Abstract
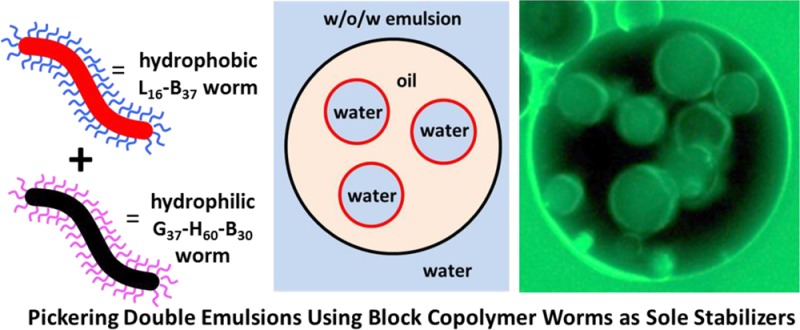
The rational formulation of Pickering double emulsions is described using a judicious combination of hydrophilic and hydrophobic block copolymer worms as highly anisotropic emulsifiers. More specifically, RAFT dispersion polymerization was utilized to prepare poly(lauryl methacrylate)–poly(benzyl methacrylate) worms at 20% w/w solids in n-dodecane and poly(glycerol monomethacrylate)–poly(2-hydroxypropyl methacrylate)–poly(benzyl methacrylate) worms at 13% w/w solids in water by polymerization-induced self-assembly (PISA). Water-in-oil-in-water (w/o/w) double emulsions can be readily prepared with mean droplet diameters ranging from 30 to 80 μm using a two-stage approach. First, a w/o precursor emulsion comprising 25 μm aqueous droplets is prepared using the hydrophobic worms, followed by encapsulation within oil droplets stabilized by the hydrophilic worms. The double emulsion droplet diameter and number of encapsulated water droplets can be readily varied by adjusting the stirring rate employed during the second stage. For each stage, the droplet volume fraction is relatively high at 0.50. The double emulsion nature of the final formulation was confirmed by optical and fluorescence microscopy studies. Such double emulsions are highly stable to coalescence, with little or no change in droplet diameter being detected over storage at 20 °C for 10 weeks as judged by laser diffraction. Preliminary experiments indicate that the complementary o/w/o emulsions can also be prepared using the same pair of worms by changing the order of homogenization, although somewhat lower droplet volume fractions were required in this case. Finally, we demonstrate that triple and even quadruple emulsions can be formulated using these new highly anisotropic Pickering emulsifiers.
Introduction
Particle-stabilized emulsions are known as Pickering or Ramsden emulsions and were first reported over a century ago.1,2 Such emulsions offer certain advantages over conventional surfactant emulsions, including enhanced long-term stability and reduced foaming during preparation.3 The emulsion type depends on the particle wettability, which is directly related to the particle contact angle:3hydrophilic particles typically stabilize oil-in-water (o/w) emulsions, whereas hydrophobic particles usually favor the formation of water-in-oil (w/o) emulsions.4−10 In principle, using both hydrophilic and hydrophobic particles should enable the preparation of so-called double emulsions, e.g., either water-in-oil-in-water (w/o/w) or oil-in-water-in-oil (o/w/o).11−20 For example, Binks and co-workers have used silane chemistry to modify the surface of silica nanoparticles in order to prepare both o/w/o and w/o/w Pickering double emulsions.21,22 Alternatively, we recently reported that the physical adsorption of a commercial water-soluble polymer, poly(ethylene imine), onto silica particles is a convenient means of tuning particle wettability in order to prepare w/o/w Pickering double emulsions23 and also the related colloidosomes-in-colloidosomes.24
Since the pioneering work by Velev and Paunov,25−27 there has been considerable interest in the use of highly anisotropic nanoparticles as Pickering emulsifiers.28,29 In particular, rod-like cellulose nanoparticles (isolated from biomass) have been utilized to prepare highly stable Pickering emulsions.30−33 In complementary studies, we recently examined both hydrophilic34 and hydrophobic35 diblock copolymer worms as emulsifiers. In principle, such anisotropic nanoparticles should adsorb much more strongly than their precursor spherical nanoparticles, while maintaining a relatively high specific surface area.34,35 Moreover, these wholly synthetic diblock copolymer worms are now readily available via polymerization-induced self-assembly (PISA). Such syntheses can be conducted via reversible addition–fragmentation chain transfer (RAFT) polymerization in water or n-alkanes to afford either hydrophilic36,37 or hydrophobic38,39 diblock copolymer worms, respectively.
Very recently, Capron and co-workers reported the preparation of Pickering double emulsions using two types of cellulose nanofibers.40 Native nanofibers proved to be sufficiently hydrophilic to stabilize o/w emulsions, whereas chemical derivatization of surface hydroxyl groups using lauroyl chloride produced highly hydrophobic nanofibers that enabled the production of w/o emulsions. Combined with knowledge of the mean droplet diameters of the two types of single emulsions, the judicious combination of both types of nanofibers enabled highly stable o/w/o emulsions to be formulated. However, o/w/o emulsions are not as useful as w/o/w emulsions for many commercial sectors (e.g., agrochemicals, laundry formulations, and home and personal care products), for which an aqueous continuous phase is strongly preferred. Accordingly, in the present work we utilize a combination of hydrophilic and hydrophobic diblock copolymer worms in order to prepare w/o/w Pickering double emulsions (see Figure 1). Our strategy involves rational design principles based on the ability to tune the mean droplet diameter by systematic variation of the shear rate (stirring rate) during homogenization. Moreover, such emulsification is conducted using significantly higher volume fractions of the droplet phase than those employed by Capron and co-workers.40 Finally, we briefly explore the feasibility of formulating complementary o/w/o emulsions and also multiple emulsions using the same methodology.
Figure 1.
