Abstract
Objective(s):
Although routinely applied in assisted reproductive technology, human sperm cryopreservation is not a completely successful procedure. Adverse effects of cryopreservation on the fertilization capacity, motility, morphology, and viability of spermatozoa have been proven; cryopreservation has also shown a role in sperm DNA fragmentation and infertility. The post-thaw survival of spermatozoa improved after addition of supplementation of antioxidant molecules to freezing media. Nerve growth factor (NGF) as one of the prosurvival substances has gained great attention in recent years. The aim of this study was the usage of NGF as prosurvival factor after cryopreservation process of human semen samples to assess the motility and viability of sperm, nitric oxide (NO) concentration, and DNA fragmentation in normozoospermic men.
Materials and Methods:
Semen samples were collected from 25 normozoospermic men and were divided into fresh semen samples as control group, frozen–thawed semen samples without addition of exogenous NGF, and three groups of semen samples cryopreserved with addition of exogenous NGF (0.5, 1, and 5 ng/ml) in freezing medium. Viability was assessed by eosin-negrosin staining technique. Motility was evaluated with inverted microscope. NO concentration and apoptosis content were measured with flow cytometry.
Results:
Results showed that exogenous NGF at 0.5 ng/ml could significantly (P-value <0.05) influence viability, motility, nitric oxide, and DNA fragmentation content.
Conclusion:
Exogenous NGF as cryoprotectant improved sperm viability and motility, increased intracellular NO concentration, and decreased apoptosis content in normal human spermatozoa.
Keywords: Apoptosis, Cryopreservation, Human sperm, Nerve growth factor, Nitric oxide
Introduction
Assisted reproductive technologies (ARTs) such as cryopreservation have been used recently for treatment of subfertility (1). Cryopreservation method is associated with some side effects including osmotic stress, cold shock, formation of intracellular ice crystal, and over production of reactive oxygen species (ROS); each might be the responsible mechanism for consequent rcryodamage (2).
Increased levels ROS above physiological defenses by sperm metabolism lead to oxidative stress (OS) (3). One of the causes for the low percentage of live birth is the susceptibility of the spermatozoa to OS during preparation and selection of sperm (4, 5). During the freezing process, osmotic effects of cryopreservation and over production of ROS may change the sperm glycocalyx, damage sperm cell membrane, and decrease the fertilizing capacity (6-9). Furthermore the mammalian chromatin structure integrity is vital for the paternal genetic contribution to normal fetus development and healthy offspring. OS induces DNA damage and creates DNA fragmentation, base oxidation, chromatin cross-linking, and other modifications. Cryopreservation also modifies sperm motility (10, 11), viability (12), penetration into cervical mucus (10), structure of acrosome (11), and activity of acrosomal protease (13). There are a number of antioxidant systems in spermatozoa and seminal plasma that scavenge ROS and prevent internal cellular damage (6, 7). A balance normally exists between ROS production and seminal antioxidants. In poor semen samples, the effects of endogenous antioxidants are often decreased while the concentration of ROS is abnormally high (6-8, 14). Therefore the addition of supplements such as antioxidant molecules, antioxidant enzymes, or both to freezing media has improved spermatozoa functions (8).
Nerve growth factor (NGF) is an antioxidant supplement that has been used recently for improving freezing medium. Four compounds of neurotrophins such as NGF, brain-derived neurotro-phic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophins 4/5 (NT-4/5) (15), not only are important for the differentiation and survival of neuronal cells, but also are needed for the development of non-neuronal systems, such as reproductive systems. The action of neurotrophins mediates by binding with high-affinity to trans-membrane tyrosine kinase receptors (Trks) (16). NTs and their receptors promote development of the gonads (17) and regulate some functions in both the female (18) and male reproductive systems (19). Although various neurotrophins have been detected in mammalian testis (20), so far only NGF seems to have a potential role in male reproduction (21). Expression of the NGF gene was demonstrated at first in the testis of rat and mouse (22). NGF has an important role in promoting the formation and development of the testis and the differentiation, maturation, and movement of the spermatozoon (23). Further, addition of exogenous NGF to freezing medium can improve viability and motility of spermatozoa (24). Moreover, endothelial cells and skeletal myocytes are protected from apoptosis (programmed cell death) by exogenous NGF (25).
