To expand the scope of antimicrobial stewardship, antimicrobial stewardship programs need to engage frontline providers at the point of antimicrobial prescription. Interventions and a pilot project are discussed.
Keywords: antimicrobial stewardship, point of prescription, frontline, antimicrobial prescribing
Abstract
Antimicrobial stewardship is pivotal to improving patient outcomes, reducing adverse events, decreasing healthcare costs, and preventing further emergence of antimicrobial resistance. In an era in which antimicrobial resistance is increasing, judicious antimicrobial use is the responsibility of every healthcare provider. Antimicrobial stewardship programs (ASPs) have made headway in improving antimicrobial prescribing using such “top-down” methods as formulary restriction and prospective audit with feedback; however, engagement of prescribers has not been fully explored. Strategies that include frontline prescribers and other unit-based healthcare providers have the potential to expand stewardship, both to augment existing centralized ASPs and to provide alternative approaches to perform stewardship at healthcare facilities with limited resources. This review discusses interventions focusing on antimicrobial prescribing at the point of prescription as well as a pilot project to engage unit-based healthcare providers in antimicrobial stewardship.
(See the Editorial Commentary by Wenzler, Rodvold, and Danziger on pages 1259–61.)
Professional societies and federal agencies have recognized the importance of antimicrobial stewardship programs (ASPs) in all healthcare facilities [1–3]. Successful programs have achieved a 20% increase in clinical cure rates and a 10%–15% reduction in treatment failures, all while decreasing antimicrobial use by 20%–35% [4]. Despite these data, less than half of all acute care hospitals have ASPs [5–7], and inappropriate use of broad-spectrum antibiotics in US hospitals remains prevalent [8]. Guidelines and recommendations for ASPs have focused primarily on the creation of top-down or centralized processes, such as antimicrobial restriction and preauthorization or postprescription audit and feedback.
Most hospitals have challenges in implementing centralized ASPs due to lack of dedicated personnel and lack of financial resources [9]. Even when implemented, centralized approaches to stewardship may fail to affect the many episodes of antimicrobial use not subject to scrutiny by the stewardship team. In addition, there are many cases in which feedback occurs subsequent to the prescribing process with an inherent lag between antimicrobial exposure and the provision of feedback, during which time antimicrobial prescribing may be inappropriate. At the point of prescription, frontline providers, which we define as multidisciplinary healthcare providers (eg, nurses, pharmacists, and physicians) that assume direct responsibility for the daily care of patients or patient care unit, have opportunities to enhance antimicrobial stewardship.
Healthcare providers and administrators must develop ways to broaden the reach of antimicrobial stewardship and to involve frontline providers to a greater degree. To this effect, recent efforts have focused on improving antimicrobial use by developing core practices that directly involve frontline healthcare providers. Healthcare facilities with or without centralized ASPs can adopt similar strategies to promote principles of antimicrobial stewardship.
The Centers for Disease Control and Prevention (CDC) recently released a report highlighting core antimicrobial prescribing practices (Table 1) [2]. Prudent antimicrobial prescribing is a complex, calculated decision process that requires understanding of the fundamental principles of microbiology, pharmacokinetics, and adverse effects of antimicrobial use. Whereas experts in microbiology, pharmacy, and infectious diseases should help to direct institutional prescribing practices, the principles outlined by the CDC can be incorporated into daily prescribing practices and can involve all members of the healthcare team. To ensure successful implementation, however, tools to effectively incorporate antimicrobial stewardship at the point of antimicrobial prescription are greatly needed [10]. Point-of-prescription interventions, or those that aim to improve antimicrobial prescribing practices, provide the opportunity to shift antimicrobial stewardship from the primary responsibility of centralized ASPs to a more universal practice among primary healthcare teams and patient care units.
Table 1.
Core Principles of Antimicrobial Prescribing
|
Adapted from the Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. 2014. Available at http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. Accessed 26 January 2015.
TRANSLATING RECOMMENDATIONS INTO PRACTICE
Strategies to promote prudent antimicrobial prescribing practices using point-of-prescription interventions are feasible to implement using a variety of approaches in a range of healthcare settings. The examples discussed in this section demonstrate that, through innovative implementation strategies, stewardship can be integrated into daily practice.