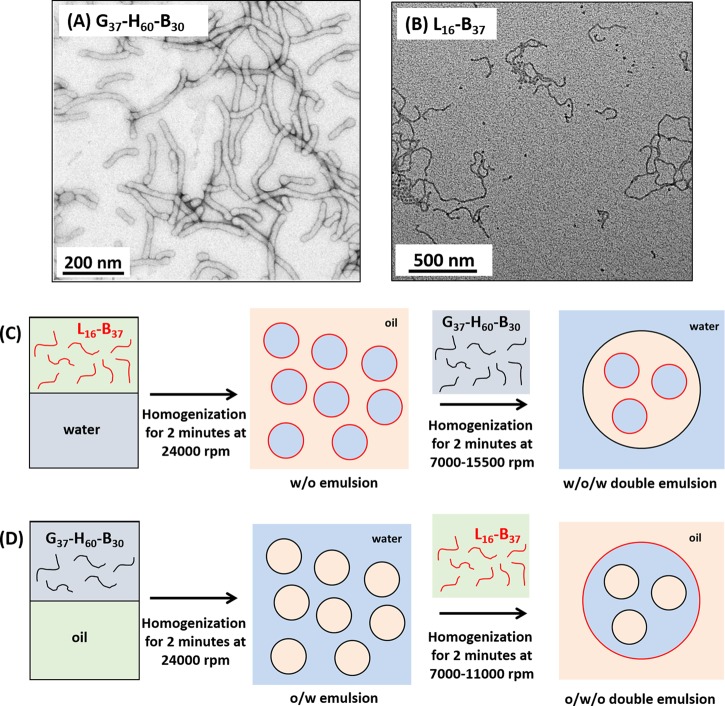
Transmission electron microscopy images recorded for (A) a dilute aqueous dispersion of G37-H60-B30 worms and (B) a dilute n-dodecane dispersion of L16-B37 worms used to prepare the Pickering double and multiple emulsions described in this work. Schematic representation of the preparation of (C) w/o/w double emulsions and (D) o/w/o double emulsions by the judicious combination of these two types of anisotropic Pickering emulsifiers.
Experimental Section
Materials
Glycerol monomethacrylate (denoted as G for brevity when describing copolymers) was obtained from GEO Specialty Chemicals (Hythe, UK) and was used as received. 2-Hydroxypropyl methacrylate (denoted hereafter as H for brevity), lauryl methacrylate (denoted as L), benzyl methacrylate (denoted as B), cumyl dithiobenzoate (CDB), 4,4′-azobis(4-cyanopentanoic acid) (ACVA), n-dodecane, fluorescein, Nile Red, D2O, and CDCl3 were purchased from Aldrich (UK). Lauryl methacrylate and benzyl methacrylate were passed through basic alumina prior to use; all other reagents were used as received unless otherwise stated. THF, n-hexane, and toluene were purchased from Fisher (UK) and CD3OD was purchased from Goss Scientific (UK). 4-Cyano-4-(2-phenylethanesulfanylthiocarbonyl)sulfanylpentanoic acid (PETTC) was prepared in-house as reported previously.41
Synthesis of G37-H60-B30 Triblock Copolymer Worms via RAFT Emulsion Polymerization
G37-H60-B30 worms (where the subscripted numbers refer to the mean degree of polymerization of each block) were prepared directly in water via polymerization-induced self-assembly (PISA) at 13% w/w solids using RAFT polymerization.42−44 More specifically, the G37-H60 diblock precursor was prepared by RAFT aqueous solution polymerization and subsequently used to polymerize benzyl methacrylate (or B) via RAFT aqueous emulsion polymerization, as described previously.34
Synthesis of L16-B37 Diblock Copolymer Worms via RAFT Dispersion Polymerization
L16-B37 diblock copolymer worms were prepared as a soft free-standing gel directly in n-dodecane via PISA at 20% w/w solids according to a previously reported protocol.39
Preparation of O/W and W/O Single Emulsions
2.0 mL of a 1.00% w/w dispersion comprising either G37-H60-B30 worms in water or L16-B37 worms in n-dodecane was placed into a 7 mL vial, followed by the addition of 2.0 mL of either n-dodecane (for the G37-H60-B30 worms) or water (for the L16-B37 worms). The two immiscible phases were homogenized for 2 min at 20 °C using a IKA Ultra-Turrax T-18 homogenizer equipped with a 10 mm dispersing tool. The stirring rate was systematically varied between 3500 and 24 000 rpm to establish the effect of this parameter on the mean droplet size. The resulting emulsions were characterized using the “drop test” to assign emulsion type (i.e., either o/w or w/o). Thus, an emulsion droplet was placed into either water or n-dodecane in turn, with rapid dispersal of the droplet signifying that the continuous phase of the emulsion was the same as that of the test solvent. These emulsions were visualized by optical microscopy and sized by laser diffraction.
Preparation of W/O/W Double Emulsions
A single w/o emulsion stabilized by L16-B37 worms was prepared at 24 000 rpm as described above. 1.0 mL of this single w/o emulsion was then homogenized at 20 °C with 1.0 mL of a 1.00% w/w aqueous dispersion of G37-H60-B30 worms for 2 min at either 7000, 11 000, or 15 000 rpm. The resulting w/o/w double emulsions were visualized by optical and fluorescence microscopy and sized by laser diffraction.
Preparation of O/W/O Double Emulsions
A single o/w emulsion stabilized by G37-H60-B30 worms was prepared at 24 000 rpm as described above. 1.0 mL of this single o/w emulsion was then homogenized at 20 °C with 2.0 mL of a 1.00% w/w aqueous dispersion of L16-B37 worms for 2 min at either 7000 or 11 000 rpm. The resulting o/w/o double emulsions were characterized by optical microscopy.
Preparation of O/W/O/W and W/O/W/O Triple Emulsions
1.0 mL of the appropriate o/w/o or w/o/w double emulsion prepared at 11 000 rpm for 2 min was further homogenized at 20 °C with 1.0 mL of either a 1.00% w/w dispersion of L16-B37 in n-dodecane or 1.00% w/w G37-H60-B30 worms in water at 7000 rpm for 2 min. The resulting o/w/o/w or w/o/w/o triple emulsions were visualized by optical microscopy.
Characterization
THF GPC
Molecular weight distributions were assessed by gel permeation chromatography (GPC) using THF eluent. The GPC set-up comprised two 5 μm (30 cm) Mixed C columns, a LC20AD ramped isocratic pump, and a WellChrom K-2301 refractive index detector operating at 950 ± 30 nm. The THF mobile phase contained 2.0% v/v triethylamine and 0.05% w/v butylhydroxytoluene (BHT), and the flow rate was fixed at 1.0 mL min–1. A series of ten near-monodisperse poly(methyl methacrylate) standards (Mp values ranging from 1280 to 330 000 g mol–1) were used for calibration.
DMF GPC
Molecular weights and polydispersities were assessed using a GPC set-up operating at 60 °C comprising a Varian 290-LC pump injection module, a Varian 390-LC refractive index detector, and two Polymer Laboratories PL gel 5 μm Mixed-C columns. The DMF mobile phase contained 0.01 M LiBr, and the constant flow rate was set at 1.0 mL min–1. DMSO was used as a flow rate marker, and calibration was achieved using a series of ten near-monodisperse poly(methyl methacrylate) standards (Mp values ranging from 1280 to 330 000 g mol–1).