Another factor that has a role in function of spermatozoa is intracellular nitric oxide (NO). NO, a highly reactive gas with a short half-life, is synthesized by NADPH-dependent NO syntheses (NOSs) from the enzymatic conversion of L-arginine to L-citrulline (26). The NO-generating system has been demonstrated in the human reproductive tract, where NO plays a role in a variety of reproductive functions (27). During capacitation and acrosomal reaction, the role of NO is documented (28). In vitro studies showed that low concentrations of NO enhanced the motility of mouse (29), hamster (30), and human spermatozoa (31), increased the acrosomal reaction (AR) of mouse (32) and bull (33) spermatozoa, and elevated the zona pellucida-binding ability of human spermatozoa (34). On the other hand, higher NO concentrations seem to have opposite effects on the motility, viability, and metabolism of human spermatozoa in vitro (5, 28).
The aim of this study was to investigate the effects of different concentrations of NGF as a cryoprotectant on semen parameters of normozoos-permic men undergone cryopreservation for assessment of sperm viability and motility as well as nitric oxide concentration and DNA fragmentation in spermatozoa.
Materials and Methods
Sample collection and preparation
Semen samples from 25 fertile men (rapid motility >25% or progression in a semen sample >50%, and fresh sperm concentration >20×106/ml) were obtained by masturbation and were collected into sterile containers, following 3–5 days’ abstinence from sexual activity. After liquefaction at 37°C and 5% CO2, 25 semen samples were examined for sperm concentration, viability, and motility according to the World Health Organization guidelines (35).
The approval of the Baqiyatallah Medical Science University Research Ethical Committee was obtained prior to the study, and all subjects were informed with respect to this study.
Semen samples were divided into 5 groups (5 samples in each group): fresh semen samples as control group, one group of frozen-thawed samples without addition of exogenous NGF and three groups of semen samples with addition of exogenous NGF (0.5, 1, and 5 ng/ml) in freezing medium.
Based on the total sperm number and concentration needed for each analysis, each semen sample was aliquoted in 4 separate cryotubes for assessment of sperm viability and motility, DNA damage, and NO concentration; then equal volume of sperm freezing solution (Vitrolife, Sweden) was added to each cryotube.
Cryopreservation of semen samples
After addition of equal volume of sperm freezing solution, each cryotube except the control group that all parameters were assessed immediately, was inserted into the liquid nitrogen vapor at –180°C (15-30 cm above the liquid nitrogen) for 20-30 min then transferred to liquid nitrogen, and stored for two weeks.
Thawing process
After two weeks the cryotubes containing semen samples were thawed at room temperature for 5 min, and incubated at 37°C for 20 min. The freezing medium was removed by centrifugation at 1000 rpm for 5 min.
Assessment of viability
The eosin–nigrosin dye exclusion staining was used for assessment of sperm viability. Briefly, one drop of the thawed spermatozoa was mixed with two drops of 1% eosin stain (BDH Laboratory Supplies, UK). After 30 sec, three drops of 10 % nigrosin (BDH) were added to each solution. A drop of each fraction was smeared onto glass microscope slides and allowed to air-dry. The smears were assessed by oil immersion light microscopy at ×1000 magnification. Live spermatozoa appear white, whereas dead spermatozoa with disrupted membranes appear red. Vitality was quantified by counting a minimum of 200 spermatozoa on each slide and the proportion of live spermatozoa was expressed as a percentage of total cell number.
Assessment of motility
For assessment of motility, 10 µl of each semen sample was put on a microscopic slide and covered with coverslip. Assessment was done with inverted microscope by 40X magnification in multiple views according to the Fifth edition (2010) of World Health Organization guidelines (35).
Measurement of nitric oxide in spermatozoa
Briefly, for NO measurements each sample was mixed with DAF-2/DA (4,5-diaminofluorescein-2/diacetate) and was incubated in the dark for120 min at 37°C before being analyzed by fluorescence-activated cell sorter (6). Excitation wavelength (488 nm) and emission wavelength (530 nm) were used at the single-cell level and data were analyzed using CellquestTM version 3.3 software (Becton Dickinson, San Jose, CA, USA). The mean fluorescence intensity of the analyzed sperm cells was determined after gating the cell population by forward and side scatter light signals.
The final gated populations usually consisted of 8000–12000 sperm cells. Fluorescence in these cells was recorded on a frequency histogram by logarithmic amplifiers.