In response to UK Department of Health recommendations on appropriate antimicrobial prescribing practices, a collaborative of healthcare facilities implemented a requirement that prescribers document an indication for all antimicrobial prescriptions and that prescriptions remain consistent with corresponding guidelines for the documented indication [11]. A multidisciplinary group including representatives from pharmacy, infectious diseases, microbiology, and nursing implemented a tiered quality improvement project to ensure compliance with the necessary documentation. Project promotion included distribution of pocket cards summarizing the policy, advertisement on patient care units, inclusion in new staff orientation, and announcement in staff meetings. Additional education efforts focused on prudent antimicrobial prescribing. Feedback was provided to both patient care units and individual physicians on compliance with hospital policy. Over the 11-month study period, compliance with hospital policy improved from 30% to 71%, and the rates of compliance remained consistently >90% after the study period [11].
Similarly, a pilot cluster randomized controlled trial in the United Kingdom evaluated the implementation of a paper form in long-term-care facilities that required documentation of antimicrobial prescribing practices. Data elements included clinical signs and symptoms, evaluation by a physician, indication for antimicrobial use, appropriate diagnostic evaluation, clinical reevaluation, and review of diagnostic tests within 48–72 hours, and duration of treatment [12]. No feedback was provided to the staff during the study, and nursing staff completed the forms with modest completion rates (31%–46%). The 12-week pilot study still demonstrated significant decrease in antimicrobial use of 4.9% in the intervention group (P = .02) compared with baseline, and a significant increase of 5.1% in the control group (P = .04) [12].
A study performed at a Montreal teaching hospital coupled an educational campaign focused on antimicrobial stewardship and antimicrobial prescribing according to institutional guidelines with an online checklist that internal medicine housestaff completed as a part of an antimicrobial “time-out” on a twice-weekly basis on selected units [13]. Housestaff were reminded to complete the checklist by unit-based pharmacists. The checklist highlighted many of the characteristics of judicious antimicrobial prescribing outlined by the CDC and focused specifically on targeted broad-spectrum antimicrobial agents, including carbapenems, fluoroquinolones, piperacillin-tazobactam, and vancomycin. Adherence with the time-out was 80%, and housestaff reported increased comfort with antimicrobial prescribing while using this tool. The study demonstrated a decrease in annual cost of antimicrobials of $149 743 [13].
Other healthcare facilities have created more sophisticated electronic medical record (EMR) systems that provide “closed loop” antimicrobial prescribing information. In one Chinese healthcare system, an EMR was created that included clinical decision support for physician order entry as well as several key principles of antimicrobial prescribing. For example, the EMR required that prescribers have culture and sensitivity data to support use of restricted antimicrobial agents within 48 hours of the prescription order [14]. After implementation of the EMR, overall antimicrobial consumption decreased by 34% (P < .001).
Other reports illustrate how frontline providers, such as hospitalists, can be the primary effectors of audit and feedback interventions. A group of hospitalists and pharmacists in a US academic medical center developed an educational campaign for treatment of skin and soft tissue infections [15]. Education was followed by audit and feedback in the form of report cards to their hospitalist colleagues. The intervention resulted in a 60% decrease in the proportion of patients exposed to broad-spectrum antimicrobials (P = .002). The hospital acquisition costs of the targeted antimicrobial, ticarcillin-clavulanate, decreased by 45% after the intervention. Hospitalists at another US academic medical center reviewed antimicrobial prescriptions to evaluate appropriate use and adherence to clinical practice guidelines [16]. Evaluation of appropriateness included key principles to antimicrobial prescribing: adherence to practice guidelines for the specific indication, narrow-spectrum therapy when possible, and utilization of available susceptibility data. Data from the audits were fed back to providers in an in-person discussion of prescribing practices. The investigators observed significant improvement in the proportion of appropriate antimicrobial prescriptions in a before–after comparison (43% improved to 74%; P < .001). Current studies are ongoing to evaluate the effects of improved documentation of an indication for antimicrobial use, expected duration of therapy, adherence to empiric treatment guidelines, and reassessment of antimicrobial prescription at 72 hours [17].
The studies discussed above indicate both feasibility of implementation and receptivity of frontline providers to incorporate enhanced antimicrobial prescribing practices into the daily care of their patients.
