Abstract
Oxanorbornadiene dicarboxylate (OND) reagents are potent Michael acceptors, the adducts of which undergo fragmentation by retro-Diels–Alder reaction at rates that vary with the substitution pattern on the OND moiety. Rapid conjugate addition between thiol-terminated tetravalent PEG and multivalent ONDs yielded self-supporting hydrogels within 1 min at physiological temperature and pH. Erosion of representative hydrogel formulations occurred with predictable and pH-independent rates on the order of minutes to weeks. These materials could be made non-degradable by epoxidation of the OND linkers without slowing gelation. Hydrogels prepared with OND linkers of equal valence had comparable physical properties, as determined by equilibrium swelling behavior, indicating similar internal network structure. Diffusion and release of entrained cargo varied with both the rate of degradation of PEG-OND hydrogels and the hydrodynamic radius of the entrained species. These results highlight the utility of OND linkers in the preparation of degradable network materials with potential applications in sustained release.
Hydrogels are water-swollen polymer networks that have found widespread use in tissue engineering, three-dimensional cell culture,1 and the controlled or sustained delivery of biologically active molecules.2 Polyethylene glycol (PEG) hydrogels have received much attention for biomedical applications due to their low toxicity and relative lack of immunogenicity.3 Additionally, PEG hydrogels are readily permeable to diffusion of both proteins and small molecules.2a,2b,4 A wide range of linear and branched PEG reagents are commercially available, and facile installation of different functional groups at chain ends has enabled the exploration of a number of bioorthogonal chemistries to form PEG networks, including strain-promoted azide–alkyne cycloaddition, Diels–Alder reaction, Michael addition, and thiol–ene reactions.5 A large body of work has been dedicated to the generation of degradable hydrogel matrices,5 with breakdown mediated by various mechanisms including enzyme-mediated bond cleavage,6 ester hydrolysis,7 photocleavage,8 β-elimination,2a retro-Diels–Alder (rDA),9 and retro-Michael reactions.10
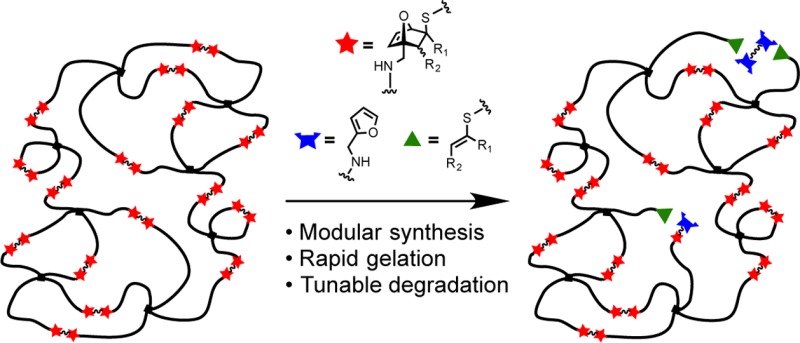
Recent reports have emphasized the generation of materials with a wide range of stabilities by changing or mixing linkers with different rates of cleavage.2a,7,10 In this study, we introduce a class of 7-oxanorbornadiene dicarboxylate (OND) linkers in which the OND moiety provides for both the connecting (conjugate addition) and cleavage (rDA) reactions, building from our prior reports of the chemistry of small-molecule variants (Scheme 1).11 The resulting modular OND-based hydrogels exhibited predictable and widely varying stabilities, with little sensitivity of decomposition rate to variations in pH. These studies lay the foundation for further development of this platform for sustained release and a range of biomedical applications.
Scheme 1. Reaction of ONDs with Thiols and Fragmentation of Adducts.
