Abstract
Reactive oxygen species play an important role in the pathogenesis of diabetic retinopathy. We studied the role of adrenergic and serotonin receptors in the generation of superoxide by retina and 661W retinal cells in high glucose and of the α1-adrenergic receptor (AR) on vascular lesions of the retinopathy in experimentally diabetic C57Bl/6J mice (and controls) after 2 and 8 months. Compared with 5 mM glucose, incubating cells or retinal explants in 30 mM glucose induced superoxide generation. This response was reduced or ablated by pharmacologic inhibition of the α1-AR (a Gq-coupled receptor) or Gs-coupled serotonin (5-HT2, 5-HT4, 5-HT6, and 5-HT7) receptors or by activation of the Gi-coupled α2-AR. In elevated glucose, the α1-AR produced superoxide via phospholipase C, inositol triphosphate-induced Ca2+ release, and NADPH oxidase, and pharmacologic inhibition of these reactions prevented the superoxide increase. Generation of retinal superoxide, expression of proinflammatory proteins, and degeneration of retinal capillaries in diabetes all were significantly inhibited with daily doxazosin or apocynin (inhibitors of α1-AR and NADPH oxidase, respectively), but increased vascular permeability was not significantly affected. Adrenergic receptors, and perhaps other GPCRs, represent novel targets for inhibiting the development of important features of diabetic retinopathy.—Du, Y., Cramer, M., Lee, C. A., Tang, J., Muthusamy, A., Antonetti, D. A., Jin, H., Palczewski, K., Kern, T. S. Adrenergic and serotonin receptors affect retinal superoxide generation in diabetic mice: relationship to capillary degeneration and permeability.
Keywords: GPCRs, NADPH oxidase, diabetic retinopathy, inflammation
GPCRs form a large diverse superfamily of membrane proteins encoded by >800 genes in the human genome (1). They detect a wide spectrum of extracellular signals, including photons, ions, small organic molecules, and proteins before undergoing conformational changes that cause activation of cytosolic signaling through activation of G proteins, including the subtypes Gs, Gi, and Gq (2). Signaling pathways regulated by these G proteins then regulate effector molecules such as calcium, potassium channels, adenylate cyclase, phospholipase C (PLC), and protein kinases. Rhodopsin is probably the best-recognized GPCR that is expressed in retinal cells, but numerous other GPCRs also are involved in maintaining retinal function and integrity (3). GPCRs also can contribute to retinal diseases. They have been implicated in the generation of reactive oxygen species, in part via regulation of intracellular calcium and NADPH oxidase in a variety of cell types, including retinal photoreceptor cells undergoing light-induced retinal degeneration (3–5).
Diabetes is known to induce oxidative stress in multiple tissues including the retina. Characteristic lesions of diabetic retinopathy in animals have been inhibited with oral antioxidants or overexpression of antioxidant enzymes (6–8), indicating that oxidative stress plays an important role in diabetes-induced retinal microangiopathy. Recently we showed that retinal photoreceptor cells generate most of the diabetes-induced increase in retinal generation of superoxide via mitochondria and NADPH oxidase (9).
Here we investigated the contribution of several GPCRs and their downstream signaling pathways to superoxide generation by retina and retinal cells. We focused initially on adrenergic receptors (ARs) and 5-hydroxytryptamine (serotonin) receptors (HTRs) because these receptors were identified in retinas from multiple species by transcriptome analysis (3), and HTR agonists were shown by others to inhibit retinal degenerative diseases (10–14). Although these receptors had not been previously implicated in diabetic retinopathy, our present findings demonstrate that pharmacologic manipulation of these receptors can regulate superoxide generation by retinas and retinal cells exposed to elevated glucose. Moreover, pharmacologic inhibition of either the α1-AR or downstream NADPH oxidase (both components of the Gq-regulated signaling pathway) lowered the diabetes-induced increase in retinal oxidative stress, expression of proinflammatory proteins by the retina, and the resulting degeneration of retinal capillaries. These results identify GPCRs and their downstream pathways as novel therapeutic targets that can reduce retinal superoxide generation and the histopathology of diabetic retinopathy.
MATERIALS AND METHODS
Chemicals
Doxazosin (Dox), apocynin (Apo), U73122, 2-aminoethoxydiphenyl borate (2-APB), ruthenium red, guanabenz (Gub), and brimonidine (Brim) were obtained from Sigma Chemicals (St. Louis, MO, USA). Lofexidine (Lof) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). LY 215840, RO 04-6790, RS 23597-190, and SQ 22536 were purchased from TOCRIS Biosciences (Bristol, United Kingdom). Sp-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-monophosphorothioate, dibutyryl cAMP, isobutylmethylxanthine, 1-methyl-3-isobutylxanthine (IBMX), and KT5720 were obtained from Enzo Life Sciences (Farmingdale, NY, USA).
In vitro studies
For initial drug candidate screening, we used a well-studied transformed cell line (661W) of retinal cells (15). The identity of these cells was confirmed by the positive identification of cone opsin mRNA and other proteins previously identified in this cell line (Supplemental Fig. S1). These cells were passaged in DMEM medium containing 5 mM glucose and 10% fetal bovine serum. For experiments, the fetal serum was reduced to 2%, and cells were incubated in either 5 or 30 mM glucose for 4 days with medium changed every other day. Test agents were added to the medium at 2–3 concentrations, each based on published reports as summarized in Table 1, with DMSO used as a control. Test drug concentrations that best reduced superoxide generation are shown in the figures. Cells were harvested by adding a trypsin-EDTA solution (0.5% and 0.02%, w/v) to the culture followed by centrifugation. In some experiments, Dox and Gub or Dox and RO 04-6790 were concurrently administered at suboptimal doses for 4 days. Effects of optimal concentrations of these drugs (selected for their ability to inhibit superoxide generation in 30 mM glucose) on cell death after 4 days are shown in Supplemental Table S1.
TABLE 1.
Agents affecting signaling pathways studied in vitro
| Agent | Mode of action | Signaling | In vitro doses (µM) |
|---|---|---|---|
| Doxazosin (Dox) | α1-AR antagonist | Gq | 10, 100, 1000 |
| Phenoxybenzamine (PBA) | α-AR antagonist | Gq | 10, 20 |
| Prazosin (PRA) | α1-AR antagonist | Gq | 0.5, 5 |
| U73122 | PLC inhibitor | — | 1, 5, 10 |
| 2-APB | IP3 receptor inhibitor | — | 10, 100, 200 |
| Ruthenium red (RR) | Calcium release from ER inhibitor | — | 5, 10 |
| Apocynin (Apo) | NADPH oxidase inhibitor | — | 10, 100, 1000 |
| Lofexidine (Lof) | α2-AR agonist | Gi | 10, 100, 1000 |
| Guanabenz (Gub) | α2-AR agonist | Gi | 1, 10 |
| Brimonidine (Brim) | α2-AR agonist | Gi | 1, 10 |
| LY 215840 (LY) | 5-HT2R/5-HT7R antagonist | Gs, Gq | 10, 100 |
| RO 04-6790 (RO) | 5-HT6 R antagonist | Gs | 10, 100 |
| RS 23597-190 (RS) | 5-HT4R antagonist | Gs | 10, 100 |
| Dibutyryl cAMP (db cAMP) | Adenylate cyclase agonist | Gs | 200, 500, 1000 |
| sp-5,6-DCI-cBIMPS | cAMP analog, PKA activator | Gs | 1, 10, 50 |
| SQ 22536 (SQ) | Adenylate cyclase antagonist | Gs | 50, 500 |
| IBMX | Phosphodiesterase inhibitor | Gs | 30, 100, 225 |
| KT5720 | PKA inhibitor | Gs | 0.05, 2 |
| Forskolin | Adenylate cyclase agonist | Gs | 10 |
Assays performed in vitro with 661W cells are described in the Materials and Methods section.
Retinal explants
Eyes were enucleated from adult C57Bl/6J mice and immediately immersed in ice-cold DMEM containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). The posterior pole (including retina, retinal pigment epithelium, and sclera) was incubated for 4 days in DMEM in humidified incubators with 5% CO2 at 37°C, thus keeping the retina in contact with the retinal pigmented epithelium. The culture medium was changed every other day. At the end of this incubation, the retina was separated from the retinal pigment epithelium prior to the assay for superoxide.
