytes).
Cell surface markers
About 90% of PLC-MSC and ADSC were positive for CD 29, CD44, CD105 and CD166, and negative for CD31, CD34, CD45, HLA-DR (the two sources were similar) (Figures 2, 3). The following are the percentages of positive cell markers. Adipose MSC: CD 105: 75.98 %; CD 29: 82.93 %; CD 44: 86.34 %; CD 166: 27.65 %. Placenta MSC: CD 105: 84.82%; CD 29: 83.52 %; CD 44: 88.59 %; CD 166: 77.67 %.
Figure 2.
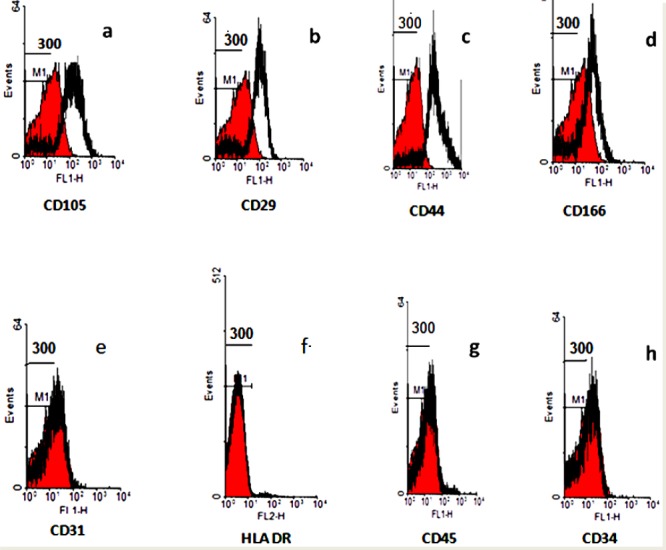
Immunophenotyping analysis of adipose mesenchymal stem cells. ADSC was positive for CD105, CD29, CD44 and CD166, and negative for CD31, HLA-DR, CD45 and CD34. Control histogram is highlighted in red.
a: Control and CD105; b: Control and CD29; c: Control and CD44; d: Control and CD166; e: Control and CD31; f: Control and HLA DR; g: Control and CD45; h: Control and CD34
Figure 3.
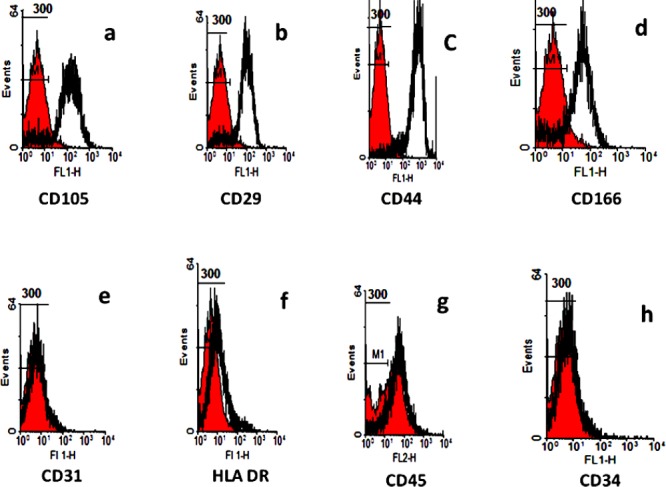
Immunophenotyping analysis of placenta mesenchymal stem cells. PLC-MSC was positive for CD105, CD29, CD44 and CD166, and negative for CD31, HL-DR, CD45 and CD34. Control histogram is highlighted in red.
a: Control and CD105; b: Control and CD29; c: Control and CD44; d: Control and CD166; e: Control and CD31; f: Control and HLA DR; g: Control and CD45; h: Control and CD34
Discussion
MSC exists in many tissues in adults (bone marrow, adipose, cartilage, peripheral blood) and in fetal tissues (placenta, cord blood, amniotic fluid). Despite bone marrow being the main source of isolation for MSC, its aspiration is an extremely invasive procedure and differentiation potential, life span and the number of MSC derived from it decreases with increasing age. Therefore, other sources of MSC isolation such as adipose tissue and placenta are being studied. The collection of PLC-MSC is less invasive and poses no side-effects for the mother or the neonate. MSC can also be obtained from adipose tissue (these cells can be isolated from cosmetic liposuction procedures in large numbers and quantities and easily cultured under standard conditions) (28).
In this study, MSC from two sources, placenta and adipose, were isolated and after characterization successfully differentiated into two lineages. The results showed that both adipocyte MSC and PLC-MSC had osteogenic and adipogenic differentiation potential, as have been shown in previous studies highlighting their pluripotency potential (3, 21, 29, 30). In this study, assessments were restricted to the mesodermal differentiation capacity (adipose tissue and bone). Based on recent reports, however, the range of differentiation for MSC does not appear to be limited to this lineage. MSC derived from both tissues have been shown able to differentiate to endo- and ectodermal lineages, too (14, 31-34).