1H NMR Spectroscopy
1H NMR spectra were recorded in either D2O, CD3OD, or CDCl3 using a Bruker Avance 400 spectrometer operating at 400 MHz. Typically 64 scans were accumulated per spectrum.
Dynamic Light Scattering (DLS)
Intensity-average hydrodynamic diameters were determined by DLS at a fixed scattering angle of 173° using a Malvern Zetasizer NanoZS instrument operating at 25 °C. Dilute (0.01% w/w) aqueous or n-dodecane worm dispersions were analyzed using either plastic or glass cuvettes, respectively, and the results were averaged over three consecutive runs. The deionized water or n-dodecane used to dilute each sample was ultrafiltered through a 0.20 μm membrane in order to remove extraneous dust.
Transmission Electron Microscopy
Transmission electron microscopy (TEM) studies were conducted using a Philips CM 100 instrument operating at 100 kV and equipped with a Gatan 1 k CCD camera. Diluted block copolymer solutions (0.50% w/w) were placed on carbon-coated copper grids and exposed to either a positive ruthenium(VIII) oxide or negative uranyl formate stain to improve contrast. The L16-B37 worms were exposed to ruthenium(VIII) oxide vapor for 7 min at 20 °C. The ruthenium(VIII) oxide was prepared as follows: ruthenium(IV) oxide (0.30 g) was added to water (50 g) to form a black slurry; addition of sodium periodate (2.0 g) with continuous stirring produced a yellow solution of ruthenium(VIII) oxide within 1 min at 20 °C. For the G37-H60-B30 worms, 9 μL of a 0.75% w/w uranyl formate aqueous solution was placed on the sample-loaded grid for 20 s prior to its removal via blotting.
Optical Microscopy
Optical microscopy images were recorded using a Motic DMBA300 digital biological microscope equipped with a built-in camera and analyzed using Motic Images Plus 2.0 ML software. Number-average (Dn or D[1,0]) and surface-average (D[3,2]) droplet diameters were estimated using ImageJ software; more than 300 droplets per sample were analyzed in each case.
Fluorescence Microscopy
Fluorescence microscopy images of the w/o precursor emulsion and various w/o/w double Pickering emulsions were recorded on a Zeiss Axio Scope A1 microscope fitted with an AxioCam 1Cm1 monochrome camera. Fluorescein was dissolved in the aqueous phase and Nile Red in the n-dodecane phase. Fluorescein-labeled droplets were imaged using Zeiss filter set 38 (excitation 470/40 nm and emission 525/50 nm). Nile Red droplets were imaged using Zeiss filter set 43 HE (excitation 550/25 nm and emission 605/70 nm). Images were captured and processed using ZEN lite 2012 software.
Laser Diffraction
The volume-average droplet (D[4,3]) diameter of the various diluted emulsions (with either water or n-dodecane used as the diluent for the continuous phase) was determined using a Malvern Mastersizer 2000 instrument equipped with a small volume Hydro 2000SM sample dispersion unit (ca. 50 mL), a He–Ne laser operating at 633 nm, and a solid-state blue laser operating at 466 nm. The stirring rate was adjusted to 1000 rpm in order to avoid sedimentation of the emulsion droplets during analysis. After each measurement, the cell was rinsed with either ethanol followed by water or with n-dodecane, depending on the emulsion type. The glass walls of the cell were carefully wiped to avoid cross-contamination, and the laser was aligned centrally to the detector prior to data acquisition.
Results and Discussion
Hydrophilic poly(glycerol monomethacrylate)–poly(2-hydroxypropyl methacrylate)–poly(benzyl methacrylate) (G37-H60-B30) worms and hydrophobic poly(lauryl methacrylate)–poly(benzyl methacrylate) (L16-B37) worms were prepared directly in either water or n-dodecane, respectively, using RAFT-mediated polymerization-induced self-assembly (PISA). Such triblock and diblock copolymer worms have been utilized previously to prepare single oil-in-water (o/w) and water-in-oil (w/o) emulsions.34,35 In the present study, these two types of block copolymer worms are used in tandem to prepare Pickering double (and multiple) emulsions. Table 1 summarizes the molecular weight distributions, sphere-equivalent DLS diameters, and mean worm thicknesses and worm contour lengths determined for both types of copolymer worms. Representative transmission electron micrographs of the G37-H60-B30 and L16-B37 worms are shown in Figure 1. In both cases the worms are highly anisotropic, with relatively well-defined worm widths but a rather broad distribution of worm contour lengths (see Table 1).
Table 1. Summary of Target Block Compositions, Molecular Weight Data, DLS Z-Average Diameters, and Estimated Worm Dimensions for the Two Block Copolymers Used in This Work.
| block copolymer | solvent medium | Mn (g mol–1) | Mw/Mn | DLS diameter (nm) (PDI) | mean worm thicknessc (nm) | worm contour length rangec (nm) |
|---|---|---|---|---|---|---|
| G30-H60-B30 | water | 21000a | 1.16 | 72 (0.21) | 23 ± 3 | 50–500 |
| L16-B37 | n-dodecane | 10800b | 1.22 | 148 (0.26) | 15 ± 3 | 90–700 |
DMF GPC (vs PMMA standards).
THF GPC (vs PMMA standards).
Estimated from TEM (∼50 worms counted per sample).