Animals
All experiments followed the guidelines set forth by the Association for Research in Vision and Ophthalmology Resolution on Treatment of Animals in Research and the Institutional Animal Care and Use Committee at Case Western Reserve University. Insulin-deficient diabetes was induced in 2-month-old fasted male C57BI/6J mice by intraperitoneal injections of streptozotocin [55 mg/kg body weight (BW)] on 5 consecutive days. Insulin was given as needed (0–0.2 units every 2–3 days) to maintain BW while allowing chronic hyperglycemia, polyuria, and hyperphagia. Blood glucose and hemoglobin A1c (HbA1c) were measured as reported previously (16, 17). All therapeutics were administered by intraperitoneal injection in DMSO. Diabetic and age-matched nondiabetic controls were studied after 2 durations of diabetes (2 and 8 months).
Drugs administered in vivo
Diabetic mice were treated with (i) the α1-AR antagonist, Dox (10 mg/kg BW, daily intraperitoneal injection in DMSO); (ii) the NADPH oxidase inhibitor, Apo (36 mg/kg BW; daily intraperitoneal injection in DMSO); (iii) the PLC inhibitor, U73122 (6.25 mg/kg BW; daily intraperitoneal injection in DMSO; or (iv) the calcium channel inhibitor, 2-APB (6.25 mg/kg BW; daily intraperitoneal injection in DMSO). The α2-AR agonist, Lof, also was given to animals (dose initially was 2 mg/kg BW daily via intraperitoneal injection in DMSO). Doses were selected based on prior publications (5) or initial dosing studies (data not shown). In all the above experiments, DMSO was injected intraperitoneally as the vehicle control.
Superoxide generation
Retinas or isolated cells were incubated in 200 µl of Krebs-[4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, pH 7.2, with 5 or 25 mM glucose for 5 minutes at 37°C in 5% CO2. Luminescence indicating the presence of superoxide was measured 5 minutes after addition of 0.54 mM (final concentration) lucigenin, as published previously (18–22). Luminescence intensity is reported in arbitrary units per milligram protein. To confirm the results obtained by the lucigenin method, we also measured reactive oxygen species with a 2′,7′ dichlorofluorescein acetate method previously reported by Best et al. (23). Results obtained with this alternate method were consistent with those found with lucigenin (data not shown).
Intracellular cAMP assay
Cells (661W) were incubated with either 5 mM glucose, 30 mM glucose, or 30 mM glucose containing drugs at their indicated concentrations for 4 days. Intracellular cAMP levels were measured with the cAMP Biotrak Enzyme Immunoassay System (GE Healthcare Life Sciences, Piscataway, NJ, USA). To ensure equal protein concentrations, cell numbers in each sample were determined, and the volume of lysis buffer was adjusted accordingly. Isobutylmethylxanthine (1 mM) was included in the lysis buffer to inhibit cAMP-dependent phosphodiesterase activity.
Immunoblots
Retinal homogenates were separated by SDS-PAGE and incubated with either anti-rat intercellular adhesion molecule-1 (1:2000 dilution; R&D Systems, Minneapolis, MN, USA) or the anti-inducible isoform of nitric oxide synthase (iNOS; 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Protein levels were quantified relative to β-actin loading controls (1:3000 dilution; Abcam, Cambridge, MA, USA) in the same samples.
RT-PCR
To confirm that 661W cells were from photoreceptor cells, we used PCR for red/green opsin. Methods and results are summarized in the Supplemental Material.
Permeability
Retinal permeability was measured in eyes from animals that were diabetic for 8 months and their age-matched controls by using a fluorescently labeled tracer as described previously (24). Briefly, sterile FITC-bovine serum albumin (BSA; 50 µg/µl) in PBS (NaCl, 0.138 M; KCl, 0.0027 M; pH 7.4) was injected into the tail vein of mice at 100 µg/g BW. The dye circulated for 20 minutes before blood samples were collected and eyes were enucleated. Eyes were fixed in ice cold 4% paraformaldehyde, infused with sucrose, and then frozen in optimal cutting temperature compound in isopentane on dry ice. Retinal cryosections were imaged by fluorescence microscopy. Two images per eye were obtained on either side of the optic disc in the inner plexiform layer, and 2 sections per eye were imaged to generate an average image pixel density in the neural retina exclusive of any vessels. Relative average value fluorescence increases were normalized to the relative plasma fluorescence for final determinations of retinal dye accumulation. Because diabetes can increase glycation and other processes that cause autofluorescence and thus might confound interpretation of fluorescence data after injection of FITC-BSA, preliminary studies of retinal sections after long-term diabetes were carried out to evaluate possible autofluorescence. Our methods failed to detect an increase in autofluorescence (fluorescence in the FITC channel in the absence of FITC-BSA) caused by diabetes, so no correction of the permeability data was made.
Diabetes-induced retinal histopathology
After 8 months of diabetes, 1 retina from each mouse was isolated for assessment of capillary histopathology, as described previously (25–27). Briefly, formalin-fixed retina was digested with 40 U/ml elastase (Calbiochem, San Diego, CA, USA) for 2–3 hours. When totally freed of neural cells, the isolated retinal vasculature was laid out on a glass microscope slide, dried, and stained with hematoxylin and periodic acid-Schiff. Degenerated (acellular) capillaries (Supplemental Fig. S2) were quantified in a masked manner in 6–7 field areas corresponding to the midretina. To evaluate possible photoreceptor degeneration in the long-term studies, the other eye was sectioned, and the number of cells in the outer nuclear layer from 2 areas on either side of the optic nerve (∼300 µm from the optic nerve) was counted, and the 4 resulting values were averaged together to compute a single estimate for each animal.
Visual function
Spatial frequency threshold and contrast sensitivity were measured after 2 and 8 months of diabetes as previously described (16), except that at 2 months, only a single spatial frequency (0.064 c/d) was measured. The grader was masked with respect to the animals’ experimental group. Although nondiabetic mice could be differentiated from diabetic animals based on BW, investigators could not discern group identity because some diabetics were treated with agents, whereas others were not.
Statistical analyses
Data are expressed as means ± sd except for the permeability and contrast sensitivity studies, where data are expressed as means ± sem. All statistical analyses were performed with ANOVA, followed by Fisher's test, except for the full contrast sensitivity curve. The latter was analyzed by repeated-measures ANOVA to account for the testing of each animal at multiple spatial frequencies. Values of P < 0.05 were considered statistically significant.
RESULTS
In vitro studies
In vitro studies were done to evaluate the contribution of Gs-, Gi-, and Gq-mediated GPCR signaling pathways to the increase in superoxide generation by 661W cells incubated in diabetes-like (30 mM) concentrations of glucose. The identities of agonists and antagonists of AR and 5-HT pathways used for these studies are summarized in Fig. 1 and Table 1. Selection of this cell line for the in vitro studies was solely because it is a well-studied cell line derived from retinal cells; results from these studies do not specifically implicate cones in the pathology of diabetic retinopathy.
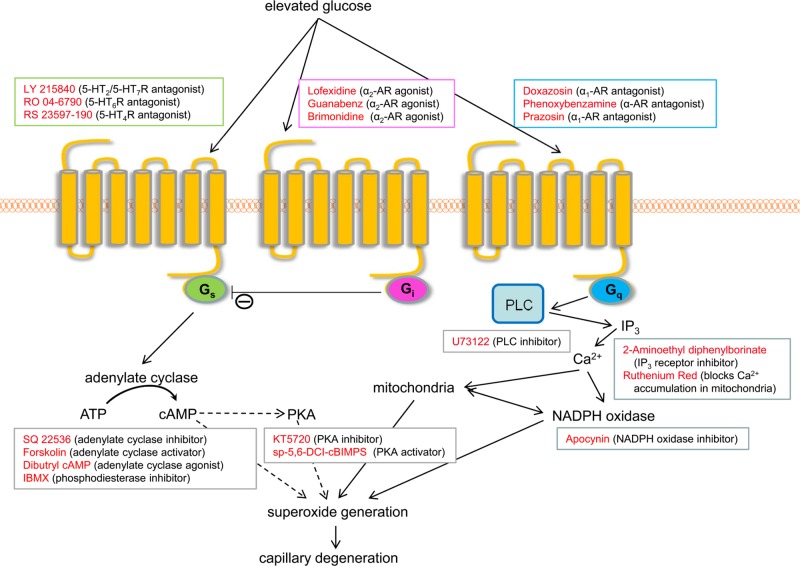
Figure 1.
Postulated relationships of major GPCR signaling pathways (Gs, Gi, and Gq) to superoxide generation and drugs used in vitro to test these relationships.