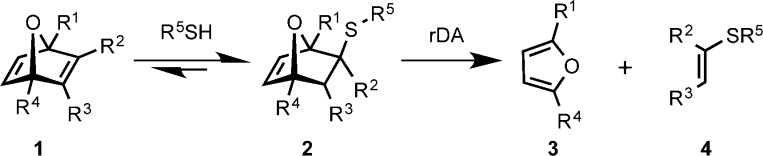
While rDA fragmentation has previously been used to promote degradation in network materials, it has typically required elevated temperatures or high organic solvent content to induce breakdown on practical time scales. Furthermore, the factors required to change degradation rates also produce gels with highly variable physical properties before decomposition.9 We anticipated that the more rapid and tunable rDA reactions of OND adducts should make them more likely to produce hydrogels with predictable and physiologically relevant erosion properties.
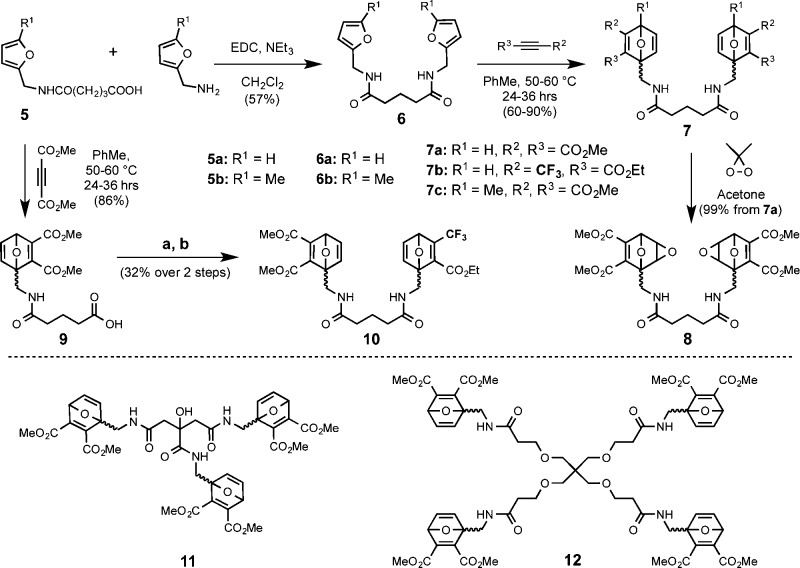
Divalent OND linkers were prepared by Diels–Alder reaction from readily accessible furan derivatives and electron-deficient acetylenes, as shown in Scheme 2. Three OND moieties were incorporated into multivalent compounds 7, 10, 11, and 12, designed to yield thiol adducts with varying stabilities to rDA fragmentation. To generate analogous non-degradable gels for comparison, the cleavage-resistant linker 8 was prepared by epoxidation of 7a with dimethyldioxirane.12 While epoxyoxanorbornene linkers exhibit rates of Michael addition similar to those of ONDs, their adducts are incapable of rDA fragmentation.13
Scheme 2. Synthesis of Symmetric and Asymmetric Bis-ONDs, and Structures of Tris- and Tetra-ONDs.
Reagents and conditions: (a) 1 equiv of furfurylamine, 1.4 equiv of DCC, CH2Cl2, 6 h; (b) 1.3 equiv of ethyl 4,4,4-trifluoro-2-butynoate, toluene, 60 °C, 40 h.
PEG-OND hydrogels were prepared by mixing tetravalent thiol-terminated PEG (4-PEG-SH, Mw ≈ 2500 Da for each arm) at 3.5 wt % and multivalent OND linkers at equimolar concentrations of thiol and electrophile in phosphate buffer containing 7% dimethylsulfoxide by volume (Scheme 3). The reactions were mixed briefly, and the gel was allowed to cure at room temperature or 37 °C. Gelation time was recorded as the point at which the sample no longer flowed upon inversion of the reaction vessel. For all linkers except 7c, self-supporting gels were observed in less than 90 s at pH 7.2 and room temperature, and in approximately 30 s at pH 7.4 and 37 °C.
Scheme 3. Formation of PEG-OND Hydrogels.