Characterization of Precursor O/W and W/O Emulsions
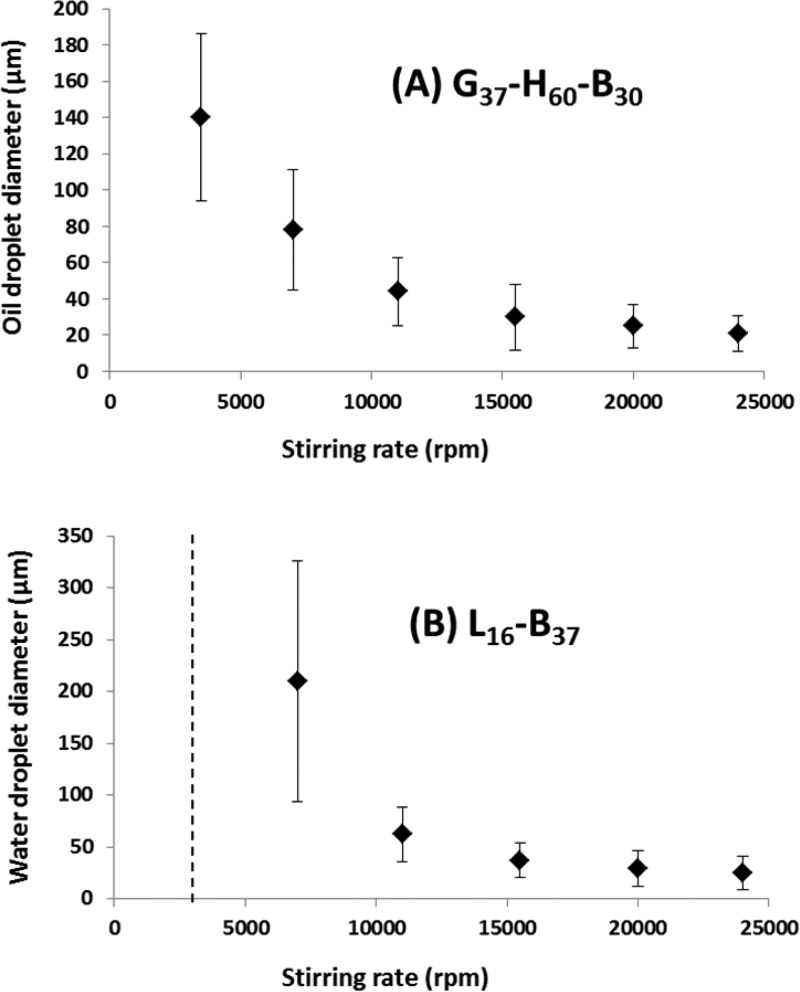
In preliminary experiments, the properties of precursor o/w and w/o emulsions were investigated in order to assess whether the hydrophobic or hydrophilic block copolymer worms should be used to stabilize the internal droplet phase when formulating the target double emulsions. Clearly, successful double emulsion formation requires that the precursor droplets should be sufficiently small to be encapsulated within the second set of droplets. In principle, the mean droplet diameter of Pickering emulsions can be tuned by (i) increasing the particle concentration, (ii) adjusting the oil (or water) volume fraction, or (iii) varying the stirring rate used for homogenization.3,45 In the present study, it was decided that the latter parameter should be the primary mechanism for controlling the emulsion size. Figure 2 depicts how systematic variation of the stirring rate at a fixed 1.00% w/w copolymer concentration affects the mean droplet diameter for o/w and w/o Pickering emulsions prepared using the G37-H60-B30 and L16-B37 worms, respectively. Previous work indicated that the adsorption efficiencies of both types of worms were relatively high (>90%) when using 0.50% w/w copolymer at 13 500 rpm for 2 min.34,35 Thus, a higher concentration (1.00% w/w) was selected in order to generate additional droplet surface area when using higher homogenization speeds (i.e., >13 500 rpm). Adjusting the stirring speed from 3500 to 24 000 rpm enabled mean droplet diameters of 21 to 140 μm to be achieved for n-dodecane-in-water emulsions prepared using the hydrophilic G37-H60-B30 worms. Similarly, mean droplet diameters of 25–210 μm could be achieved for water-in-n-dodecane emulsions formulated using the hydrophobic L16-B37 worms under the same conditions. Exceptionally, stable emulsions could not be obtained at a stirring rate of 3500 rpm when using the L16-B37 worms. The droplet size distributions are relatively broad, but this is typical of Pickering emulsions prepared by conventional homogenization.46 Recently, we reported that hydrophilic G45-H140 diblock copolymer worms are unstable when subjected to high shear.34 These relatively delicate self-assembled nanoparticles dissociated in situ to generate individual copolymer chains, which then acted as a polymeric surfactant and adsorbed strongly at the oil/water interface. Thus, this formulation leads to the formation of stable emulsions, but not the intended Pickering emulsions.
Figure 2.
Variation of droplet diameter with stirring rate for n-dodecane-in-water and water-in-n-dodecane precursor emulsions prepared at 20 °C using (A) G37-H60-B30 worms and (B) L16-B37 worms, respectively. The worm copolymer concentration was fixed at 1.00% w/w in both series of experiments. The vertical dashed line shown in (B) indicates that complete phase separation was observed when homogenization was attempted at 3500 rpm.
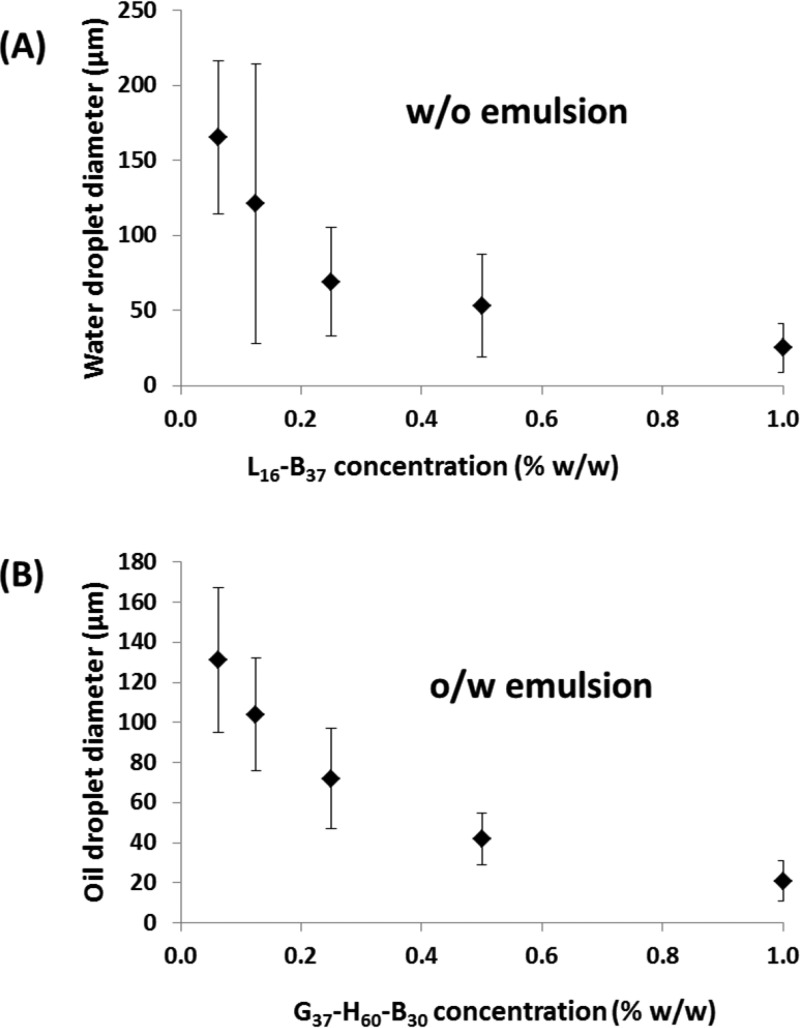
In contrast, prior work34,35 had already established that the G37-H60-B30 and L16-B37 block copolymer worms used in the present study survive homogenization at shear rates of up to 13 500 rpm, since in both cases the core-forming poly(benzyl methacrylate) block confers sufficient stability. Experimental evidence supporting the formation of genuine Pickering emulsions was obtained by TEM studies of the dried emulsion droplets, which revealed the presence of an adsorbed worm layer.34,35 Moreover, the strong concentration dependence that was observed for the mean droplet diameter is characteristic of a Pickering emulsifier, whereas essentially zero concentration dependence is invariably observed when block copolymer nano-objects dissociate under shear.34,47 In the present work, a significant increase in droplet diameter was observed on lowering the copolymer concentration, indicating adsorption of intact G37-H60-B30 or L16-B37 block copolymer worms even when employing stirring rates of up to 24 000 rpm (see Figure 3). Thus both the precursor o/w and w/o precursor emulsions are genuine Pickering emulsions. Herein we focus on the judicious combination of these hydrophilic and hydrophobic worms to prepare w/o/w double emulsions, since an aqueous continuous phase is attractive for commercial encapsulation applications. However, we briefly demonstrate that the complementary o/w/o emulsions can also be formulated (see later).