PILOT STUDY OF A POINT-OF-PRESCRIPTION TOOL TO IMPROVE ANTIMICROBIAL PRESCRIBING
Methods
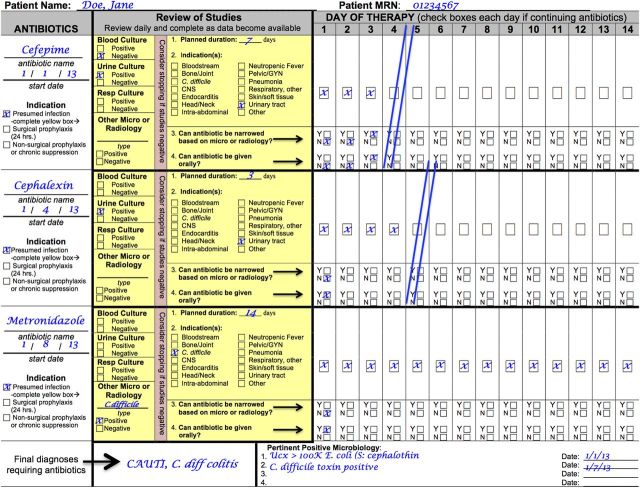
The authors of this article, a collaborative of university medical centers that include the University of Pennsylvania, Harvard University, Duke University, Washington University, and Rush University, in conjunction with the CDC Prevention Epicenters Program, developed and piloted a tool to promote antimicrobial stewardship in smaller hospitals without existing stewardship programs. To develop this tool, the collaborators reviewed available literature on antimicrobial stewardship and quality measures surrounding antimicrobial use to determine the most feasible characteristics of a tool to implement at the point of antimicrobial prescription. These characteristics included (1) implementation without centralized ASP infrastructure, (2) generalizability to a variety of healthcare facilities, (3) focus on evidence-based practices, (4) minimal workflow disruption for frontline providers, and (5) minimal associated cost. The authors convened a panel of experts in antimicrobial stewardship to identify core principles of antimicrobial prescribing. These principles were identical to those proposed by the CDC (Table 1). The collaborative developed a daily rounding flowsheet that incorporated the core principles and fulfilled the characteristics of an ideal point-of-prescription tool. The flowsheet was revised through an iterative process with input from 13 adult and pediatric hospitals without existing stewardship programs from a wide geographical distribution to create the final version (Figure 1). Four hospitals were selected to pilot the flowsheet on selected units; characteristics of these sites are detailed in Table 2.
Figure 1.
Sample of flowsheet to facilitate core antimicrobial prescribing practices. Abbreviations: CNS, central nervous system; GYN, gynecology; MRN, medical registration number.
Table 2.
Description of Hospitals Participating in the Pilot Study and Corresponding Implementation Strategies
| Name | Location | Hospital Size, No. of Beds | Prior ASP Activities | Unit | Flowsheet Completion | Information on Back of Flowsheet |
|---|---|---|---|---|---|---|
| Duke Raleigh | Raleigh, North Carolina | 186 | None | 12-bed medical/ surgical ICU | Unit pharmacist | None |
| Faulkner Hospital | Boston, Massachusetts | 150 | Prospective audit, prior authorization | 10-bed medical ICU | Nurse initiated, housestaff completed | None |
| Nash Hospital | Rocky Mount, North Carolina | 280 | None | 15-bed medical/ surgical ICU | Unit pharmacist | Antibiogram |
| Virtua Vorhees | Vorhees, New Jersey | 398 | None | 30-bed pediatric floor | Hospitalist | Empiric treatment guidelines |
Abbreviations: ASP, antimicrobial stewardship program; ICU, intensive care unit.
Participating sites were instructed to implement the flowsheet by taking advantage of existing infrastructure. Study sites chose which type of healthcare worker was best suited to complete the flowsheet. The sites also had the option of placing antimicrobial prescribing information such as hospital treatment guidelines and antimicrobial susceptibility data on the back of the flowsheet. The flowsheet was utilized for 30 days on selected units as determined by site leaders at each of the hospitals (Table 2). An information sheet was distributed to relevant prescribers on these units prior to implementation.
The primary objective of the pilot project was to determine the feasibility of implementation of the flowsheet. The outcomes studied included (1) the proportion of patients on antimicrobial medications that had a flowsheet completed satisfactorily and (2) the perceptions of medical providers regarding the benefits of completion of the flowsheet. The outcome for the first objective was measured using the proportion of patients on antimicrobial agents that had a flowsheet completed by a member of the healthcare team. Numerator data were collected by counting the total number of forms completed over the study period. Denominator data were collected by determining the total number of patients who received an antimicrobial agent during the study period, assessed using hospital-specific medication administration data. The proportion of flowsheets completed on eligible patients was determined by participating hospitals.
The second objective was evaluated using an optional survey to providers and site leaders. At the end of the 30-day pilot period, medical providers involved in completing the flowsheets were requested to complete an optional, anonymous Web-based survey. The site leaders also completed a survey at the end of the pilot period. We collected and managed these surveys using Research Electronic Data Capture, a secure Web-based application and electronic data capture tool, hosted at the University of Pennsylvania [18].
The project was approved by the institutional review boards at the participating universities and hospitals.