Gq-mediated signaling is known to activate NADPH oxidase (5), making this signaling pathway of special interest as a potential contributor to the generation of superoxide during elevated glucose concentrations and diabetes. Pharmacologic inhibition of the α1-AR with either Dox, PBA, or PRA (Fig. 2) significantly inhibited glucose-induced generation of superoxide by 661W cells. Because the α1-AR is known to regulate the activity of NADPH oxidase via activation of PLC, generation of inositol triphosphate (IP3), and calcium release from the endoplasmic reticulum (ER), we pharmacologically inhibited each of these steps in vitro (Fig. 2).
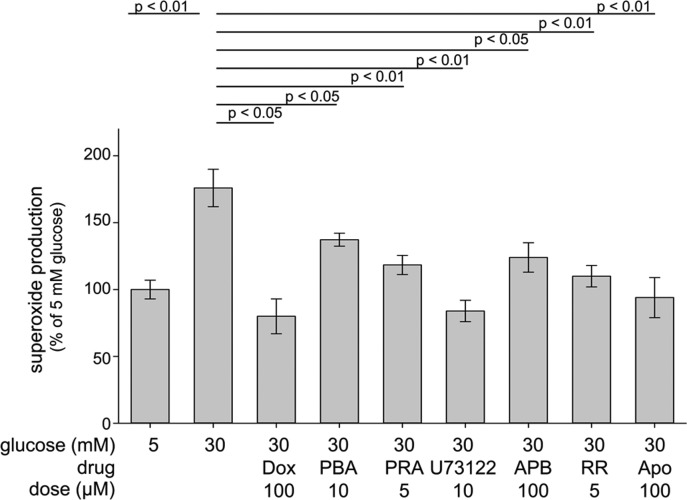
Figure 2.
Pharmacological inhibition of the α1-AR and Gq pathway (including downstream PLC, IP3, Ca2+, and NADPH oxidase) decreased superoxide formation in 661W cells. Cells were incubated for 4 days in 30 mM glucose in the presence of therapies at the concentrations listed. Then cells were harvested, concentrated by centrifugation, and assayed for superoxide by the lucigenin method. Dox, PBA (phenoxybenzamine), and PRA (prazosin) are α1-AR antagonists. Other drugs tested were U73122 (a PLC inhibitor); APB, (2-APB or 2-aminoethoxydiphenyl borate, an inhibitor of IP3-induced Ca2+ release); RR (ruthenium red, a Ca2+ release inhibitor); and Apo (apocynin, a NADPH oxidase inhibitor). n ≥ 3 for all groups.
Pharmacologic inhibition of PLC by U73122, IP3 receptors with 2-APB, calcium release from the ER by ruthenium red, or NADPH oxidase with Apo also significantly inhibited the glucose-induced increase in superoxide generation by these cells. α1-AR signaling has not been found to affect cAMP, and consistent with this observation, Dox did not inhibit the glucose-induced increase in cAMP (Fig. 3).
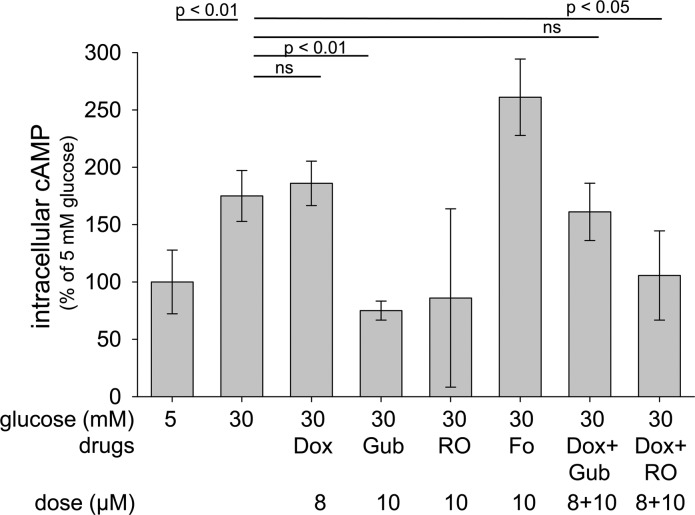
Figure 3.
Effects of inhibitors of α1-ARs or serotonin receptors (5-HTRs), or activators of α2-ARs on cAMP levels in 661W cells. Studies were performed as described in the legend for Fig. 2. In some cases, drugs were combined for the full 4 days of study. Fo (forskolin) activates adenylyl cyclase and thus increases intracellular levels of cAMP. RO (RO 04-6790) is a 5-HT6R antagonist. n = 3 for all groups, except n = 2 for RO and Fo groups.
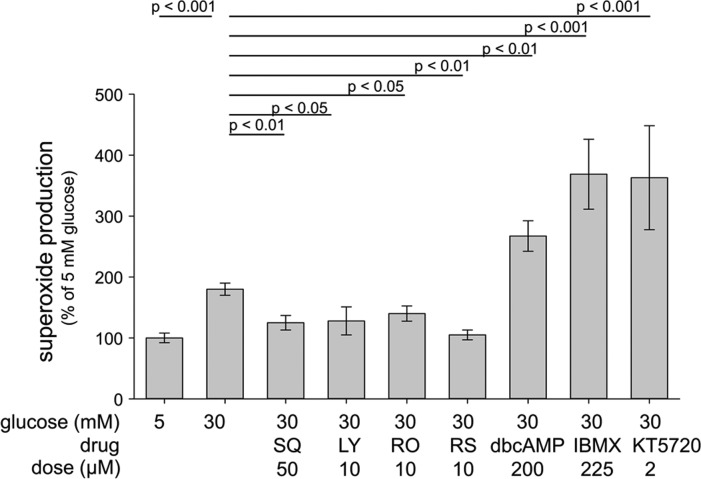
Activation of the Gs pathway leads to accumulation of cAMP. In addition to β-ARs, several 5-HTRs, including 5-HT4R and 5-HT7R, are known to signal via the Gs pathway. The cAMP mimic, dibutyryl cAMP, and the phosphodiesterase inhibitor, IBMX, both increased the generation of superoxide in vitro. Inhibition of signaling with the 5-HT2/5-HT7R antagonist, LY 215840, or antagonists of the 5-HT6R (RO 04-6790) or 5-HT4R (RS 23597-190) significantly inhibited superoxide production by 661W cells in high glucose, as did the adenylate cyclase inhibitor, SQ 22536. The most effective doses tested in vitro are summarized in Fig. 4. These results demonstrate that elevating cAMP levels increased the production of superoxide in hyperglycemia in a retinal cell culture system. Because the effects of cAMP accumulation are often mediated via cAMP-dependent protein kinase (PKA), we tested the effect of PKA inhibition by KT5720 on superoxide generation. Here we found that PKA inhibition did not decrease superoxide production, but instead significantly increased it (Fig. 4), suggesting that superoxide generation by retinal cells in high glucose does not require PKA signaling.
Figure 4.
Pharmacologic inhibition of Gs-coupled 5-HTRs lowered superoxide formation in 661W cells incubated for 4 days in 30 mM glucose, whereas a cAMP analog (db cAMP, dibutyryl cAMP), a phosphodiesterase inhibitor (IBMX), or a PKA inhibitor (KT5720) increased it. SQ, SQ 22536, an adenylate cyclase inhibitor; LY, LY 215840, a 5-HT2R/5-HT7R antagonist; RO, RO 04-6790, a 5-HT6R antagonist; RS, RS 23597-190, a 5-HT4R antagonist. Studies were performed as described in the legend for Fig. 2. n ≥ 3 for all groups.
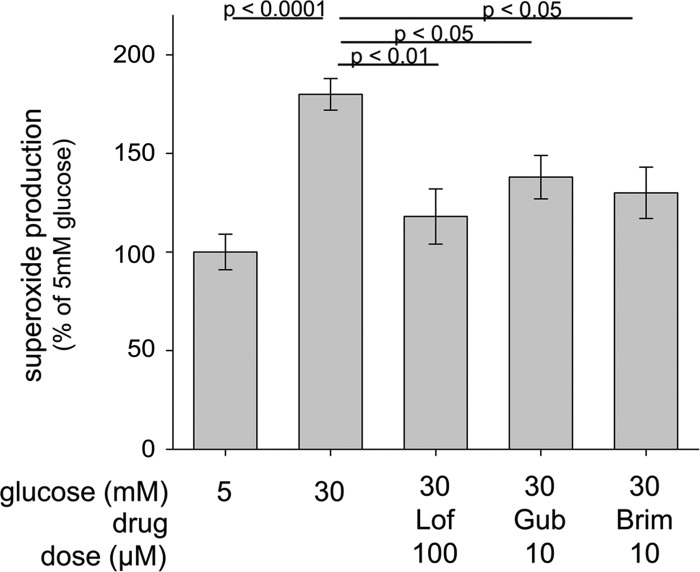
Activation of Gi-mediated signaling is known to inhibit adenylate cyclase. Consistent with this observation, activation of α2-AR signaling by Gub significantly inhibited cAMP generation in high glucose (Fig. 3). Thus, we tested whether stimulation of α2-AR signaling would also inhibit superoxide generation in high glucose. As shown in Fig. 5, several agonists of the α2-AR (Lof, Gub, and Brim) did indeed significantly inhibit superoxide generation by 661W cells in 30 mM glucose.