Figure 3.
Droplet diameter vs copolymer concentration for (A) w/o emulsions prepared using L16-B37 worms and (B) o/w emulsions prepared using G37-H60-B30 worms. All emulsions were homogenized at 24 000 rpm for 2 min at 20 °C.
Preparation of W/O/W Double Emulsions
Pickering w/o/w double emulsions were prepared in two steps such that the inner water droplets are stabilized by L16-B37 worms and the outer oil droplets are stabilized by G37-H60-B30 worms. First, a L16-B37 worm-stabilized precursor w/o emulsion was prepared at an n-dodecane volume fraction of 0.50 using the maximum stirring rate of 24 000 rpm to produce the smallest possible mean droplet diameter (25 ± 16 μm). This strategy was adopted to enable maximum variation in the stirring rate during the second homogenization step. Figure 4 depicts fluorescent microscopy images recorded for the precursor w/o emulsion using (a) an oil-soluble fluorescent dye (Nile Red) and (b) a water-soluble fluorescent dye (fluorescein), which confirms that it is indeed of the w/o type.
Figure 4.
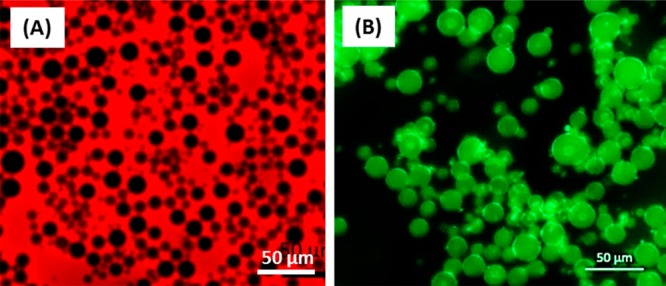
Fluorescence microscopy images of the precursor water-in-oil emulsions prepared using the L16-B37 worms where (A) the n-dodecane continuous phase is labeled using an oil-soluble fluorescent dye (Nile Red) and (B) the aqueous droplet phase is labeled using a water-soluble fluorescent dye (fluorescein).
This precursor w/o emulsion was then further homogenized using an equal volume of a 1.00% w/w aqueous dispersion of G37-H60-B30 worms to form a w/o/w double emulsion (as indicated in Figure 1C). The stirring rate utilized for this second step was either 7000, 11 000, or 15 500 rpm to afford secondary droplets of various sizes. Optical microscopy studies indicated the successful preparation of stable Pickering double emulsions (see Figure 5). Slower stirring rates produced larger droplets, as expected (see Table 2). Moreover, the largest droplets prepared at 7000 rpm were able to encapsulate many more of the initial 25 μm water droplets. Significantly fewer encapsulated water droplets are discernible within the double emulsions prepared at 15 500 rpm, with some of the smaller oil droplets remaining empty. This is attributed to the similar mean diameters observed for the inner water and outer oil droplets (25 ± 16 μm vs 30 ± 18 μm, respectively). Taking into account the relatively broad droplet size distributions, it is clear that a certain fraction of oil droplets are simply too small to encapsulate some of the larger water droplets. Conversely, the number of water droplets encapsulated within each oil droplet can be conveniently controlled simply by adjusting the stirring rate used for homogenization. Figure 6 shows two fluorescence microscopy images recorded for one such w/o/w Pickering double emulsion prepared at 11 000 rpm. The aqueous phase has been dyed with fluorescein in Figure 6A, while in Figure 6B the oil phase has been dyed with Nile Red. Aqueous droplets can be clearly identified within the oil droplets, and hence these emulsions are confirmed to be of the w/o/w type. Table 2 summarizes the mean oil droplet diameters as determined by laser diffraction for a series of both fresh and aged w/o/w double emulsions. Within experimental error, the initial double emulsion droplet diameter is essentially identical to the equivalent single o/w droplet diameter prepared at the same stirring rate. This confirms that the mean oil droplet diameter obtained for the w/o/w double emulsion is simply characteristic of the stirring rate and is not affected by the presence of the encapsulated 25 μm water droplets.
Figure 5.
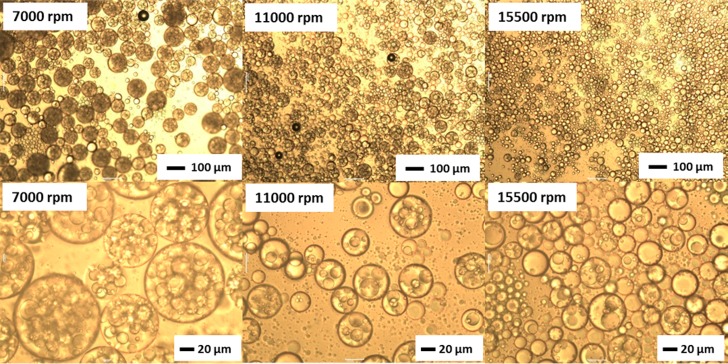
Optical microscopy images recorded at low magnification (upper row) and high magnification (lower row) for Pickering w/o/w double emulsions prepared at stirring rates of 7000, 11 000, or 15 500 rpm using equal volume fractions of an aqueous dispersion containing 1.00% w/w G37-H60-B30 worms and the precursor w/o emulsion, respectively. In each case, the precursor w/o emulsion was prepared at a water volume fraction of 0.50 using 1.00% w/w L16-B37 worms in n-dodecane at a stirring rate of 24 000 rpm.