Results
Two of the 4 hospitals used unit-based pharmacists to complete the daily flowsheets during multidisciplinary rounds in the intensive care unit (ICU). One of the 4 hospitals used clinical nurses to prompt physicians to complete the flowsheet during multidisciplinary rounding in the ICU. One of the 4 hospitals required individual hospitalists to complete the flowsheet on a pediatric ward (Table 2). Of all eligible patients across the 4 pilot hospital units, 167 of 174 (96.0%) patients treated with antimicrobial agents had a flowsheet completed satisfactorily (range among hospitals, 92.5%–100%).
Survey response rate was 18 of 19 (94.7%). Most providers found the flowsheet easy to use, with 6 (33%) finding it very easy to use, 8 (44%) finding it somewhat easy to use, 2 (11%) remaining neutral, 2 (11%) finding it somewhat difficult to use, and 0 (0%) finding it very difficult to use. The flowsheet did not significantly impact workflow, with providers reporting a median completion time of 3 minutes per patient per day (range, 1–10 minutes).
Perceived benefit of the flowsheet by prescribers was variable. Of those hospitals that had providers primarily responsible for completion of the flowsheet, these providers estimated that a median of 5% of antimicrobial prescriptions were affected by the flowsheet (range, 0%–50%). Of the 2 hospitals in which unit-based pharmacists were responsible for completing the form, an estimated 40% of antimicrobial prescriptions were affected by the flowsheet. One hospital using unit-based pharmacists estimated that the flowsheet resulted in 33 interventions on 35 patients receiving antimicrobial agents: 20 antimicrobial discontinuations, 8 intravenous-to-oral conversions, 3 de-escalations, and 2 dose optimizations.
DISCUSSION
Centralized ASPs focused on top-down interventions have markedly improved antimicrobial prescribing and patient outcomes [1, 19]. However, many centralized approaches to stewardship are incomplete in scope because they do not reach all instances of antimicrobial use, as in the cases of nontargeted drugs with prior authorization programs and days of therapy prior to intervention in prospective audit and feedback approaches. Centralized ASP models often place the onus of reviewing the rationale and appropriateness for antimicrobials on an external team rather than attempting to improve antimicrobial use more generally by providers at the point of antimicrobial prescription. Designing interventions that emphasize principles of prudent antimicrobial prescribing at the point of prescription can help to expand antimicrobial stewardship to all healthcare settings and to supplement existing centralized ASPs.
Many principles of antimicrobial prescribing outlined by the CDC have already been applied successfully in different healthcare facilities. In our pilot project, a daily flowsheet incorporating these principles was feasible to implement in a variety of hospital settings and was well accepted by clinical staff. Study hospitals chose to implement the flowsheet using a variety of methods. Some hospitals utilized unit-based pharmacists to complete the forms and to prompt core antimicrobial prescribing practices during multidisciplinary rounds; others relied on individual prescribers to incorporate the flowsheet into their daily workflow.
Provider perceptions of the effect of the flowsheet appeared to depend on which healthcare provider completed it, but our pilot study was not designed to detect these differences. Likewise, the relative merits of implementation of the flowsheet in ICU vs non-ICU settings remains to be determined and should be the focus of future work. The difference between pharmacists and other clinicians may have occurred because when pharmacists completed the flowsheet, an active discussion with prescribers was required. Placing the responsibility for oversight of flowsheet completion with unit-based personnel has the advantage of standardizing the practice and prompting a conversation about antimicrobial choice and duration. Involving prescribers in the completion of the flowsheet has the advantage of starting these processes immediately at the time of antimicrobial prescription. Further research is needed to assess the impact of more prolonged implementation of this intervention on the quality, quantity, and cost of antimicrobial use and on associated clinical outcomes.
Our pilot study and the other studies summarized above illustrate some important characteristics of point-of-prescription interventions (Table 3). Data suggest that prescriber-level interventions can be sustained if they are carefully designed to harness existing resources and processes [11, 12]. If interventions to promote core principles to antimicrobial prescribing are viewed as disruptive to patient care processes, then they may not be assimilated by clinicians. Hospitals may increase success of these point-of-prescription interventions by reviewing the daily rounds workflow and selecting an implementation plan that is least disruptive while still empowering change.
Table 3.
Important Characteristics of Point-of-Prescription Stewardship Interventions
|
Integration of strategies to reinforce judicious antimicrobial prescribing practices is also helpful. Although developing and disseminating guidelines is essential to improve prescribing practices, it is often insufficient to produce a sustained improvement unless antimicrobial prescribing practices are reinforced among frontline providers [20–22]. Basic tenets of quality improvement can be used to guide the implementation of interventions by using antimicrobial prescribing practices as key drivers. Effective implementation requires policy awareness, education, and periodic feedback to providers to achieve sustainable effects.