Measurement of DNA fragmentation in spermatozoa
In situ cell death detection kit, fluorescein (Roche, 11684795910, Germany) was used for detection and quantification of apoptosis at single cell level, per manufacturer’s instruction. Briefly, for apoptosis measurement, test sample was washed 3 times in PBS, adjusted to 2×107 cells/ml, and transferred 100 µl/well cell suspension into a V-bottomed 96-well microplate. Freshly prepared fixation solution was added to cell suspension, and then resuspended well and incubated 60 min at 15-25°C. After that, microplate was centrifuged and fixative was removed by flicking off or suction. Then cells were washed once with PBS and microplate was centrifuged and PBS was removed by flicking off or suction. Cells were resuspended in permeabilisation solution for 2 min on ice and were washed twice with PBS. Then cells were resuspended in TUNEL reaction mixture. Lid was added and incubated for 60 min at 37°C in a humidified atmosphere in the dark. Samples were washed twice in PBS and cells were transferred in a tube to a final volume of 250-500 µl in PBS. Samples can directly be analyzed by flow cytometry.
Statistical analysis
Data are expressed as the mean ± SEM. For detection of NGF effects, data of viability, nitric oxide, and apoptosis content in different groups were analyzed by one-way ANOVA. When variances in Levene test were statistically different Dunnett T3 for multiple comparison post hoc tests was used. If variances in Levene test were not statistically different, Tukey HSD for multiple comparison post hoc tests was used. Differences were regarded as statistically significant at P-value <0.05. Data of motility was analyzed by Chi-Square Test.
Results
Effect of exogenous NGF on viability
In normozoospermic men (n= 25) viability of frozen-thawed group (28±2.54) was significantly reduced compared with fresh group (56±4.30) (P-value <0.00). The addition of NGF in freezing medium at 0.5 ng/ml (55±3.53) and 1 ng/ml (43±2.54) significantly increased viability vs. frozen-thawed group (28±2.54) (P-value <0.05). But viability in frozen-thawed samples with exogenous NGF at 5 ng/ml (13±2.54) had no significant difference compared with frozen-thawed group (28±2.54) (P-value> 0.02) (Figure 1a).
Figure 1.
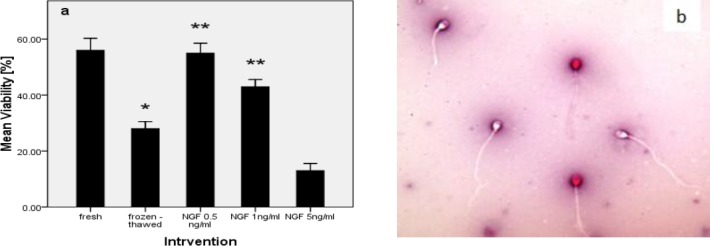
Effect of nerve growth factor on sperm viability in normozoospermic men (a). Human spermatozoa staining with eosin-nigrosin dye assessed by oil immersion light microscopy at ×1000 magnification. Live spermatozoa appeared white whilst dead spermatozoa with disrupted membranes have taken up the eosin stain and appeared red (b). P-values <0.05 were considered significant.*: Significant difference vs. fresh group (P-value <0.05). **: Significant difference vs. frozen-thawed group (P-value <0.05). Error bars: ±1 SE
Effect of exogenous NGF on motility
Progressive motility percentage of sperm in fresh group was 90% whereas in frozen-thawed group was 74% of samples. Progressive motility percentage of sperm with addition of exogenous NGF at 0.5 ng/ml increased (78%), but other dosage of NGF (1 and 5 ng/ml) could not increase progressive motility.
The lowest percentage of non-progressive motility was related to fresh group (7%) whereas the highest percentages were seen in frozen-thawed group (14%) and frozen-thawed with addition of exogenous NGF at 5 ng/ml (17%).
Immotility percentage of sperm in fresh group was 3% whereas in frozen-thawed group was 12%. Addition of NGF at 0.5 ng/ml could decrease immotility percentage vs. frozen-thawed group (Table 1).
Table 1.
Effect of nerve growth factor on sperm motility percentage in normozoospermic men. PR: Progressive motility. NP: Non-progressive motility. IM: Immotility
| Groups | Grade | |||
|---|---|---|---|---|
| PR% | NP% | IM% | ||
| Fresh | 90 | 7 | 3 | |
| Frozen-thawed | 74 | 14 | 12 | |
| Normozoospermic men | NGF 0.5ng/ml | 78 | 12 | 10 |
| NGF 1ng/ml | 75 | 11 | 14 | |
| NGF 5ng/ml | 69 | 17 | 14 | |
Effect of exogenous NGF on nitric oxide
In normozoospermic men (n=35) NO content of frozen-thawed group (9.76 ± 1.38) showed significant elevation vs. fresh group (0.96 ± 0.12) (P-value <0.01). The addition of NGF in freezing medium at 0.5 ng/ml (5.14 ± 1.35) significantly reduced NO concentration compared with frozen-thawed group (9.76 ± 1.38) (P-value <0.05). But NO concentration in group of frozen-thawed samples with exogenous NGF at 1 ng/ml (6.57 ± 0.69) and 5 ng/ml (11.67 ± 1.85) had no significant difference vs. frozen-thawed group (9.76 ± 1.38) (P-value >0.01) (Figure 2a). Direct NO concentration in human spermatozoa was measured by flow cytometry using the NO-specific probe DAF-2/DA, is shown in Figure 2b, c, d.