Table 2. Relationship between Mean Volume-Average Droplet Diameter and Stirring Rate for Single, Double, and Triple Emulsions, Where Water Is the Continuous Phase in Each Case.
| emulsion type | 7000 rpm | 11 000 rpm | 15 500 rpm |
|---|---|---|---|
| single o/w | 78 ± 33 | 44 ± 19 | 30 ± 18 |
| double w/o/w (initial) | 77 ± 32 | 44 ± 20 | 30 ± 14 |
| w/o/w (after 4 weeks) | 76 ± 32 | 45 ± 19 | 28 ± 14 |
| w/o/w (after 10 weeks) | 87 ± 78 | 46 ± 19 | 28 ± 13 |
| triple o/w/o/w | 76 ± 27 |
Figure 6.
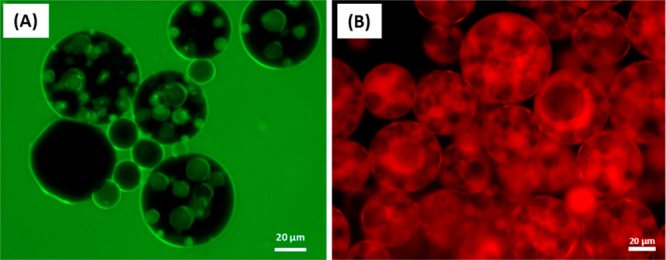
Fluorescence microscopy images recorded for w/o/w double emulsions where (A) the aqueous phase is labeled with fluorescein and (B) the n-dodecane phase is labeled with Nile Red. Each precursor w/o emulsion was prepared at a stirring rate of 24 000 rpm, while the second-stage homogenization was conducted at 11 000 rpm; the L16-B37 and G37-H60-B30 worm concentrations were 1.00% w/w in both cases.
In addition, laser diffraction studies indicate that these Pickering w/o/w double emulsions are relatively stable, with little or no change in droplet diameter being observed for at least four weeks in all cases. In contrast to the work of Cunha et al., no initial coalescence of the secondary droplets is observed.40 There is some evidence for limited coalescence of the emulsion prepared at 7000 rpm, with a modest increase in mean diameter from 76 to 87 μm being observed for an emulsion after storage at 20 °C for ten weeks. It is perhaps also worth emphasizing that the volume fractions of the encapsulated primary water droplets and secondary oil droplets are relatively high (0.25 and 0.50, respectively) compared to double Pickering emulsions reported by others.40 These findings suggest that the block copolymer worms described herein offer superior performance for the stabilization of Pickering double emulsions.
Pickering O/W/O Double Emulsions
Preparation of the complementary o/w/o double emulsions was also investigated. In these experiments, the precursor o/w emulsion was prepared using an aqueous volume fraction of 0.50 containing 1.00% w/w G37-H60-B30 worms at a stirring rate of 24 000 rpm. This protocol afforded a mean emulsion droplet diameter of 21 ± 10 μm (see Figure 7A). This precursor emulsion was then homogenized with an equal volume of a 1.00% w/w L16-B37 worm dispersion in n-dodecane to produce a final emulsion with a water volume fraction of 0.25 (see Figure 1D). However, this formulation did not result in the intended stable double emulsion, but rather produced an o/w emulsion with a larger mean droplet diameter. In fact, such emulsions are actually examples of so-called high internal phase emulsions (HIPEs),48−50 since the final oil volume fraction is 0.75. We hypothesize that the hydrophilic G37-H60-B30 worms have a stronger affinity for the oil/water interface than the hydrophobic L16-B37 worms. Indeed, if a 1.00% w/w aqueous G37-H60-B30 worm dispersion is homogenized with an equal volume of 1.00% w/w L16-B37 worms in n-dodecane, a stable o/w emulsion is produced, rather than a w/o emulsion. This observation indicates that the G37-H60-B30 worms are preferentially adsorbed at the oil/water interface. Thus, in the above series of experiments, the additional oil introduced for the second-stage homogenization is simply stabilized by the G37-H60-B30 worms, despite the presence of the L16-B37 worms within it. This problem was circumvented by increasing the volume fraction of L16-B37 worms in n-dodecane from 0.50 to 0.67 (i.e., by employing an aqueous volume fraction of 0.33 rather than 0.50 to prepare the precursor o/w emulsion). This increases the number of L16-B37 worms available for adsorption at the oil/water interface and also increases the volume fraction of n-dodecane to 0.83, making it impossible for the G37-H60-B30 worms to stabilize the additional interfacial area. The optical microscopy images for the o/w/o double emulsions prepared at this volume fraction are shown in Figures 7B and 7C. Like the w/o/w emulsions, the aqueous droplets of the o/w/o emulsion prepared at 7000 rpm are significantly larger and contain more encapsulated oil droplets than those prepared at 11 000 rpm.
Figure 7.
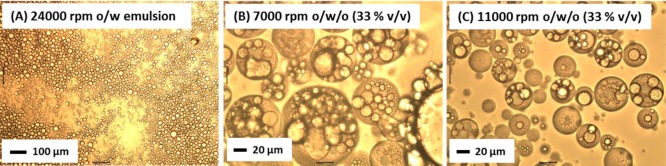
Optical microscopy images recorded for initial o/w and o/w/o double emulsions. The precursor o/w emulsion was prepared at an n-dodecane volume fraction of 0.50 using 1.00% w/w G37-H60-B30 worms and a stirring rate of 24 000 rpm for 2 min. The second-stage homogenization was conducted at either (B) 7000 rpm or (C) 11 000 rpm using a precursor emulsion volume fraction of 0.33.
Triple and Quadruple Emulsions
Inspired by the recent reports of multiple emulsions prepared via microfluidic techniques by Weitz and co-workers,51,52 we decided to explore the feasibility of the preparation of triple and quadruple emulsions by judicious combination of hydrophilic and hydrophobic worms. It was found that both o/w/o/w and w/o/w/o triple emulsions could be produced by using progressively slower stirring rates for each homogenization. Thus initial emulsification was conducted at 24 000 rpm, the intermediate double emulsion was produced at 11 000 rpm, and the final triple emulsion was obtained at 7000 rpm (see Figures 8A and 8B).
Figure 8.