Figure 5.
Pharmacologic activation of α2-ARs (Gi pathway) inhibited the glucose-induced increase in superoxide generation by 661W cells. Studies were performed as described in the legend for Fig. 2. Lof, lofexidine; Gub, guanabenz; Brim, brimonidine. n ≥ 3 for all groups.
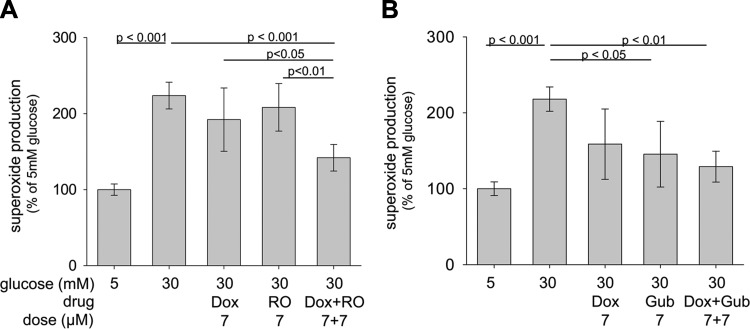
To further investigate the impact of G-coupled signaling pathways on superoxide generation by retinal cells in high glucose, we tested whether suboptimal doses of therapeutics that affected different G protein signaling pathways would have additive effects on superoxide inhibition (Fig. 6). Simultaneous inhibition of 5-HTRs (that signal through the Gs pathway) and α1-AR (Gq pathway) with suboptimal doses of Dox and RO 04-6790 caused significantly greater inhibition of superoxide generation than either drug alone (Fig. 6). Simultaneous activation of the Gi- and inhibition of Gq-mediated signaling pathways with suboptimal doses of Dox and Gub showed less than an additive inhibitory effect.
Figure 6.
Combinations of suboptimal doses of compounds that act on different G protein signaling pathways show additive effects on superoxide inhibition in 661W cells. Simultaneous inhibition of the Gq and Gs pathways with Dox and RO (RO-04-6790) or inhibition of the Gi and activation of the Gq pathways with Dox and Gub resulted in greater suppression of superoxide generation than single therapies. Studies were performed as described in the legend for Fig. 2. n = 4–5 for all groups.
Retinal explants
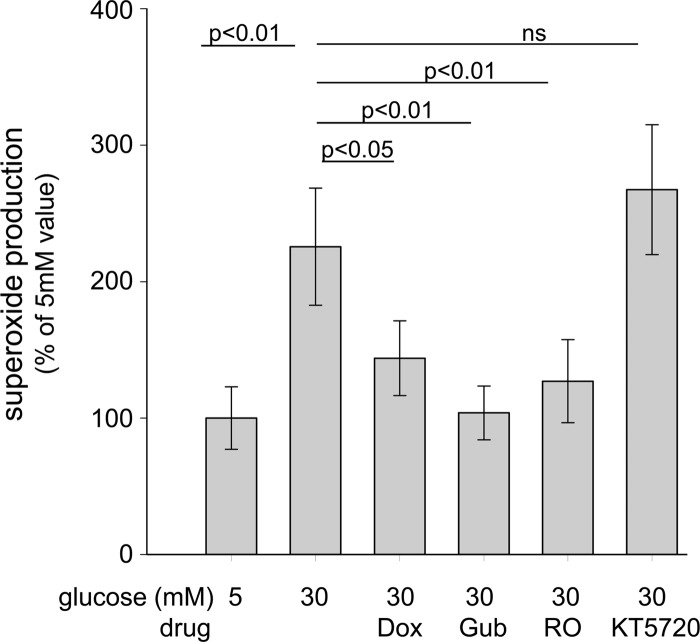
Incubation of retinal explants in 30 mM glucose for 4 days resulted in a significant increase in superoxide generation compared with their incubation in 5 mM glucose (Fig. 7). This increase was significantly inhibited by either Dox, Gub, or RO and was marginally increased by the PKA inhibitor, KT5720. These results were very consistent those obtained with 661W cells, again indicating that the cAMP-driven increase in superoxide was not mediated via PKA.
Figure 7.
Effect of therapies on retinal explants ex vivo. Pharmacologic inhibition of the α1-AR and Gq pathways in retinal explants decreased superoxide generation by retinas from nondiabetic mice incubated 4 days in 30 mM glucose, whereas inhibition of cAMP-regulated protein kinase (PKA) failed to reduce superoxide production. Dox, doxazosin; Gub, guanabenz; RO, RO 04-6790, a 5-HT6R antagonist; KT5720, a PKA inhibitor. n = 4–5 for all groups.
In vivo studies
Based on our encouraging in vitro screening results, we proceeded to in vivo studies focusing initially on the α1- and α2-ARs known to be prevalent in the retina (3). Dox was used to inhibit the α1-ARs, and Lof was used as an agonist for the α2-ARs. Both compounds were administered daily for 2 months, starting promptly after the initiation of diabetes. Lof (initial daily dose of 2 mg/kg BW) was toxic to a number of diabetic animals, so was not studied further in vivo.
Diabetic mice from all experimental groups in the long-term experiment had levels of HbA1c and blood glucose that were significantly greater (P < 0.05) than levels found in age-matched nondiabetic controls. Average BWs and nonfasting glucose and HbA1c levels for the animal groups in the 8-month experiment are summarized in Table 2. Clinical data for diabetic groups studied for 2 months were similar. All mice appeared healthy and none had lost BW, although diabetic mice did not gain weight at a normal rate.
TABLE 2.
Therapeutics not affecting metabolic control over an 8 month period of diabetes in mice
| Condition and treatment | n | Final BW (g) | Blood glucose (nonfasting; mg/dl) | HbA1c (%) |
|---|---|---|---|---|
| Non-diabetic control | 8 | 46 ± 3 | 153 ± 25 | 3.3 ± 0.2 |
| Diabetic (8 mo) | 8 | 29 ± 2 | 519 ± 34 | 11.1 ± 0.6 |
| Diabetic (8 mo) Dox | 8 | 28 ± 2 | 483 ± 69 | 10.8 ± 1.2 |
| Diabetic (8 mo) Apo | 8 | 26 ± 3 | 487 ± 68 | 11.1 ± 0.5 |
Except for final BW, clinical parameters were measured over the 8 month duration of diabetes as described in the Materials and Methods section.
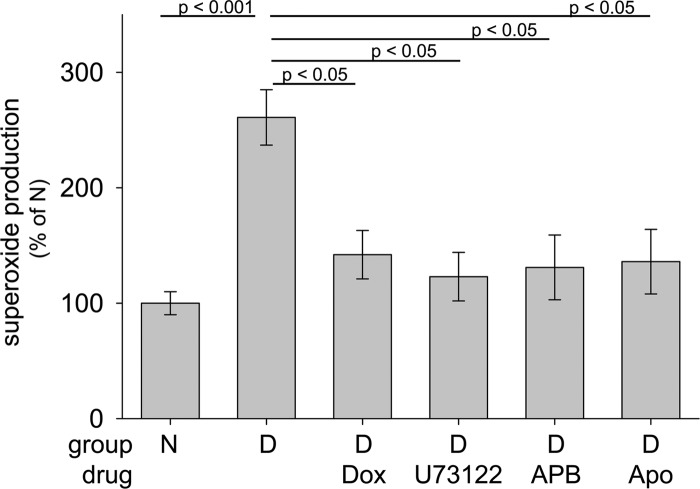
In the 2-month studies, administration of Dox significantly suppressed the diabetes-induced increase in retinal superoxide generation (Fig. 8). Similar to our in vitro studies, the signaling mechanism by which the α1-AR initiated retinal superoxide generation in diabetes also was studied in vivo. As shown in Fig. 8, inhibition of α1-ARs, PLC, IP3 receptors, or NADPH oxidase each significantly reduced superoxide generation by retinas from diabetic mice.