Figure 2.
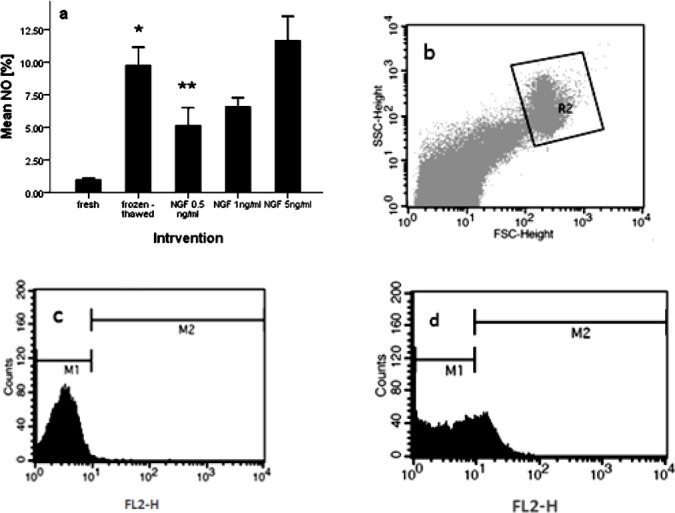
Effect of nerve growth factor on sperm nitric oxide content in normozoospermic men (a). Dot plot represents total acquired events and final gated population of spermatozoa (b). Histogram of unstaining semen sample (c). Histogram of semen sample, incubated with baseline 4,5-diaminofluorescein-2/diacetate fluorescence (d). P-values <0.05 were considered significant.*: Significant difference vs. fresh group (P-value <0.05). **: Significant difference vs. frozen-thawed group (P-value <0.05). Error bars: ±1 SE
Effect of exogenous NGF on apoptosis
In normozoospermic men (n = 15) DNA damage rate of frozen-thawed group (34.94± 2.43) was significantly elevated vs. fresh group (7.58 ± 1.30) (P-value <0.01). The addition of NGF at 0.5 ng/ml (12.92 ± 3.20) in freezing medium showed significant difference in DNA fragmentation compared with frozen-thawed group (34.94± 2.43) (P<0.01). But NGF at 1 ng/ml (31.80 ± 3.60) and 5 ng/ml (32.97 ± 1.16) had no significant difference vs. frozen-thawed group (34.94± 2.43) (P-value >0.1) (Figure 3a). Dot plot and histogram of semen samples that was analyzed by flow cytometry is shown in Figure 3b, c, d.
Figure 3.
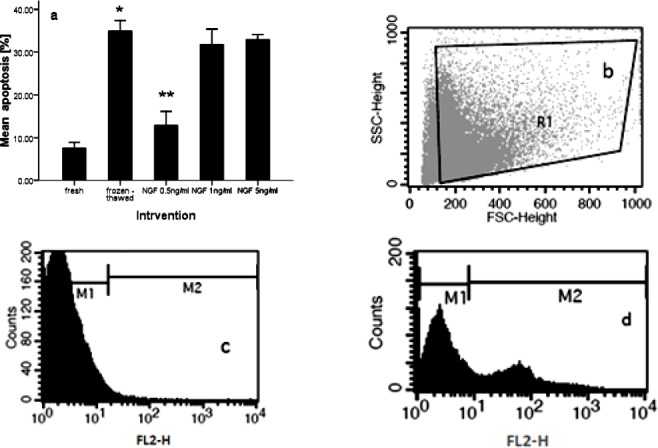
Effect of nerve growth factor on sperm DNA fragmentation in normozoospermic men (a). Dot plot represents total acquired events and final gated population of spermatozoa (b). Histogram of unstaining semen sample (c). Histogram of semen sample, incubated with TUNEL reaction mixture (d). P-values <0.05 were considered significant.*: Significant difference vs. fresh group (P-value <0.05). **: Significant difference vs. frozen-thawed group (P-value <0.05). Error bars: ±1 SE
Discussion
In this study we detected that NGF supplementation of semen cryopreservation medium at a concentration of 0.5 ng/ml significantly improved post-thaw viability, motility, and nitric oxide content and reduced DNA fragmentation.