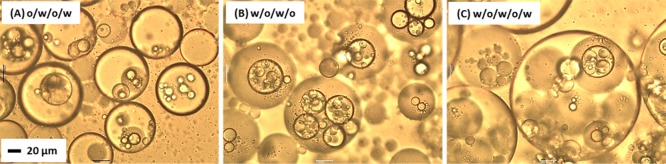
Optical microscopy images recorded for (A) an o/w/o/w triple emulsion, (B) an w/o/w/o triple emulsion, and (C) a w/o/w/o/w quadruple emulsion prepared by using progressively slower stirring rates for each subsequent homogenization step. The single emulsions were prepared at 24 000 rpm, the double at 11 000 rpm, the triple at 7000 rpm, and finally the quadruple w/o/w/o/w emulsion at 3500 rpm. The copolymer concentrations were 1.00% w/w for each stage of emulsification.
Finally, a w/o/w/o/w quadruple emulsion was generated via homogenization of a w/o/w/o triple emulsion with an equal volume of an aqueous phase comprising 1.00% w/w G37-H60-B30 worms at a stirring rate of 3500 rpm (see Figure 8C). These multiple emulsions exhibit good long-term stability over time scales of months with no signs of demulsification, although some creaming or sedimentation occurs (depending on the nature of the continuous phase). Table 2 confirms that an o/w/o/w triple emulsion has essentially the same droplet diameter as the single and double emulsions prepared using the same stirring rate. Again, this indicates that the final emulsion droplet diameter is simply dictated by the homogenization conditions.
Conclusions
Both w/o/w and o/w/o double emulsions can be prepared using a judicious combination of hydrophilic and hydrophobic block copolymer worms as Pickering emulsifiers. Systematic variation of the stirring rate during homogenization enables stable w/o/w double emulsions of adjustable mean diameter to be prepared at volume fractions of up to 0.50 for the precursor w/o emulsion. Pickering double emulsions are reasonably stable over time scales of several months with no discernible change in the mean droplet diameter. The complementary o/w/o double emulsions can also be prepared, albeit at somewhat lower volume fractions for the precursor emulsion. Finally, we demonstrate that triple and even quadruple emulsions can also be prepared using these new highly anisotropic Pickering emulsifiers. Given their straightforward and rather efficient synthesis at high solids via polymerization-induced self-assembly, such block copolymer worms are expected to be useful wholly synthetic alternatives to naturally occurring anisotropic nanoparticles derived from biomass for the rational design of bespoke Pickering emulsions.
Acknowledgments
S.P.A. thanks EPSRC (Platform grant EP/J007846/1) for partial postdoctoral support of K.L.T. and also acknowledges a five-year Advanced Investigator ERC grant (PISA 320372).
Author Present Address
J.A.L.: Department of Chemical and Biological Engineering, The University of Sheffield, Mappin Street, Sheffield, Yorkshire S1 3JD, UK.
The authors declare no competing financial interest.
References
- Pickering S. U. Emulsions. J. Chem. Soc. 1907, 91, 2001. [Google Scholar]
- Ramsden W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation). -- Preliminary account. Proc. R. Soc. London 1903, 72, 156. [Google Scholar]
- Binks B. P. Particles as surfactants - similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21. [Google Scholar]
- Binks B. P.; Lumsdon S. O. Stability of oil-in-water emulsions stabilised by silica particles. Phys. Chem. Chem. Phys. 1999, 1, 3007. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Lumsdon S. O. Pickering emulsions stabilized by monodisperse latex particles. Effects of particle size. Langmuir 2001, 17, 4540. [Google Scholar]
- Binks B. P.; Lumsdon S. O. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622. [Google Scholar]
- Binks B. P.; Isa L.; Tyowua A. T. Direct measurement of contact angles of silica particles in relation to double inversion of Pickering emulsions. Langmuir 2013, 29, 4923. [DOI] [PubMed] [Google Scholar]
- Dinsmore A. D.; Hsu M. F.; Nikolaides M. G.; Marquez M.; Bausch A. R.; Weitz D. A. Colloidosomes: Selectively permeable capsules composed of colloidal particles. Science 2002, 298, 1006. [DOI] [PubMed] [Google Scholar]
- Ashby N. P.; Binks B. P. Pickering emulsions stabilised by laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2, 5640. [Google Scholar]
- Aveyard R.; Binks B. P.; Clint J. H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503. [Google Scholar]
- Garti N. Double emulsions - scope, limitations and new achievements. Colloids Surf., A 1997, 123, 233. [Google Scholar]
- Lee D.; Weitz D. A. Nonspherical colloidosomes with multiple compartments from double emulsions. Small 2009, 5, 1932. [DOI] [PubMed] [Google Scholar]
- Utada A. S.; Lorenceau E.; Link D. R.; Kaplan P. D.; Stone H. A.; Weitz D. A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537. [DOI] [PubMed] [Google Scholar]
- Garti N.; Bisperink C. Double emulsions: Progress and applications. Curr. Opin. Colloid Interface Sci. 1998, 3, 657. [Google Scholar]
- Lee D.; Weitz D. A. Double emulsion-templated nanoparticle colloidosomes with selective permeability. Adv. Mater. 2008, 20, 3498. [Google Scholar]
- Zou S.; Wang C.; Gao Q.; Tong Z. Surfactant-free multiple pickering emulsions stabilized by combining hydrophobic and hydrophilic nanoparticles. J. Dispersion Sci. Technol. 2013, 34, 173. [Google Scholar]
- Carrillo C. A.; Nypelö T. E.; Rojas O. J. Cellulose nanofibrils for one-step stabilization of multiple emulsions (W/O/W) based on soybean oil. J. Colloid Interface Sci. 2015, 445, 166. [DOI] [PubMed] [Google Scholar]
- Ning Y.; Wang C.; Ngai T.; Tong Z. Fabrication of tunable Janus microspheres with dual anisotropy of porosity and magnetism. Langmuir 2013, 29, 5138. [DOI] [PubMed] [Google Scholar]
- Ning Y.; Yang Y.; Wang C.; Ngai T.; Tong Z. Hierarchical porous polymeric microspheres as efficient adsorbents and catalyst scaffolds. Chem. Commun. 2013, 49, 8761. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Wang C.; Tong Z. Facile, controlled, large scale fabrication of novel capsule clusters. RSC Adv. 2013, 3, 4514. [Google Scholar]
- Barthel H.; Binks B. P.; Dyab A.; Fletcher P. Multiple emulsions. US 2003/0175317 A l, 2003.