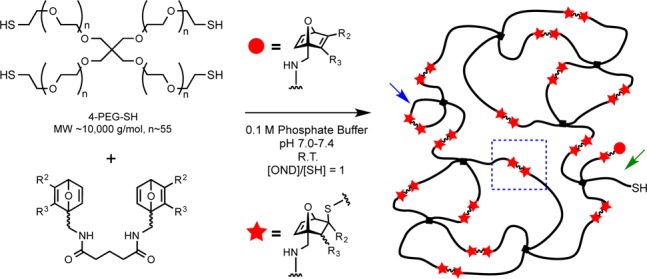
The blue arrow marks a loop defect, the green arrow indicates an unreacted chain end, and the blue box highlights a productive linkage.
Oscillatory rheology in the linear regime at 37 °C showed the storage modulus (G′) to be larger than the loss modulus (G″) throughout the gelation process (Supporting Information, Figure S2). G′ was found to be frequency-independent and much larger than G″ at all frequencies, confirming the solid-like character of these gels (Figure 1C). No remaining OND linker was observed by 1H NMR after 10 min of curing at room temperature (Figure S7). At pH 6.5, gelation was much slower, with self-supporting gels observed after 20 min. The 1,4-disubstituted linker 7c formed only viscous solutions at pH 6.5–7.4. Increasing the buffer pH to 8.0 gave gels with 7c within 1.5 min at room temperature. These were fairly stable at 4 °C, but reverted to the liquid state within 30 min at room temperature, as indicated by the crossover between G′ and G″ in Figure 1D. This behavior is consistent with the slower rate of conjugate addition, and the faster rate of fragmentation, of the thiol adduct of this 1,4-dialkyl-substituted OND electrophile.11,13 Gels derived from cleavable OND linkers 7a and 7b collapsed with extended heating at 50 °C (assessed by periodically inverting samples during incubation), while the gel formed using epoxide linker 8 remained intact (Figure 1B). 1H NMR analysis of representative hydrogels confirmed the conversion of OND-thiol adducts to furan and thiomaleate fragments with the expected first-order kinetic behavior at 37 °C (Figures S7–S10).11 We therefore conclude that breakdown of the PEG-OND gels occurred by rDA fragmentation of the OND-thiol adducts rather than an alternative process such as amide hydrolysis (Figure 1A).
Figure 1.
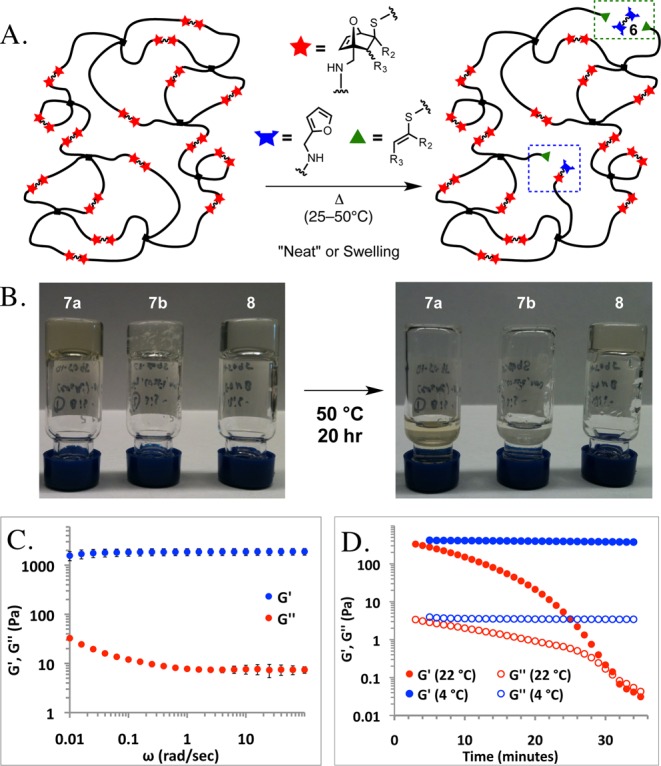
(A) Species formed during degradation of PEG-OND hydrogels. (B) Inversion test for hydrogel degradation; gels derived from 8 were stable indefinitely. (C) Angular frequency dependence of G′ and G″ at a fixed strain of 1% at 37 °C for gel formed with 8. (D) Time dependence of G′ and G″ for gel formed with linker 7c at a constant strain of 5% and an angular frequency of 1 rad/s, at 4 and 22 °C.