The aim of the present study was to determine whether the addition of an antioxidant to the semen freezing medium could improve the post-thaw viability, motility, intracellular nitric oxide, and rDNA integrity of cryopreserved human spermatozoa in men with normal sperm parameters.
Mammalian spermatozoa are one of the first successfully cryopreserved cells (9). Cryopreservation of semen is routinely used in a variety of circumstances, including assisted reproduction, pre-radiation or chemotherapy treatment, for men undergoing vasectomy and for storage of donor semen until seronegativity for HIV and hepatitis is confirmed. It is also used for storage of sperms retrieved from azoospermic patients who have undergone testicular sperm extraction. For further use of assisted reproductive techniques such as intrauterine insemination, in vitro fertilization and intracytoplasmic sperm injection, storage of spermatozoa provides an important fertility reserve (36).
The cells are exposed to severe stresses in both freezing and thawing process (9). Cryopreservation can induce excessive lipid peroxidation in the sperm plasma membrane and cause an overall increase in oxygen radical concentration of the samples. Exposure to high ROS concentrations impairs the sperm motility (10, 11, 14) and viability (12), DNA integrity (37), penetration into cervical mucus (10), and acrosomal structure (11).
Spermatozoa and seminal plasma, to counteract the harmful effects of ROS, have a number of antioxidant systems that scavenge ROS and prevent internal cellular damage. Antioxidant systems consist of enzymatic and non-enzymatic molecules (6, 7). Enzymatic antioxidant defense mechanisms include the glutathione peroxidase/reductase system, superoxide dismutase, and catalase (38), whereas non-enzymatic antioxidants include reduced glutathione, urate, ascorbic acid, vitamin E, carotenoids, ubiquinones, taurine, and hypotaurine (6, 7, 38). Addition of antioxidant molecules, enzymes, or both to freezing media improved survival and DNA integrity of the post-thaw spermatozoa (8). Antioxidants have been used not only as dietary supplements, but also in the culture medium in vitro to counteract the adverse effects of sperm oxidative stress (38). Some antioxidant compounds such as ascorbate, genistein, catalase, and vitamin E have been used for improving semen freezing protocols (6, 14, 37, 39).
NGF, discovered by Rita Levi-Montalcini, is a member of the neurotrophin protein family and probably the most extensively studied member of NTs, mediated through TrkA and possesses the ability to stimulate growth, differentiation and survival of neurons during development and after damage (22). Many studies have shown that NGF not only is present in all stages of germinal cells from primary spermatocytes to mature spermatozoids, but also is detected in the Leydig cells and may play an important role in the development of reproductive tissues such as viability and motility of spermatozoa and DNA integrity. Presence of ROS following freezing and thawing process changes sperm viability and mitochondrial activity that induce sperm apoptosis (24, 41). Great difference between fresh and frozen sperm regarding the generation rate of O2- and H2O2 or in the intracellular concentration of free calcium ions (Ca2+) leads to shorter lifespan and lower fertility of frozen sperm compared to the raw fresh semen (40). Studies showed that exogenous NGF is able to improve viability of cells (24, 41). Melanie Abram suggested that in the allergic airway inflammation, NGF/TrkA-mediated pulmonary IgE production contributes significantly to serum-IgE levels. She concluded that the neurotrophins NGF and NT3 act as survival factors for pulmonary plasma cells (42). Moreover, based on flow cytometry, addition of exogenous NGF to incubation medium could increase sperm viability (24). In present study exogenous NGF (0.5 and 1 ng/ml) also significantly increased the viability of normal human spermatozoa.
Motility is one of the most important features that participates in fertilizing ability of spermatozoa and is regulated by a number of hormones and growth factors such as testosterone, epidermal growth factor, and fibroblast growth factor (7, 43). Exposure to high concentration of ROS can lead to disruption of mitochondrial and plasma membranes, which results in chromosomal and DNA fragmentation and causes a reduction in sperm motility (44). The addition of antioxidants such as vitamin E and NGF to frozen semen medium significantly improved post-thaw motility; this finding may promote the clinical application of NGF in ARTs (14, 43, 45). In this line, our study also suggested that motility of sperm with addition of exogenous NGF at 0.5ng/ml was increased (78%).