All MSC derived from these two sources showed typical MSC features with a fibroblastoid morphology, the formation of single separated fibroblastoid colonies, differentiation ability and the expression of classical markers of MSC, but without the expression of hematopoietic markers.
We were able to differentiate MSC from two different sources to adipogenic and osteogenic tissues with similar capabilities. However, in a study by Kern et al (2006), which compared isolation of MSC from bone marrow, umbilical cord blood and adipose tissue, the researchers were unable to differentiate cord blood MSC to adipose tissue. This indicates that MSC from different sources can have different abilities for multilineage differentiation.
Neither MSC derived from the two sources expressed hematopoietic stem cell markers (CD45, CD34), but they expressed MSC markers (CD29, CD44, CD105, CD166) with similar intensity. As in other studies, none of the MSC expressed HLA II (28). Definitive cell markers of ADSC or PL-MSC will help not only to separate them from other cell populations in cultures, but will also enable their purification from stromal vascular fraction. However, we have not distinguished these markers in the current study; this separation can be done by functional assay (35). The reproduction rate of adipose-derived MSC was much faster than placenta derived MSC. It took seven days for adipocyte- derived MSC to reach confluence of 80%, whereas this took 12 to 15 days for PLC-MSC (at passage 0). However, this difference was minimized in the following passages (PLC-MSC reached similar duplication rate in the third passage). Reliable, rapid and efficient methods for MSC differentiation are also necessary. The study showed that adipogenesis differentiation was relatively quick, occurring in nearly two weeks, but that chondrogenic and osteogenic differentiation took notably longer (roughly 21 days) (36). According to some studies, bone marrow MSC has been recognized as the most important source of MSC for medical applications (37); however due to limitations in obtaining bone marrow, as we have mentioned previously, and its reduced differentiation with age (38, 39), other sources of MSC such as adipose tissue and placenta, with easy isolation and sufficient MSC numbers, should be considered for clinical application (4, 28, 40-42). Addition to the regenerative function of ADSC and PL-MSC, is their important potential immunomodulatory effects. CP-MSC may be more effective than other MSC in relation to immunomodulation and have been suggested as useful sources for cell therapy (25).
According to some studies, isolated MSC from different sources, including peripheral blood, adipose and Wharton’s jelly have different immunomodulatory characteristics (43, 44). Studies have also shown that these characteristics result in membrane or secretory MSC molecules (45). Of course, these studies demonstrate that MSC could identify environmental signals and through understanding these signals, as well as the cell’s needs, stimulatory or inhibitory functions can be achieved (45). Our study, as in a number of others, showed similar immunophenotypic and differentiation properties for neonatal (e.g., placenta) and adult MSC sources (e.g., adipose) (46, 47). These results differ from some studies that indicate differentiated potential for neonatal MSC sources (48).
Conclusion
The current study showed that PLC-MSC and ADSC show similar mesenchymal cell surface markers, morphology and differentiation potential, and due to their multipotency for differentiation into another lineage, like osteocytes and adipocytes, they can be considered an attractive source of MSC for use in regenerative medicine.
Acknowledgment
This study was the result of an MSc student thesis supported financially by the Vice Chancellor for research at Mashhad University of Medical Sciences, Mashhad, Iran. We would like to convey our thanks to him. We also thank Dr Jalali, Mr Khodadoust and Mrs Ganjali for their help in performing tests.
Footnotes
Conflict of interest
The authors confirm that there is no conflict of interest to disclose.
References
- 1.Nikoozad Z, Ghorbanian MT, Rezaei A. Comparison of the liver function and hepatic specific genes expression in cultured mesenchymal stem cells and hepatocytes. Iran J Basic Med Sci. 2014;17:27–33. [PMC free article] [PubMed] [Google Scholar]
- 2.Maqbool M, Vidyadaran S, George E, Ramasamy R. Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol Int. 2011;35:1247–1251. doi: 10.1042/CBI20110070. [DOI] [PubMed] [Google Scholar]
- 3.Raynaud CM, Maleki M, Lis R, Ahmed B, Al-Azwani I, Malek J, et al. Comprehensive characterization of mesenchymal stem cells from human placenta and fetal membrane and their response to osteoactivin stimulation. Stem Cells Int. 2012;2012:658356. doi: 10.1155/2012/658356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenov OV, Koestenbauer S, Riegel M, Zech N, Zimmermann R, Zisch AH, et al. Multipotent mesenchymal stem cells from human placenta: critical parameters for isolation and maintenance of stemness after isolation. Am J Obstet Gynecol. 2010;202:193 e1–193.e13. doi: 10.1016/j.ajog.2009.10.869. [DOI] [PubMed] [Google Scholar]
- 5.Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ED. Bone marrow transplantation: prospects for leukemia and other conditions. Proc Inst Med Chic. 1975;30(8):256–8. [PubMed] [Google Scholar]