Degradation rates of the PEG-OND hydrogels in the absence of swelling buffer varied with temperature and with the identity of OND used to form the gel. Gels derived from OND 7a were stable for more than 2 weeks at 4 °C by the inversion test, but collapsed after 12 h at 37 °C, and within 3 h at 50 °C. In comparison, gels formed using 7b were stable for more than 60 h at 37 °C, and approximately 13 h at 50 °C. The same trend was observed by time-lapse photography, in which gels were subjected to heating while supporting a glass bead (Supporting Information, Movie S1): gels derived from 8 remained intact, and gels derived from 7b were more stable than those made with 7a.
The swelling of the PEG-OND hydrogels in deionized water was measured at 4 °C to prevent significant fragmentation during the experiment. The swollen mass of the gel after 24 h was compared to the mass at curing to obtain the equilibrium swelling ratio, and residual mass of the gel components after lyophilization was used to calculate the gel fraction, which was found to be >90% for all formulations examined. An independent experiment measuring soluble fraction thiol content yielded results complementing those obtained gravimetrically (Table S3, see Supporting Information for details). With the knowledge that small molecules can readily permeate swollen PEG-OND hydrogels (see below), we attempted to detect and modify residual thiols in gels prepared from divalent linkers 7a and 8 by swelling in the presence of a fluorogenic OND derivative (Figure S12, see Supporting Information for details).11 This treatment yielded fluorescently labeled hydrogels, revealing the presence of residual thiols in the gel network equal to 11.8 ± 3.3% of input macromer thiol content. This result suggests that residual thiols in these materials can be modified post-gelation for the attachment of functional cargo.
Apparent molecular weights between elastic cross-links (Mc) between 3700 and 4100 g/mol were determined using Flory–Rehner theory (Table 1) from swelling results.14 These Mc values for gels formed with divalent ONDs (entries 1–5) are lower than the ideal Mc expected for network polymers formed by step-growth polymerization.15 Similar behavior has been described previously for networks with low chemical cross-linking density, where molecular weight between cross-links exceeds the entanglement molecular weight for the macromer (∼4400 for PEG).16 In contrast, the observed Mc values for gels made with ONDs 11 and 12 are greater than the theoretical Mc, indicating a lower cross-link density than expected in an ideal network. This may be due to the formation of network defects, such as loops, or incomplete conversion of reactive end groups, as depicted in Scheme 3, made more likely by the increasing valency of these linkers.15
Table 1. Comparative Hydrogel Swelling Results.
| entry | linker | equilibrium swelling ratioa | gel fractionb | Mc (g/mol)c |
|---|---|---|---|---|
| 1 | 7a | 1.70 ± 0.04d | 0.91 ± 0.03 | 4080 ± 57 |
| 2 | 7b | 1.40 ± 0.02 | 0.96 ± 0.02 | 3698 ± 16 |
| 3 | 8 | 1.60 ± 0.10 | 0.97 ± 0.01 | 3900 ± 93 |
| 4 | 10 | 1.87 ± 0.12 | 0.98 ± 0.04 | 4112 ± 94 |
| 5 | 7a+7b | 1.71 ± 0.04 | 0.99 ± 0.04 | 3981 ± 36 |
| 6 | 11 | 1.54 ± 0.07 | 0.98 ± 0.08 | 3846 ± 102 |
| 7 | 12 | 1.44 ± 0.08 | 0.96 ± 0.05 | 3740 ± 95 |
Mass post-swelling/mass pre-swelling.