NO has a well-known role in the spermatozoal function regarding capacitation and acrosomal reaction (28). NO is a free radical generated from the oxidation of Larginine to L-citrulline by nicotinamide adenine dinucleotide phosphate (NADPH)-dependent NO synthases (NOS) (26). Miraglia et al confirmed that NO stimulates human sperm motility via the activation of soluble guanylate cyclase, the subsequent synthesis of cGMP, and the activation of cGMP-dependent protein kinases (46). In vitro studies have shown that low concentrations of NO enhance the motility of mouse (29), hamster (30), and human spermatozoa (31), increase the acrosomal reaction (AR) of mouse (32) and bull (33) spermatozoa, and elevate the zona pellucida-binding ability of human spermatozoa (34). On the other hand, higher NO concentrations seem to have opposite effects on the motility, viability, and metabolism of human spermatozoa in vitro (5, 28).
Our results showed that the addition of NGF in freezing medium at 0.5ng/ml significantly reduced NO concentration compared with frozen-thawed group. Moreover, with reduction of NO content, sperm viability and motility (progressive motility) were significantly elevated. Apoptosis as a result of sperm cryopreservation may reduce life span of the surviving population and change the permeability, integrity, and symmetry of the plasma membrane (14). These conditions are related to the creation of OS and ROS. To decrease the impact of ROS, prosurvival factors such as antioxidants have an important role. Withdrawal of prosurvival factors could induce apoptosis in human spermatozoa. Survival factors, through activation of phosphatidylinositol 3-kinase–Akt phosphorylation, prevent spermatozoa from apoptosis. In vivo long life span of human spermatozoa may be related to the presence of prosurvival factors in the epididymal plasma and uterotubal fluids that prevent apoptosis state. But, in vitro incubation of spermatozoa in simple culture medium without any prosurvival factors leads to apoptosis. Because most ARTs culture media only have balanced salt solutions supplemented with energy substrates and possibly serum albumin without any supplementation (1). Previous study have shown that during freezing and thawing of sperm in buffalo, human, bull, and stallion DNA integrity was unchanged (14). But, many other researches indicated that generation of excessive OS during cryopreservation leads to DNA fragmentation (37, 40). As a result, because of the damaging effects of cryopreservation on sperm chromatin, assessment of the DNA integrity of frozen/thawed spermatozoa is important (14). In recent years different supplementations and antioxidants have been added to cryopreservation medium and the DNA integrity has been assessed. NGF is one supplementation which its effect has been determined on different cells. Studies showed that NGF prevents STS-induced apoptotic morphology and caspase-3 activity in hippocampus by upregulating phosphorylation of the tropomyosin receptor kinase (Trk) receptor (47). Further, exogenous NGF not only protects endothelial cells and skeletal myocytes from apoptosis, but also induces neovascularization in murine ischemic limb muscles and diabetic skin wounds (25). NGF in non-neuronal osteoblastic cells may play an important role in cell survival as an anti-apoptotic factor and that suppresses the apoptosis of rat peritoneal mast cells and may act as a key factor to promote survival of connective tissue-type mast cells (48). Furthermore NGF prevents rotenone-induced apoptosis through the activation of the PI 3-kinase pathway in dopaminergic cells (49). In this study we detected that exogenous NGF (0.5 ng/ml) decreased apoptosis, but the other doses of exogenous NGF (1 and 5 ng/ml) could not reduce apoptosis in normal human spermatozoa.
Conclusion
Generally, our results clearly demonstrated that exogenous NGF as a cryoprotectant improved sperm viability and motility, increased intracellular NO concentration, and reduced apoptosis in normal human spermatozoa. Further examination of the possible interactions between antioxidants and semen parameters may elucidate detailed mechanisms of antioxidant functions.
Acknowledgment
None of the authors declare probable conflicts of interest. This project financially supported by Research and Technology Foundation (Iran National Science foundation (INSF)). Thus, we thank for the facilities provided. The authors are also grateful to all participants, without whom this work would have been impossible.