- 7.Bacigalupo A. Mesenchymal stem cells and haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2004;17:387–399. doi: 10.1016/j.beha.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Talaei-Khozani T, Heidari F, Esmaeilpour T, Vojdani Z, Mostafavi-Pour Z, Rohani L. Cardiomyocyte marker expression in mouse embryonic fibroblasts by cell-free cardiomyocyte extract and epigenetic manipulation. Iran J Med Sci. 2014;39:203–212. [PMC free article] [PubMed] [Google Scholar]
- 9.Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kan I, Melamed E, Offen D. Integral therapeutic potential of bone marrow mesenchymal stem cells. Curr Drug Targets. 2005;6:31–41. doi: 10.2174/1389450053344902. [DOI] [PubMed] [Google Scholar]
- 11.Shyu KG, Wang BW, Hung HF, Chang CC, Shih DT. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J Biomed Sci. 2006;13:47–58. doi: 10.1007/s11373-005-9038-6. [DOI] [PubMed] [Google Scholar]
- 12.Baghaban Eslaminejad M, Fallah N. Small molecule-BIO accelerates and enhances marrow-derived mesenchymal stem cell in vitro chondrogenesis. Iran J Med Sci. 2014;39:107–116. [PMC free article] [PubMed] [Google Scholar]
- 13.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieback K, Kern S, Kocaomer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18:S71–S76. [PubMed] [Google Scholar]
- 16.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 17.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 18.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 19.Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 20.De Francesco F, Tirino V, Desiderio V, Ferraro G, D’Andrea F, Giuliano M, et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One. 2009;4:e6537. doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmins NE, Kiel M, Gunther M, Heazlewood C, Doran MR, Brooke G, et al. Closed system isolation and scalable expansion of human placental mesenchymal stem cells. Biotechnol Bioeng. 2012;109:1817–1826. doi: 10.1002/bit.24425. [DOI] [PubMed] [Google Scholar]
- 22.Sun NZ, Ji H. In vitro differentiation of osteocytes and adipocytes from human placenta-derived cells. J Int Med Res. 2012;40:761–767. doi: 10.1177/147323001204000242. [DOI] [PubMed] [Google Scholar]
- 23.Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168–182. doi: 10.1900/RDS.2010.7.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vellasamy S, Sandrasaigaran P, Vidyadaran S, George E, Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J Stem Cells. 2012;4:53–61. doi: 10.4252/wjsc.v4.i6.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, Jung J, Lee HJ, Jeong SJ, Cho KJ, Hwang SG, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Kim BS, Kim JS, Chung YS, Sin YW, Ryu KH, Lee J, et al. Growth and osteogenic differentiation of alveolar human bone marrow-derived mesenchymal stem cells on chitosan/hydroxyapatite composite fabric. J Biomed Mater Res A. 2013;101:1550–1558. doi: 10.1002/jbm.a.34456. [DOI] [PubMed] [Google Scholar]
- 27.Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12–23. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 28.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 29.Sabapathy V, Ravi S, Srivastava V, Srivastava A, Kumar S. Long-term cultured human term placenta-derived mesenchymal stem cells of maternal origin displays plasticity. Stem Cells Int. 2012;2012:174328. doi: 10.1155/2012/174328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XF, He X, He J, Zhang LH, Su XJ, Dong ZY, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. doi: 10.1186/1423-0127-18-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 33.Loya K, Eggenschwiler R, Ko K, Sgodda M, Andre F, Bleidissel M, et al. Hepatic differentiation of pluripotent stem cells. Biol Chem. 2009;390:1047–1055. doi: 10.1515/BC.2009.120. [DOI] [PubMed] [Google Scholar]
- 34.Chou MT, Chang SN, Ke C, Chang HI, Sung ML, Kuo HC, et al. The proliferation and differentiation of placental-derived multipotent cells into smooth muscle cells on fibrillar collagen. Biomaterials. 2010;31:4367–4375. doi: 10.1016/j.biomaterials.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Behravan E, Moallem SA, Khateri S, Maraghi E, Jowsey P, Blain PG, et al. Deoxyribonucleic acid damage in Iranian veterans 25 years after wartime exposure to sulfur mustard. J Res Med Sci. 2013;18:239–244. [PMC free article] [PubMed] [Google Scholar]
- 36.Caviggioli F, Vinci V, Salval A, Klinger M. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79:856. doi: 10.1111/j.1445-2197.2009.05122.x. [DOI] [PubMed] [Google Scholar]
- 37.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 39.Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- 40.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 43.Najar M, Raicevic G, Boufker HI, Kazan HF, Bruyn CD, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 2010;264:171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 45.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2012;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 46.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Song CH, Sung HJ, Yoo YD, Geum DH, Park SH, et al. Human placenta-derived feeders support prolonged undifferentiated propagation of a human embryonic stem cell line, SNUhES3: comparison with human bone marrow-derived feeders. Stem Cells Dev. 2007;16:421–428. doi: 10.1089/scd.2006.0098. [DOI] [PubMed] [Google Scholar]
- 48.Mikkola HK, Gekas C, Orkin SH, Dieterlen-Lievre F. Placenta as a site for hematopoietic stem cell development. Exp Hematol. 2005;33:1048–1054. doi: 10.1016/j.exphem.2005.06.011. [DOI] [PubMed] [Google Scholar]