Mass of dry residue post-swelling/input mass of OND and 4-PEG-SH.
Calculated using the Flory–Rehner equation, see Supporting Information.
Error represents standard deviation for n = 3 separate gels.
Degradation behavior of PEG-OND hydrogels under swelling conditions was investigated by labeling 3% of the 4-PEG-SH thiols with a BODIPY fluorophore via maleimide coupling prior to gelation. Gels prepared from this material are expected to have slightly lower cross-link densities and to degrade sooner when compared to gels formed without the erosion probe (Figure S16). However, the probe-labeled macromer allowed convenient monitoring and comparison of erosion behavior under conditions relevant for biological applications. An increase in absorbance (504 nm) of the swelling buffer was observed as pieces containing the labeled PEG-thiol components were detached from the gel by rDA fragmentation (Figure 2).2a,17 Slow release of the BODIPY-labeled PEG was observed, followed by rapid solubilization at the reverse gelation point, which was reached at later times for gels formed with more stable OND adducts, consistent with the relative stabilities observed in unswelled gels (Figure 2B). Using combinations of linkers, it was possible to produce hydrogels with release profiles tuned between those of gels prepared from a single linker (Figure 2C). Analysis of supernatants collected during erosion by gel permeation chromatography revealed the presence of monomeric macromer species prior to reverse gelation, and a larger number of oligomeric macromer species after gel collapse (Figure S14).
Figure 2.
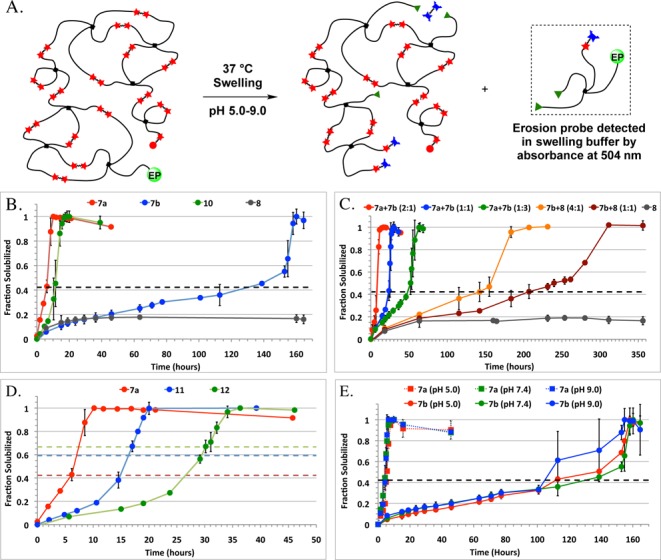
(A) Schematic of release of erosion probe (EP) during gel degradation. Erosion profiles resulting from varying (B) OND linker identity, (C) ratios of linkers, and (D) OND valence. (E) Demonstrated insensitivity of erosion behavior to pH of swelling buffer. Dotted lines represent theoretical reverse gelation thresholds. See Supporting Information for details.
PEG-OND hydrogels prepared with divalent OND linkers reached the reverse gelation point at a conversion close to the value predicted by Flory and Rehner for step growth gels (dotted lines in Figure 2B–E).14b,18 The higher-valent ONDs 11 and 12 produced gels that were more stable than those formed from divalent ONDs, but reverse gelation occurred earlier than predicted by theory (Figure 2D). As with the Mc values noted above, this suggests a less than ideal cross-linking density for these systems in which more attachment points are possible.
In contrast to other hydrogels designed to degrade by hydrolysis, β-elimination, or retro-Michael reactions, we expected gels formed with ONDs to exhibit stability profiles largely independent of changes in pH due to the nature of the rDA reaction. Degradation in swelling buffers at pH values ranging from 5.0 to 9.0 yielded very similar erosion profiles (Figure 2E) and degradation times measured by time-lapse photography (Supporting Information, Movie S2 and Figure S17).