- Binks B. P.; Dyab A. K. F.; Fletcher P. D. I. Novel emulsions of ionic liquids stabilised solely by silica nanoparticles. Chem. Commun. 2003, 2540. [DOI] [PubMed] [Google Scholar]
- Williams M.; Warren N. J.; Fielding L. A.; Armes S. P.; Verstraete P.; Smets J. Preparation of double emulsions using hybrid polymer/silica particles: New Pickering emulsifiers with adjustable surface wettability. ACS Appl. Mater. Interfaces 2014, 6, 20919. [DOI] [PubMed] [Google Scholar]
- Williams M.; Armes S. P.; Verstraete P.; Smets J. Double emulsions and colloidosomes-in-colloidosomes using silica-based Pickering emulsifiers. Langmuir 2014, 30, 2703. [DOI] [PubMed] [Google Scholar]
- Noble P. F.; Cayre O. J.; Alargova R. G.; Velev O. D.; Paunov V. N. Fabrication of “hairy” colloidosomes with shells of polymeric microrods. J. Am. Chem. Soc. 2004, 126, 8092. [DOI] [PubMed] [Google Scholar]
- Paunov V. N.; Noble P. F.; Cayre O. J.; Alargova R. G.; Velev O. D. Fabrication off novel types of colloidosome microcapsules for drug delivery applications. Nanoscale Mater. Sci. Biol. Med. 2005, 845, 279. [Google Scholar]
- Alargova R. G.; Warhadpande D. S.; Paunov V. N.; Velev O. D. Foam superstabilization by polymer microrods. Langmuir 2004, 20, 10371. [DOI] [PubMed] [Google Scholar]
- Madivala B.; Fransaer J.; Vermant J. Self-assembly and rheology of ellipsoidal particles at interfaces. Langmuir 2009, 25, 2718. [DOI] [PubMed] [Google Scholar]
- Madivala B.; Vandebril S.; Fransaer J.; Vermant J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717. [Google Scholar]
- Kalashnikova I.; Bizot H.; Bertoncini P.; Cathala B.; Capron I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952. [Google Scholar]
- Kalashnikova I.; Bizot H.; Cathala B.; Capron I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471. [DOI] [PubMed] [Google Scholar]
- Zoppe J. O.; Venditti R. A.; Rojas O. J. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202. [DOI] [PubMed] [Google Scholar]
- Andresen M.; Stenius P. Water-in-oil emulsions stabilized by hydrophobized microfibrillated cellulose. J. Dispersion Sci. Technol. 2007, 28, 837. [Google Scholar]
- Thompson K. L.; Mable C. J.; Cockram A.; Warren N. J.; Cunningham V. J.; Jones E. R.; Verber R.; Armes S. P. Are block copolymer worms more effective Pickering emulsifiers than block copolymer spheres?. Soft Matter 2014, 10, 8615. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Fielding L. A.; Mykhaylyk O. O.; Lane J. A.; Derry M. J.; Armes S. P.. Vermicious thermo-responsive Pickering emulsifiers. Chem. Sci. 2015, submitted [DOI] [PMC free article] [PubMed]
- Blanazs A.; Verber R.; Mykhaylyk O. O.; Ryan A. J.; Heath J. Z.; Douglas C. W. I.; Armes S. P. Sterilizable gels from thermoresponsive block copolymer worms. J. Am. Chem. Soc. 2012, 134, 9741. [DOI] [PubMed] [Google Scholar]
- Warren N. J.; Armes S. P. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J. Am. Chem. Soc. 2014, 136, 10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding L. A.; Derry M. J.; Ladmiral V.; Rosselgong J.; Rodrigues A. M.; Ratcliffe L. P. D.; Sugihara S.; Armes S. P. RAFT dispersion polymerization in non-polar solvents: facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 2013, 4, 2081. [Google Scholar]
- Fielding L. A.; Lane J. A.; Derry M. J.; Mykhaylyk O. O.; Armes S. P. Thermo-responsive diblock copolymer worm gels in non-polar solvents. J. Am. Chem. Soc. 2014, 136, 5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha A. G.; Mougel J.-B.; Cathala B.; Berglund L. A.; Capron I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 2014, 30, 9327. [DOI] [PubMed] [Google Scholar]
- Semsarilar M.; Ladmiral V.; Blanazs A.; Armes S. P. Anionic polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization. Langmuir 2011, 28, 914. [DOI] [PubMed] [Google Scholar]
- Chiefari J.; Chong Y. K.; Ercole F.; Krstina J.; Jeffery J.; Le T. P. T.; Mayadunne R. T. A.; Meijs G. F.; Moad C. L.; Moad G.; Rizzardo E.; Thang S. H. Living free-radical polymerization by reversible addition–fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559. [Google Scholar]
- Moad G.; Chiefari J.; Chong Y. K.; Krstina J.; Mayadunne R. T. A.; Postma A.; Rizzardo E.; Thang S. H. Living free radical polymerization with reversible addition – fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49, 993. [Google Scholar]
- Perrier S.; Takolpuckdee P. Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/Xanthates (MADIX) polymerization. J. Polym. Sci., Part A: Polym. Chem. 2005, 43, 5347. [Google Scholar]
- Thompson K. L.; Armes S. P.; Howse J. R.; Ebbens S.; Ahmad I.; Zaidi J. H.; York D. W.; Burdis J. A. Covalently cross-linked colloidosomes. Macromolecules 2010, 43, 10466. [Google Scholar]
- Thompson K. L.; Armes S. P.; York D. W. Preparation of Pickering emulsions and colloidosomes with relatively narrow size distributions by stirred cell membrane emulsification. Langmuir 2011, 27, 2357. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Chambon P.; Verber R.; Armes S. P. Can polymersomes form colloidosomes?. J. Am. Chem. Soc. 2012, 134, 12450. [DOI] [PubMed] [Google Scholar]
- Cameron N. R. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer 2005, 46, 1439. [Google Scholar]
- Cameron N. R.; Sherrington D. C. High internal phase emulsions (HIPEs) - Structure, properties and use in polymer preparation. Biopolym. Liq. Cryst. Polym. Phase Emulsion 1996, 126, 163. [Google Scholar]
- Lissant K. J. The geometry of high-internal-phase-ratio emulsions. J. Colloid Interface Sci. 1966, 22, 462. [Google Scholar]
- Adams L. L. A.; Kodger T. E.; Kim S.-H.; Shum H. C.; Franke T.; Weitz D. A. Single step emulsification for the generation of multi-component double emulsions. Soft Matter 2012, 8, 10719. [Google Scholar]
- Abate A. R.; Weitz D. A. High-order multiple emulsions formed in poly(dimethylsiloxane) microfluidics. Small 2009, 5, 2030. [DOI] [PubMed] [Google Scholar]