References
- 1.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassalle B, Testart J. Human zona pellucida recognition associated with removal of sialic acid from human sperm surface. J Reprod Fertil. 1994;101:703–711. doi: 10.1530/jrf.0.1010703. [DOI] [PubMed] [Google Scholar]
- 3.Cheema RS, Bansal AK, Bilaspuri GS. Manganese provides antioxidant protection for sperm cryopreservation that may offer new consideration for clinical fertility. Oxid Med Cell Longev. 2009;2:152–159. doi: 10.4161/oxim.2.3.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Plessis SS, Makker K, Desai NR, Agarwal A. The impact of oxidative stress on in vitro fertilization. Expert Rev Obstet Gynecol. 2008;3:539–554. [Google Scholar]
- 5.Du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, Lampiao F. Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–210. doi: 10.1111/j.1439-0272.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 6.Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC. Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology. 2011;62:40–46. doi: 10.1016/j.cryobiol.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2010;60:235–237. doi: 10.1016/j.cryobiol.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Kalthur G, Raj S, Thiyagarajan A, Kumar S, Kumar P, Adiga SK. Vitamin E supplementation in semen-freezing medium improves the motility and protects sperm from freeze-thaw–induced DNA damage. Fertil Steril. 2011;95:1149–1151. doi: 10.1016/j.fertnstert.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Talaei T, Esmaeelpour T, Aekiyash F, Bahmanpour S. Effects of cryopreservation on plasma membrane Glycoconjugates of human spermatozoa. Iran J Reprod Med. 2010;8:119–124. [Google Scholar]
- 10.Critser JK, Arneson BW, Aaker DV, Huse-Benda AR, Ball GD. Cryopreservation of human spermatozoa. II. Post-thaw chronology of motility and zona-free hamster ova penetration. Fertil Steril. 1987;47:980–984. doi: 10.1016/s0015-0282(16)59233-4. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin EA, Ford WC, Hull MG. Motility characteristics and membrane integrity of cryopreserved human spermatozoa. J Reprod Fertil. 1992;95:527–534. doi: 10.1530/jrf.0.0950527. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez JG, Storey BT. Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: glycerols and other polyols as sole cryoprotectant. J Androl. 1993;14:199–208. [PubMed] [Google Scholar]
- 13.Mack SR, Zaneveld LJ. Acrosomal enzymes and ultrastructure of unfrozen and cryotreated human spermatozoa. Gamete Res. 1987;18:375–383. doi: 10.1002/mrd.1120180411. [DOI] [PubMed] [Google Scholar]
- 14.Taylor K, Roberts P, Sanders K, Burton P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod Biomed Online. 2009;8:184–189. doi: 10.1016/s1472-6483(10)60254-4. [DOI] [PubMed] [Google Scholar]
- 15.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 16.Barbacid M. Structural and functional properties of the TRK family of neurotrophin Receptors. Ann N Y Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 17.Levine E, Cupp AS, Skinner MK. Role of neurotrophins in rat embryonic testis morphogenesis (cord formation) Biol Reprod. 2000;62:132–142. doi: 10.1095/biolreprod62.1.132. [DOI] [PubMed] [Google Scholar]
- 18.Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, et al. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- 19.Muller D, Paust HJ, Middendorff R, Davidoff MS. Nerve growth factor (NGF) receptors in male reproductive organs. Adv Exp Med Biol. 1997;424:157–158. doi: 10.1007/978-1-4615-5913-9_29. [DOI] [PubMed] [Google Scholar]
- 20.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zheng L, Wang C, Zhou X. Absence of nerve growth factor and comparison of tyrosine kinase receptor A levels in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clinica Chimica Acta. 2010;411:1482–1486. doi: 10.1016/j.cca.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Mutter D, Middendorff R, Davidoff MS. Neurotrophic factors in the testis. Biomed Reviews. 1999;10:25–30. [Google Scholar]
- 23.Lipps BV. Isolation of nerve growth factor (NGF) from human body fluid;saliva, serum and urine: comparison between cobra venom and cobra serum NGF. J Nat Toxins. 2000;9:349–356. [PubMed] [Google Scholar]