Finally, the diffusion of entrained, rather than covalently anchored, cargos of different sizes out of OND hydrogels of differing stabilities was measured. This was done with a small molecule (carboxyfluorescein), a globular protein (fluorescein-labeled bovine serum albumin, BSA), and a 30 nm protein nanoparticle (fluorescein-labeled bacteriophage Qβ virus-like particle) (Figure 3). The first and last species have no available thiol groups; the second was reacted with N-ethylmaleimide prior to gelation to cap cysteine-34 and avoid tethering of the protein cargo to the hydrogel network.11 Hydrogels derived from 4-PEG-SH and 7a, 7b, or 8 were formed in the presence of each cargo; the speed of this reaction made it highly unlikely that OND connectors were addressed by protein amine groups under these conditions.
Figure 3.
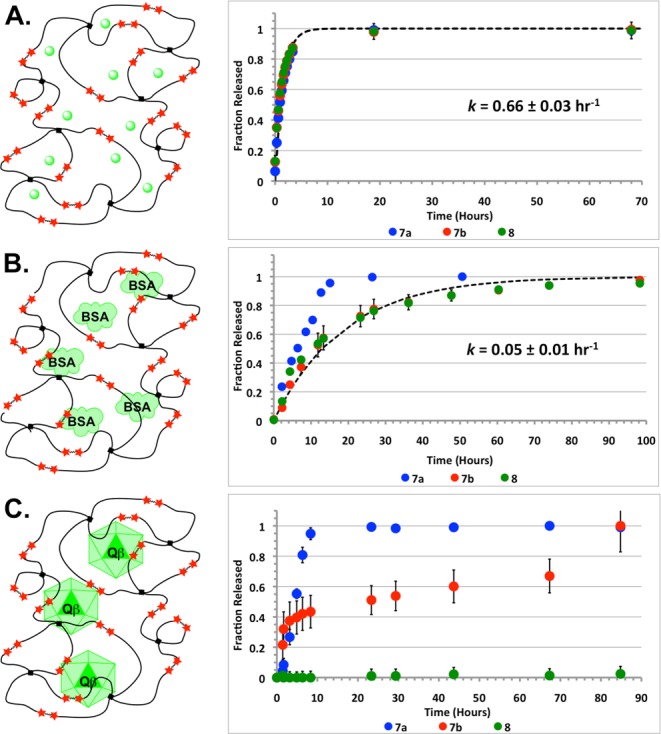
Comparative release of entrained carboxyfluorescein (A), bovine serum albumin (B), and bacteriophage Qβ virus-like particle (C) cargos from PEG-OND hydrogels. Dotted lines represent fit for diffusion of cargo from PEG-OND gel 8.
Carboxyfluorescein rapidly diffused out of all gels at the same rate (kdiffusion = 0.66 ± 0.03 h–1, Figure 3A), apparently unhindered by the hydrogel matrix. Bovine serum albumin diffusion was also observed, but was slower (kdiffusion = 0.05 ± 0.01 h–1, Figure 3B). The decomposition rate of the least stable hydrogel (made with 7a) was competitive with this diffusion, so BSA release from that gel was markedly faster than from the other, more stable, matrices. In contrast, the release of the trapped virus-like particles was largely governed by hydrogel degradation for all of the materials (Figure 3C), indicating that the particle diameter exceeded the hydrogel mesh size (see Supporting Information for details). As a result, nanoparticles were not released from gels derived from linker 8.
In summary, the conjugate addition and retro-Diels–Alder properties of electron-deficient oxanorbornadienes have been used for the first time to prepare degradable hydrogels with two rare and valuable properties: predictable, widely varying stabilities and insensitivity of decomposition toward variations in pH. These gels exhibited comparable equilibrium swelling behaviors, indicating similar internal structure and physical properties regardless of the built-in degradation rate. While the most stable degradable hydrogel studied here decomposes after approximately 2 weeks at 37 °C, more stable formulations may be desirable for some biomedical applications. Based on the observed erosion behavior, such highly stable gels should be readily accessible by using higher valence analogues of linkers that produce more stable or non-degradable thiol adducts (i.e., 7b, and 8), as well as increasing the valence of thiol-modified reaction partners. The ease of synthesis of OND linkers, the tunability of material erosion behavior using combinations of a small subset of linkers, and the rapid gelation at physiological pH show great promise for applications of these materials as injectable depots for sustained release.