Figure 8.
Two months of diabetes in mice (D) increased retinal superoxide production compared with nondiabetic mice (N) through a GPCR/PLC/IP3/Ca2+/NADPH oxidase signaling cascade, and inhibition of any of these downstream steps reduced the excess superoxide generation by isolated retina. Dox (10 mg/kg BW), U73122 (6.25 mg/kg BW), APB (2-APB; 6.25 mg/kg BW), and Apo (36 mg/kg BW) were injected i.p. in DMSO daily for the 2 months of diabetes. n = 3–5 per group.
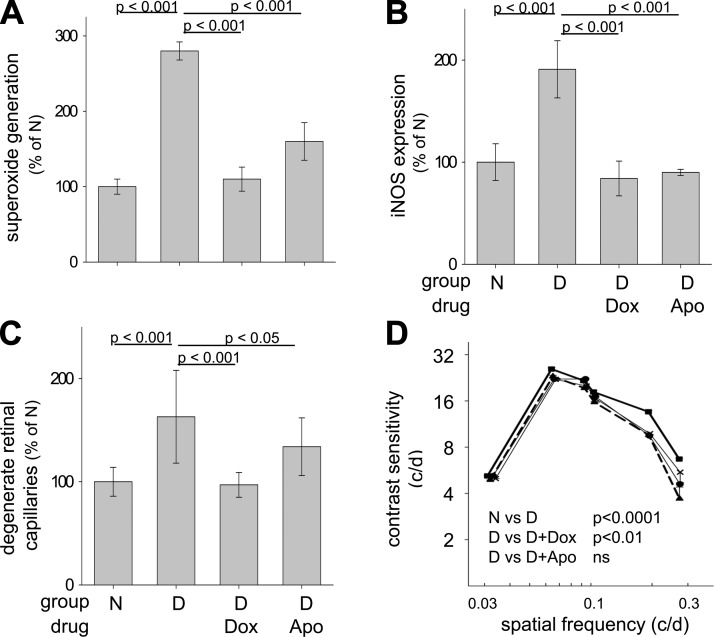
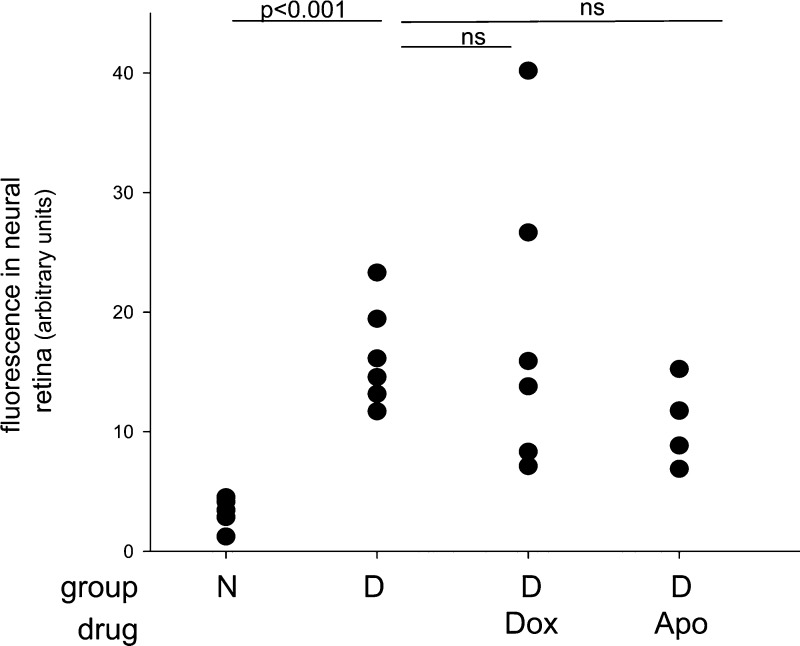
To determine whether Gq-mediated signaling pathways are involved in the long-term vascular pathology of diabetic retinopathy, we administered Dox or Apo to diabetic mice daily for 8 months. As expected, diabetes caused a significant increase in the number of degenerated retinal capillaries, leakage of FITC-BSA in the neural retina, and a significant impairment of visual function compared with age-matched nondiabetic controls (Figs. 9 and 10). The number of degenerated capillaries was significantly reduced in diabetic mice treated with either Dox or Apo, as was the retinal generation of superoxide and expression of proinflammatory proteins (Fig. 9A, B). These studies clearly implicate the α1-AR signaling pathway in the oxidative stress affecting mouse retina in diabetes and show that Dox and Apo are valid pharmacologic agents capable of suppressing diabetic vascular degeneration. In contrast to the beneficial effect of both Dox and Apo on diabetes-induced degeneration of retinal capillaries recorded at 8 months, neither therapy had a significant beneficial effect on the accumulation of FITC-albumin into the neural retina (a parameter of vascular leakage) (Fig. 10). Dox had a statistically significant effect on contrast sensitivity, but neither Dox nor Apo showed a beneficial effect on the spatial frequency threshold or maintained contrast sensitivity at normal levels (Fig. 9; Table 3). Neither diabetes of 8 months duration nor any tested therapeutic produced a loss of photoreceptor cells (Table 4), and diabetic controls actually had more photoreceptors than did age-matched normal mice.
Figure 9.
Daily administration of inhibitors of α1-ARs (Dox) and NADPH oxidase (Apo) for 8 months to mice significantly reduced diabetes-induced increases in retinal superoxide (A), expression of iNOS (B), and capillary degeneration (C). Dox (but not Apo) significantly inhibited the diabetes-induced defect in contrast sensitivity at 8 months of diabetes, but the functional effect was modest (D). Nondiabetic, solid squares, solid line; diabetic, solid triangles, dashed line; diabetic+doxazosin, solid circles, thin solid line; diabetic+apocynin, X, thin solid line. Contrast sensitivity was determined at the same spatial frequencies in all groups, but group means are offset slightly to allow easier visualization of the data. n = 5 in each group. Mean ± sem.
Figure 10.
Diabetes of 8 months duration significantly increased accumulation of FITC-BSA in the inner plexiform layer of mouse retina. FITC-albumin was injected intravenously and allowed to circulate for 20 minutes, and then fluorescence in areas of the inner plexiform layer was quantitated from retinal cross sections. Neither compound achieved a statistically significant difference from the control at 8 months of diabetes. n = 4–6.
TABLE 3.
Effect of Dox or Apo on diabetes-induced defects in the visual function of mice after 2 or 8 months duration of diabetes
| Category | Duration (mo) | n | Spatial frequency threshold (c/d) | Contrast sensitivity |
|---|---|---|---|---|
| Nondiabetic control | 2 | 4 | 0.399 ± 0.009 | 26.9 ± 0.9a |
| Diabetic control | 2 | 4 | 0.379 ± 0.003b | 21.8 ± 1.3a,b |
| Diabetic + Dox | 2 | 4 | 0.375 ± 0.008b | 23.1 ± 0.8a,b |
| Diabetic + Apo | 2 | 4 | 0.374 ± 0.006b | 23.3 ± 1.0a,b |
| Nondiabetic | 8 | 5 | 0.396 ± 0.006 | Fig. 9D |
| Diabetic control | 8 | 5 | 0.358 ± 0.006b | Fig. 9D |
| Diabetic + Dox | 8 | 5 | 0.349 ± 0.006b | Fig. 9D |
| Diabetic + Apo | 8 | 5 | 0.351 ± 0.004b | Fig. 9D |
Assays were performed in vivo as described in the Materials and Methods section. All animals were 2 months of age at initiation of diabetes.
At 0.064 c/d.
P < 0.01 compared with nondiabetic control.
TABLE 4.
Diabetes of 8 months duration did not cause loss of retinal photoreceptor cells in C57Bl/6J mice
| Category | Duration (mo) | n | Number of cell layers in outer nuclear layer |
|---|---|---|---|
| Nondiabetic control | 8 | 7 | 11.2 ± 0.7 |
| Diabetic control | 8 | 6 | 12.6 ± 0.8a |
| Diabetic + Dox | 8 | 7 | 11.6 ± 0.3 |
| Diabetic + Apo | 8 | 7 | 11.9 ± 0.4 |
P < 0.05 compared with nondiabetic control.
DISCUSSION
Reactive oxygen species are important in the pathogenesis of the vascular histopathology known as diabetic retinopathy (6–8). The diabetes-induced increase in retinal generation of superoxide has been attributed to several intracellular molecular sources, including mitochondria (7–9), NADPH oxidase (9, 28), and pathways regulated by iNOS (29), arginase (30), and aldose reductase (31).