- 24.Li C, Sun Y, Yi K, Ma Y, Sun Y, Zhang W, Zhou X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology. 2010;74:1615–1622. doi: 10.1016/j.theriogenology.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, et al. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 26.Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 27.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of Reproduction. Hum Reprod Update. 1998;4:3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Roessner C, Paasch U, Glander HJ, Grunewald S. Activity of nitric oxide synthase in mature and immature human spermatozoa. Andrologia. 2010;42:132–137. doi: 10.1111/j.1439-0272.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 29.Herrero MB, Cebral E, Boquet M, Viggiano JM, Vitullo A, Gimeno MA. Effect of nitric oxide on mouse sperm hyperactivation. Acta Physiol Pharmacol Ther Latinoam. 1994;44:65–69. [PubMed] [Google Scholar]
- 30.Yeoman RR, Jones WD, Rizk BM. Evidence for nitric oxide regulation of hamster sperm. hyperactivation. J Androl. 1998;19:58–64. [PubMed] [Google Scholar]
- 31.Hellstrom WJG, Bell M, Wang R, Sikka SC. Effects of sodium nitroprusside on sperm motility, viability, and lipid Peroxidation. Fertil Steril. 1994;61:1117–1122. doi: 10.1016/s0015-0282(16)56766-1. [DOI] [PubMed] [Google Scholar]
- 32.Herrero MB, Viggiano JM, Perez-Martinez S, De Gimeno MF. Evidence that nitric oxide synthase is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Reprod Fertil Dev. 1997;9:433–439. doi: 10.1071/r96044. [DOI] [PubMed] [Google Scholar]
- 33.Zamir N, Barkan D, Keynan N, Naor Z, Breitbart H. Atrial natriuretic peptide induces acrosomal exocytosis in bovine spermatozoa. Am J Physiol. 1995;269:E216–E221. doi: 10.1152/ajpendo.1995.269.2.E216. [DOI] [PubMed] [Google Scholar]
- 34.Sengoku K, Tamate K, Yoshida T, Takaoka Y, Miyamoto T, Ishikawa M. Effects of low concentrations of nitric oxide on the zona pellucida binding ability of human spermatozoa. Fertil Steril. 1998;69:522–527. doi: 10.1016/s0015-0282(97)00537-2. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. 5th ed. Geneva, Switzerland: WHO Press; 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 36.Kläver R, Bleiziffer A, Redmann K, Mallidis C, Kliesch S, Gromoll J. Routine cryopreservation of spermatozoa is safe —Evidence from the DNA methylation pattern of nine spermatozoa genes. J Assist Reprod Genet. 2012;29:943–950. doi: 10.1007/s10815-012-9813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–2070. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez JG, Storey BT. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989;23:77–90. doi: 10.1002/mrd.1120230108. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Soto JC, De DiosHourcade J, Gutierrez-Adan A, Landeras JL, Gadea J. Effect of genistein supplementation of thawing medium on characteristics of frozen human spermatozoa. Asian J Androl. 2010;12:431–441. doi: 10.1038/aja.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemma A. Effect of cryopreservation on sperm quality and fertility. Artificial Insemination Farm Animals. 2011;12:191–216. [Google Scholar]
- 41.Chen Y, Dicou E, Djakiew D. Characterization of nerve growth factor precursor protein expression in rat round spermatids and the trophic effects of nerve growth factor in the maintenance of Sertoli cell viability. Mol Cell Endocrinol. 1997;127:129–136. doi: 10.1016/s0303-7207(96)04001-4. [DOI] [PubMed] [Google Scholar]
- 42.Abram M, Wegmann M, Fokuhl V, Sonar S, Luger EO, Kerzel S, et al. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J Immunol. 2009;182:4705–4712. doi: 10.4049/jimmunol.0802814. [DOI] [PubMed] [Google Scholar]
- 43.Jin WZ, Taya K. Effect of NGF on the motility and acrosome reaction of golden hamster spermatozoa in vitro. J Reprod Dev. 2010;56:437–443. doi: 10.1262/jrd.09-219n. [DOI] [PubMed] [Google Scholar]
- 44.Baumber J, Ball BA, Linfor JJ, Meyers SA. Reactive oxygen species and cryopreservation promote DNA fragmentation in equine spermatozoa. J Androl. 2003;24:621–628. doi: 10.1002/j.1939-4640.2003.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 45.Shi CG, Lin K, Xu XB, Zhang SC, Wang N, Fan M. Evidence for the involvement of NGF in human sperm motility. J Biomed Sci Eng. 2012;5:534–541. [Google Scholar]
- 46.Miraglia E, De Angelis F, Gazzano E, Hassanpour H, Bertagna A, Aldieri E, et al. Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway. Reproduction. 2011;141:47–54. doi: 10.1530/REP-10-0151. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TL, Kim CK, Cho JH, Lee KH, Ahn JY. Neroprotection signaling pathway of nerve growth factor and brain-derived neurotrophic factor against staurosporine induced apoptosis in hippocampal H19-7 cells. Exp Mol Med. 2010;42:583–595. doi: 10.3858/emm.2010.42.8.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trk proto-oncogene receptor. Blood. 1995;86:4638–4644. [PubMed] [Google Scholar]
- 49.Hirata Y, Meguro T, Kiuchi K. Differential effect of nerve growth factor on dopaminergic neurotoxin-induced apoptosis. J Neurochem. 2006;99:416–425. doi: 10.1111/j.1471-4159.2006.04006.x. [DOI] [PubMed] [Google Scholar]