Acknowledgments
This work was supported by the NSF (CHE 1011796), NIH (GM101421), the Skaggs Institute for Chemical Biology, and the Georgia Institute of Technology. C.J.H. gratefully acknowledges support by the NSF Graduate Research Fellowship Program. We thank S. Crooke for Qβ VLPs, and G. Berg, C. Bowman, A. Kislukhin, and H. Wisniewska for helpful discussions.
Supporting Information Available
Details of materials, experimental procedures, and spectral characterization; rheological characterization; calculations; and time-lapse videos comparing gel stabilities. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Lee K. Y.; Mooney D. J. Chem. Rev. 2001, 101, 1869. [DOI] [PubMed] [Google Scholar]
- a Ashley G. W.; Henise J.; Reid R.; Santi D. V. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Koehler K. C.; Anseth K. S.; Bowman C. N. Biomacromolecules 2013, 14, 538. [DOI] [PubMed] [Google Scholar]
- Drury J. L.; Mooney D. J. Biomaterials 2003, 24, 4337. [DOI] [PubMed] [Google Scholar]
- Mellott M. B.; Searcy K.; Pishko M. V. Biomaterials 2001, 22, 929. [DOI] [PubMed] [Google Scholar]
- Kharkar P. M.; Kiick K. L.; Kloxin A. M. Chem. Soc. Rev. 2013, 42, 7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M. P.; Raeber G. P.; Zisch A. H.; Tirelli N.; Hubbell J. A. Adv. Mater. 2003, 15, 888. [Google Scholar]
- Xu J.; Feng E.; Song J. J. Am. Chem. Soc. 2014, 136, 4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloxin A. M.; Kasko A. M.; Salinas C. N.; Anseth K. S. Science 2009, 324, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wei H.-L.; Yang Z.; Zheng L.-M.; Shen Y.-M. Polymer 2009, 50, 2836. [Google Scholar]; b Kirchhof S.; Brandl F. P.; Hammer N.; Goepferich A. M. J. Mater. Chem. B 2013, 1, 4855. [DOI] [PubMed] [Google Scholar]
- Baldwin A. D.; Kiick K. L. Polym. Chem. 2013, 4, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislukhin A. A.; Higginson C. J.; Hong V. P.; Finn M. G. J. Am. Chem. Soc. 2012, 134, 6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. W.; Singh M. Org. Synth. 1997, 74, 91. [Google Scholar]
- Hong V.; Kislukhin A. A.; Finn M. G. J. Am. Chem. Soc. 2009, 131, 9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Flory P. J. Chem. Rev. 1946, 39, 137. [DOI] [PubMed] [Google Scholar]; b Flory P. J. J. Chem. Phys. 1950, 18, 108. [Google Scholar]; c Flory P. J.; Rehner J. J. Chem. Phys. 1943, 11, 521. [Google Scholar]
- Metters A.; Hubbell J. Biomacromolecules 2004, 6, 290. [DOI] [PubMed] [Google Scholar]
- Zdyrko B.; Varshney S. K.; Luzinov I. Langmuir 2004, 20, 6727. [DOI] [PubMed] [Google Scholar]
- DuBose J. W.; Cutshall C.; Metters A. T. J. Biomed. Mater. Res. Part A 2005, 74A, 104. [DOI] [PubMed] [Google Scholar]
- Adzima B. J.; Aguirre H. A.; Kloxin C. J.; Scott T. F.; Bowman C. N. Macromolecules 2008, 41, 9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.