In the present study, we investigated the possibility that some extracellular GPCRs contribute to superoxide generation by the retina in diabetes. When binding to appropriate ligands, GPCRs transduce extracellular stimuli into intracellular second messengers through activation of one or several G proteins, including the Gs, Gi, and Gq subtypes. Previous studies have demonstrated that the Gs subtype activates adenylate cyclase, thus increasing intracellular cAMP, a secondary messenger that signals through activation of PKA or exchange proteins activated by cAMP (32). In contrast, activation of the Gi subtype inhibits adenylate cyclase and suppresses signaling of the cAMP/PKA pathway. The Gq subtype activates PLC, which increases the second messengers IP3 and diacylglycerol. Water-soluble IP3 diffuses through the cytoplasm into the ER, where it binds to and opens calcium channels, releasing calcium stores into the cytoplasm (5, 33–35). Ionized calcium affects many cellular processes, including activation of NADPH oxidase, an enzyme capable of generating large amounts of superoxide. Having used pharmacologic approaches to study these pathways in the retina, we acknowledge that some drugs studied here could have pleiotropic effects. Although our previous studies indicate that the diabetes-induced increase in retinal superoxide arises predominantly from retinal neuronal cells (photoreceptor cells), adrenergic and serotonin receptors occur on many cell types, so the contribution of various other retinal cell types to this superoxide generation in diabetes requires further study.
Previous studies have shown that the Gq-regulated pathway plays an important role in the pathogenesis of light-induced photoreceptor cell degeneration (3, 5). Diabetes differs from retinal degenerations in that photoreceptor loss is not a reproducible finding, and this degeneration was absent in our study. Nevertheless, Dox, a selective α1-AR blocker, was effective in suppressing the diabetes-induced increase in superoxide generation, expression of inflammatory proteins, and degeneration of retinal capillaries. This work demonstrates that the α1-AR/PLC/IP3/NADPH oxidase pathway plays a critical role in diabetes-induced generation of superoxide and the degeneration of retinal capillaries. Importantly, Dox is already U.S. Food and Drug Administration-approved for treating patients with benign prostatic hyperplasia and associated hypertension or urinary retention defects. Whether or not our tested therapies also affected antioxidant enzymes is presently unknown.
Altered vascular permeability also is characteristic of diabetic retinopathy. In our long-term studies, neither Dox nor Apo significantly inhibited FITC-albumin accumulation in the neural retina with the sample sizes tested [although the effect of Apo was almost significant (P = 0.065)]. The contribution of NADPH oxidase to the retinal permeability defect after prolonged diabetes may be less than after diabetes of shorter durations; shorter-term studies by others have reported that inhibition of NADPH oxidase in diabetes protected against increased vascular permeability (28, 36). Moreover, catalytic subunits of NADPH oxidase have been implicated because of vascular injury in ischemic retinopathy and retinal neovascularization, as well as in nonocular complications of diabetes (37–40). Differences in the duration of diabetes between our 8 month study and shorter (weeks-long) studies could have resulted in additional structural or functional changes that adversely affect vascular permeability with increasing duration of diabetes. Nevertheless, the present results suggest that conclusion of short-term studies might not predict outcome of long-term trials with respect to vascular permeability and suggest that the pathogenesis of diabetes-induced degeneration of retinal capillaries could differ in some respects from the defects causing long-term changes in vascular permeability. It is premature to conclude that the failure of the drugs tested herein to inhibit the permeability defect limits their potential value in patients, because therapeutic effects on the clinical end point that matters most (macular edema/thickening) cannot be tested in rodents, and it remains unclear whether or not retinal thickening is caused solely by a permeability defect.
Our studies show that Dox or Apo had only a slight or no beneficial effect on diabetes-induced defects in visual function. The finding that therapies that inhibit diabetes-induced degeneration of retinal capillaries have little effect on visual function was previously observed (16). This observation is consistent with the possibility that vascular and neural cells have different susceptibilities to these therapies or that the pathogenesis of the capillary degeneration differs in some ways from that of the visual function defect in diabetic mice. Nevertheless, it seems premature to conclude that this means that neither therapy warrants further testing in diabetic patients. Indeed, retinal edema, a major cause of visual impairment in diabetic patients, does not even develop in most rodent models of diabetic retinopathy, so the effects of these drugs on defects in visual function and retinal edema in patients remains to be determined.
The location of α1-ARs in the retina could provide clues as to which cell types are involved in the effects observed. α1-ARs reportedly are most abundant in retinal photoreceptor cells (41). Others reported that the distribution of α1-AR binding sites was concentrated in the outer and inner plexiform layers (42). In the rat retina, all 3 receptor subtypes (2A, B, C) of the α2-AR are present, but are localized to different cell types. The α2A-AR, α2B-AR, and α2C-AR are present in cells of the ganglion cell layer and inner nuclear layer, the neurons and glia, and the photoreceptors, respectively (43). We recently reported that retinal photoreceptors generate or regulate most of the diabetes-induced increase in retinal generation of superoxide (9).
β-ARs signal via the Gs pathway, leading to the accumulation of cAMP. Dibutyryl-cAMP increased superoxide even in the absence of high glucose, indicating that elevating cAMP levels increases the production of superoxide and other reactive oxygen species, as also noted in other conditions (44). Thus, based on our previous studies which demonstrated a strong association between the generation of superoxide and development of diabetic retinopathy, we postulated that inhibition of β-AR signaling should inhibit this retinopathy. Steinle et al., however, demonstrated that a β-AR agonist (isoproterenol) reduced the formation of degenerated capillaries in diabetes, inhibited apoptosis of cells in the ganglion cell layer, and decreased TNF-α and inflammatory mediators (45–48). Additionally, β2-AR knockout mice or mice treated the AR antagonist, propranolol, developed features similar to those of diabetic retinopathy even in the absence of diabetes (45, 49). Thus, these studies seem to contradict our hypothesis about the role of cAMP (and superoxide) in the pathogenesis of diabetic retinopathy. It is worth noting that gene profiling revealed few β-ARs in the retina compared with α-ARs (3), raising the possibility that effects of β-AR inhibition on the retina could be mediated systemically, as opposed to locally. This idea would not have been tested by our in vitro studies. HTR agonists (which also signal through the Gs pathway) similarly have been shown by others to inhibit oxidative changes that lead to photoreceptor degenerative diseases (10–14).
Activation of α2-ARs, known to signal via a Gi-mediated pathway, inhibits adenylate cyclase activation, thus preventing cAMP accumulation. Activation of these receptors by blood pressure-lowering drugs like Gub and Lof or the neuroprotectant, Brim, strongly reduced superoxide generation caused by elevated glucose in vitro. Activation of this signaling pathway is pertinent to diabetes because treatment of diabetic rats with Brim significantly inhibited the diabetes-induced increase in vitreo-retinal VEGF expression and blood-retinal barrier breakdown (50).
Diabetes-induced changes in signaling through GPCRs could result from alterations in ligands presented to these receptors, but diabetes also is known to alter the expression of GPCRs, G proteins, and adenylate cyclase. Reduced expression of Gi proteins has been reported in platelets, aorta, and retina from diabetic patients or rats (51–53). Expression of Gqα and Gi proteins was altered in aortas or aortic smooth muscle from diabetic rodents (54–56), the change apparently caused by reactive oxygen species. Sciatic nerves of diabetic rats evidenced subnormal adenylate cyclase activity and increased expression of Gq, Gs, and Gi proteins (57). Two months of diabetes or experimental galactosemia activated the Harvey rat sarcoma viral oncogene homolog (H-Ras), a small-molecular-weight G protein in the retina and retinal microvessels of diabetic rats (58).
The present results are the first to show overactive Gq signaling playing a causal role in the development of diabetic retinopathy. Our results with 661W cells indicated increased cAMP levels in elevated glucose, but inhibition of the α1-AR by Dox did not alter cAMP in elevated glucose, and therefore presumably Dox did not reduce the retinopathy by affecting cAMP-regulated pathways. Retinal levels of cAMP were reported to be subnormal in diabetic rats (59), but no data were presented to show whether those animals were also catabolic (an extreme manifestation of diabetes with little relevance to most diabetic patients). The in vivo contribution of retinal cAMP changes to the observed effects of drugs in diabetic retinopathy remains to be determined.
Experimental results described here identified a series of intrinsically linked events in which 3 signaling pathways mediated by GPCRs (Gs, Gi, and Gq) play important roles in the hyperglycemia-induced generation of superoxide by retinal cells. Whether these findings are unique to the cells we studied or also pertain to other retinal cells and receptors that use these signaling pathways remains to be learned. Links between different GPCR pathways with respect to superoxide generation could stem from hyperglycemia-induced increases in cytosolic Ca2+ concentration, which are known to induce superoxide generation in other conditions. The therapeutic importance of demonstrating that GPCRs regulate retinal oxidative stress in diabetes is suggested by our evidence that the diabetes-induced degeneration of retinal capillaries could be inhibited by blocking either of 2 steps in the Gq signaling pathway that lead to superoxide generation through NADPH oxidase. We acknowledge that NADPH oxidase is unlikely to be the only contributor to such oxidative stress and that the pathogenesis of different lesions (such as capillary degeneration or increased permeability) might differ, especially with longer durations of diabetes.
These observations raise the possibility that important lesions of diabetic retinopathy, and perhaps other complications of diabetes, could be inhibited by therapies selectively targeting a subset of GPCRs or their signaling pathways. Moreover, because all 3 GPCR signaling pathways regulate superoxide generation by retinal cells, combinations of therapies at safe low doses that target several GPCR signaling pathways could even inhibit diabetic retinopathy without undesirable side effects. Many therapeutics targeting GPCRs are already U.S. Food and Drug Administration-approved, so this approach can be readily tested in patients. Identifying specific drugs to advance for patient studies will require further studies. Undesirable side effects have been reported with at least some related drugs. For example, AZD3783, a potent inhibitor of the 5-HT1B receptor, was neurotoxic in dogs (60). Dox was found to be highly effective in our studies, but it also can evidence negative side effects (61), and whether a more selective α blocker would be safer but just as effective remains to be determined.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health, National Eye Institute (R01EY00300, R01EY022938, and R24EY021126), the Medical Research Service of the Department of Veteran Affairs, and the Office of Research and Technology Management at Case Western Reserve University. Services of the Case Western Reserve University Visual Science Research Center Core Facilities are acknowledged (P30EY11373). K.P. is John H. Hord Professor of Pharmacology. The authors declare no conflicts of interest.
Glossary
- 2-APB
2-aminoethoxydiphenyl borate
- APO
apocynin
- AR
adrenergic receptor
- Brim
brimonidine
- BSA
bovine serum albumin
- BW
body weight
- Dox
doxazosin (an α1-adrenergic receptor antagonist)
- ER
endoplasmic reticulum
- Gub
guanabenz
- HbA1c
hemoglobin A1c
- HTR
5-hydroxytryptamine (serotonin) receptor
- IBMX
1-methyl-3-isobutylxanthine
- IP3
inositol triphosphate
- Lof
Lofexidine
- ONL
outer nuclear layer
- PKC
cAMP-dependent protein kinase
- PLC
phospholipase C
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Fredriksson R., Lagerström M. C., Lundin L. G., Schiöth H. B. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 [DOI] [PubMed] [Google Scholar]
- 2.Marinissen M. J., Gutkind J. S. (2001) G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 22, 368–376 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Palczewska G., Mustafi D., Golczak M., Dong Z., Sawada O., Maeda T., Maeda A., Palczewski K. (2013) Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J. Clin. Invest. 123, 5119–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usui S., Oveson B. C., Lee S. Y., Jo Y. J., Yoshida T., Miki A., Miki K., Iwase T., Lu L., Campochiaro P. A. (2009) NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 110, 1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Okano K., Maeda T., Chauhan V., Golczak M., Maeda A., Palczewski K. (2012) Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 287, 5059–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowluru R. A., Tang J., Kern T. S. (2001) Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50, 1938–1942 [DOI] [PubMed] [Google Scholar]
- 7.Kanwar M., Chan P. S., Kern T. S., Kowluru R. A. (2007) Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest. Ophthalmol. Vis. Sci. 48, 3805–3811 [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz B. A., Gradianu M., Bissig D., Kern T. S., Roberts R. (2009) Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest. Ophthalmol. Vis. Sci. 50, 2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y., Veenstra A., Palczewski K., Kern T. S. (2013) Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 110, 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier R. J., Wang Y., Smith S. S., Martin E., Ornberg R., Rhoades K., Romano C. (2011) Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5-HT1A agonist. Invest. Ophthalmol. Vis. Sci. 52, 8108–8116 [DOI] [PubMed] [Google Scholar]
- 11.Collier R. J., Patel Y., Martin E. A., Dembinska O., Hellberg M., Krueger D. S., Kapin M. A., Romano C. (2011) Agonists at the serotonin receptor (5-HT(1A)) protect the retina from severe photo-oxidative stress. Invest. Ophthalmol. Vis. Sci. 52, 2118–2126 [DOI] [PubMed] [Google Scholar]
- 12.Shen J., Ghai K., Sompol P., Liu X., Cao X., Iuvone P. M., Ye K. (2012) N-acetyl serotonin derivatives as potent neuroprotectants for retinas. Proc. Natl. Acad. Sci. USA 109, 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thampi P., Rao H. V., Mitter S. K., Cai J., Mao H., Li H., Seo S., Qi X., Lewin A. S., Romano C., Boulton M. E. (2012) The 5HT1a receptor agonist 8-Oh DPAT induces protection from lipofuscin accumulation and oxidative stress in the retinal pigment epithelium. PLoS ONE 7, e34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renganathan K., Gu J., Rayborn M. E., Crabb J. S., Salomon R. G., Collier R. J., Kapin M. A., Romano C., Hollyfield J. G., Crabb J. W. (2013) CEP biomarkers as potential tools for monitoring therapeutics. PLoS ONE 8, e76325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.al-Ubaidi M. R., Font R. L., Quiambao A. B., Keener M. J., Liou G. I., Overbeek P. A., Baehr W. (1992) Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 119, 1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C. A., Li G., Patel M. D., Petrash J. M., Benetz B. A., Veenstra A., Amengual J., Von Lintig J., Burant C., Tang J., Kern T. S. (2013) Diabetes-induced impairment in visual function in mice: contributions of p38 MAPK, RAGE, leukocytes, and aldose reductase. Invest. Ophthalmol. Vis. Sci. 93, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang J., Allen Lee C., Du Y., Sun Y., Pearlman E., Sheibani N., Kern T. S. (2013) MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS ONE 8, e68871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Y., Tang J., Li G., Berti-Mattera L., Lee C. A., Bartkowski D., Gale D., Monahan J., Niesman M. R., Alton G., Kern T. S. (2010) Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest. Ophthalmol. Vis. Sci. 51, 2158–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kern T. S., Du Y., Miller C. M., Hatala D. A., Levin L. A. (2010) Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am. J. Pathol. 176, 2550–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubitosi-Klug R. A., Talahalli R., Du Y., Nadler J. L., Kern T. S. (2008) 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 57, 1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern T. S., Miller C. M., Du Y., Zheng L., Mohr S., Ball S. L., Kim M., Jamison J. A., Bingaman D. P. (2007) Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes 56, 373–379 [DOI] [PubMed] [Google Scholar]
- 22.Du Y., Miller C. M., Kern T. S. (2003) Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med. 35, 1491–1499 [DOI] [PubMed] [Google Scholar]
- 23.Best T. M., Fiebig R., Corr D. T., Brickson S., Ji L. (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J. Appl. Physiol. 87, 74–82 [DOI] [PubMed] [Google Scholar]
- 24.Antonetti D. A., Barber A. J., Khin S., Lieth E., Tarbell J. M., Gardner T. W.; Penn State Retina Research Group (1998) Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes 47, 1953–1959 [DOI] [PubMed] [Google Scholar]
- 25.Li G., Tang J., Du Y., Lee C. A., Kern T. S. (2011) Beneficial effects of RAGE-Ig fusion protein on early diabetic retinopathy and tactile allodynia. Mol. Vis. 17, 3156–3165 [PMC free article] [PubMed] [Google Scholar]
- 26.Li G., Veenstra A. A., Talahalli R. R., Wang X., Gubitosi-Klug R. A., Sheibani N., Kern T. S. (2012) Marrow-derived cells regulate the development of early diabetic retinopathy and tactile allodynia in mice. Diabetes 61, 3294–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veenstra A. A., Tang J., Kern T. S. (2013) Antagonism of CD11b with neutrophil inhibitory factor (NIF) inhibits vascular lesions in diabetic retinopathy. PLoS ONE 8, e78405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Shabrawey M., Rojas M., Sanders T., Behzadian A., El-Remessy A., Bartoli M., Parpia A. K., Liou G., Caldwell R. B. (2008) Role of NADPH oxidase in retinal vascular inflammation. Invest. Ophthalmol. Vis. Sci. 49, 3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng L., Du Y., Miller C., Gubitosi-Klug R. A., Ball S., Berkowitz B. A., Kern T. S. (2007) Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia 50, 1987–1996 [DOI] [PubMed] [Google Scholar]
- 30.Elms S. C., Toque H. A., Rojas M., Xu Z., Caldwell R. W., Caldwell R. B. (2013) The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia 56, 654–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang J., Du Y., Petrash J. M., Sheibani N., Kern T. S. (2013) Deletion of aldose reductase from mice inhibits diabetes-induced retinal capillary degeneration and superoxide generation. PLoS ONE 8, e62081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billington C. K., Hall I. P. (2012) Novel cAMP signalling paradigms: therapeutic implications for airway disease. Br. J. Pharmacol. 166, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue T., Suzuki Y., Yoshimaru T., Ra C. (2008) Reactive oxygen species produced up- or downstream of calcium influx regulate proinflammatory mediator release from mast cells: role of NADPH oxidase and mitochondria. Biochim. Biophys. Acta 1783, 789–802 [DOI] [PubMed] [Google Scholar]
- 34.Brown G. C. (2007) Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 35, 1119–1121 [DOI] [PubMed] [Google Scholar]
- 35.Yamamori T., Inanami O., Nagahata H., Cui Y., Kuwabara M. (2000) Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 467, 253–258 [DOI] [PubMed] [Google Scholar]
- 36.Al-Shabrawey M., Bartoli M., El-Remessy A. B., Ma G., Matragoon S., Lemtalsi T., Caldwell R. W., Caldwell R. B. (2008) Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 49, 3231–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson-Berka J. L., Deliyanti D., Rana I., Miller A. G., Agrotis A., Armani R., Szyndralewiez C., Wingler K., Touyz R. M., Cooper M. E., Jandeleit-Dahm K. A., Schmidt H. H. (2014) NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid. Redox Signal. 20, 2726–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray S. P., Di Marco E., Okabe J., Szyndralewiez C., Heitz F., Montezano A. C., de Haan J. B., Koulis C., El-Osta A., Andrews K. L., Chin-Dusting J. P., Touyz R. M., Wingler K., Cooper M. E., Schmidt H. H., Jandeleit-Dahm K. A. (2013) NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127, 1888–1902 [DOI] [PubMed] [Google Scholar]
- 39.Sedeek M., Nasrallah R., Touyz R. M., Hébert R. L. (2013) NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 24, 1512–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan E. C., van Wijngaarden P., Liu G. S., Jiang F., Peshavariya H., Dusting G. J. (2013) Involvement of Nox2 NADPH oxidase in retinal neovascularization. Invest. Ophthalmol. Vis. Sci. 54, 7061–7067 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki F., Taniguchi T., Nakamura S., Akagi Y., Kubota C., Satoh M., Muramatsu I. (2002) Distribution of alpha-1 adrenoceptor subtypes in RNA and protein in rabbit eyes. Br. J. Pharmacol. 135, 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarbin M. A., Wamsley J. K., Palacios J. M., Kuhar M. J. (1986) Autoradiographic localization of high affinity GABA, benzodiazepine, dopaminergic, adrenergic and muscarinic cholinergic receptors in the rat, monkey and human retina. Brain Res. 374, 75–92 [DOI] [PubMed] [Google Scholar]
- 43.Woldemussie E., Wijono M., Pow D. (2007) Localization of alpha 2 receptors in ocular tissues. Vis. Neurosci. 24, 745–756 [DOI] [PubMed] [Google Scholar]
- 44.Prabu S. K., Anandatheerthavarada H. K., Raza H., Srinivasan S., Spear J. F., Avadhani N. G. (2006) Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J. Biol. Chem. 281, 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Y., Walker R. J., Kern T. S., Steinle J. J. (2010) Application of isoproterenol inhibits diabetic-like changes in the rat retina. Exp. Eye Res. 91, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinle J. J., Chin V. C., Williams K. P., Panjala S. R. (2008) Beta-adrenergic receptor stimulation modulates iNOS protein levels through p38 and ERK1/2 signaling in human retinal endothelial cells. Exp. Eye Res. 87, 30–34 [DOI] [PubMed] [Google Scholar]
- 47.Walker R. J., Steinle J. J. (2007) Role of beta-adrenergic receptors in inflammatory marker expression in Müller cells. Invest. Ophthalmol. Vis. Sci. 48, 5276–5281 [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y., Steinle J. J. (2010) Systemic propranolol reduces b-wave amplitude in the ERG and increases IGF-1 receptor phosphorylation in rat retina. Invest. Ophthalmol. Vis. Sci. 51, 2730–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y., Zhang Q., Liu L., Tang J., Kern T. S., Steinle J. J. (2013) β2-adrenergic receptor knockout mice exhibit A diabetic retinopathy phenotype. PLoS ONE 8, e70555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusari J., Zhou S. X., Padillo E., Clarke K. G., Gil D. W. (2010) Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest. Ophthalmol. Vis. Sci. 51, 1044–1051 [DOI] [PubMed] [Google Scholar]
- 51.Hadjiconstantinou M., Qu Z. X., Moroi-Fetters S. E., Neff N. H. (1988) Apparent loss of Gi protein activity in the diabetic retina. Eur. J. Pharmacol. 149, 193–194 [DOI] [PubMed] [Google Scholar]
- 52.Livingstone C., McLellan A. R., McGregor M. A., Wilson A., Connell J. M., Small M., Milligan G., Paterson K. R., Houslay M. D. (1991) Altered G-protein expression and adenylate cyclase activity in platelets of non-insulin-dependent diabetic (NIDDM) male subjects. Biochim. Biophys. Acta 1096, 127–133 [DOI] [PubMed] [Google Scholar]
- 53.Abbracchio M. P., Cattabeni F., Di Giulio A. M., Finco C., Paoletti A. M., Tenconi B., Gorio A. (1991) Early alterations of Gi/Go protein-dependent transductional processes in the retina of diabetic animals. J. Neurosci. Res. 29, 196–200 [DOI] [PubMed] [Google Scholar]
- 54.Descorbeth M., Anand-Srivastava M. B. (2010) Role of oxidative stress in high-glucose- and diabetes-induced increased expression of Gq/11α proteins and associated signaling in vascular smooth muscle cells. Free Radic. Biol. Med. 49, 1395–1405 [DOI] [PubMed] [Google Scholar]
- 55.Li Y., Lappas G., Anand-Srivastava M. B. (2007) Role of oxidative stress in angiotensin II-induced enhanced expression of Gi(alpha) proteins and adenylyl cyclase signaling in A10 vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 292, H1922–H1930 [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Descorbeth M., Anand-Srivastava M. B. (2008) Role of oxidative stress in high glucose-induced decreased expression of Gialpha proteins and adenylyl cyclase signaling in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 294, H2845–H2854 [DOI] [PubMed] [Google Scholar]
- 57.Goraya T. Y., Wilkins P., Douglas J. G., Zhou J., Berti-Mattera L. N. (1995) Signal transduction alterations in peripheral nerves from streptozotocin-induced diabetic rats. J. Neurosci. Res. 41, 518–525 [DOI] [PubMed] [Google Scholar]
- 58.Kanwar M., Kowluru R. A. (2008) Diabetes regulates small molecular weight G-protein, H-Ras, in the microvasculature of the retina: implication in the development of retinopathy. Microvasc. Res. 76, 189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do Carmo Buonfiglio D., Peliciari-Garcia R. A., do Amaral F. G., Peres R., Nogueira T. C., Afeche S. C., Cipolla-Neto J. (2011) Early-stage retinal melatonin synthesis impairment in streptozotocin-induced diabetic wistar rats. Invest. Ophthalmol. Vis. Sci. 52, 7416–7422 [DOI] [PubMed] [Google Scholar]
- 60.Chang J. C., Ciaccio P., Schroeder P., Wright L., Westwood R., Berg A. L. (2014) Pathology and neurotoxicity in dogs after repeat dose exposure to a serotonin 5-HT1B inhibitor. J. Toxicol. Pathol. 27, 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gavras I., Gavras H. (2001) Benefits and side effects of blood pressure lowering treatment: what was wrong with doxazosin in the ALLHAT? Curr. Control. Trials Cardiovasc. Med. 2, 257–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.