As healthcare moves into an era when most hospitals use EMR, clinicians can leverage the meaningful use of these systems to integrate antimicrobial prescribing practices into prescribing workflow. Most directly, EMRs may allow incorporation of these core principles into electronic ordering of antimicrobial agents [23, 24]. EMRs also have the potential of creating closed-loop systems that require prescribers to evaluate the appropriateness of antimicrobial agents after they are prescribed. Software vendors have already taken steps to incorporate antimicrobial stewardship into daily prescribing. Initial data suggest that these systems are cost effective and have beneficial effects for antimicrobial stewardship [25–28].
Successful point-of-prescription interventions often benefit from clinical champions who oversee implementation and audit of interventions, such as a unit-based pharmacist, nurse, or non-ASP physician. Improving collaboration among different members of the healthcare team may provide the continuity, expertise, and sustainability necessary to make these interventions successful. Nurses and other nonphysician frontline providers have taken leadership on many quality improvement initiatives shown to successfully impact prescriber behavior [29, 30]. These key members of the medical team can assist in unit-level data collection to help track antimicrobial use and performance on key process measures. For example, many acute care facilities have multidisciplinary patient care rounding structures in place. Responsibility for assurance of prescribing principles can be integrated into these existing team decision-making structures.
Although centralized ASPs are useful to guide and support the efforts of frontline providers with expert guidance from infectious diseases physicians, microbiologists, and pharmacists, point-of-prescription interventions have the potential to engage prescribers directly in the fundamentals of judicious antimicrobial prescribing. Ultimately, dissemination of these practice habits among frontline clinicians will require improved training, ready access to standards of care that are particular to the formulary options and local infection resistance patterns, integration of key information into charting and order entry processes, and appropriate surveillance for and response to outlier prescribing patterns. All healthcare facilities are encouraged to begin to make responsible utilization of antimicrobial drugs the responsibility of every healthcare provider.
Notes
Acknowledgments. The authors acknowledge the contributions of Neil Fishman, MD, and Gregory Mayro, MD, of the University of Pennsylvania; Sara Cosgrove, MD, MS, and Pranita Tamma, MD, MHS, of Johns Hopkins University; and Adam Hersh, MD, PhD, of the University of Utah for their expertise and thoughtful contributions in the development of the antimicrobial flowsheet for the pilot study referenced in the article. The authors also acknowledge Luke Huets, PharmD, BCPS, of Nash Healthcare; Charles Wingerson III, PharmD candidate from the University of North Carolina Eschelman School of Pharmacy; Crystal Hahn, PharmD, and Christopher Stein, PharmD, BCPS, of Duke Raleigh Hospital; Ana Mann, MD, of Virtua Vorhees Medical Center; and Roger Clark, DO, of the Brigham and Women's Faulkner Hospital for their outstanding work conducting the pilot study.
Disclaimer. The opinions expressed by the authors do not necessarily reflect the opinions of the US Department of Health and Human Services, the Public Health Service, the CDC, or the authors’ affiliated institutions. GlaxoSmithKline or its affiliates did not contribute any funding or have any input in the study or manuscript preparation.
Financial support. This work was supported by the CDC (Cooperative Agreement FOA#CK11-001–Epicenters for the Prevention of Healthcare Associated Infections).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 3.Executive Office of the President, President's Council of Advisors on Science and Technology. Report to the president on combating antibiotic resistance. Available at: http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf Accessed 30 September 2014.
- 4.Septimus EJ, Owens RC., Jr Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis 2011; 53:S8–14. [DOI] [PubMed] [Google Scholar]
- 5.Gross R, Morgan AS, Kinky DE, et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis 2001; 33:289–95. [DOI] [PubMed] [Google Scholar]
- 6.Pope SD, Dellit TH, Owens RC, et al. Results of survey on implementation of Infectious Diseases Society of America and Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Infect Control Hosp Epidemiol 2009; 30:97–8. [DOI] [PubMed] [Google Scholar]
- 7.Newland JG, Gerber JS, Weissman SJ, et al. Prevalence and characteristics of antimicrobial stewardship programs at freestanding children's hospitals in the United States. Infect Control Hosp Epidemiol 2014; 35:265–71. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312:1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc 2011; 86:1113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; doi:10.1002/14651858.CD003543.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar K, Gilchrist M, Dickinson E, et al. A quality improvement programme to increase compliance with an anti-infective prescribing policy. J Antimicrob Chemother 2011; 66:1916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleet E, Rao GG, Patel B, et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother 2014; 69:2265– 73. [DOI] [PubMed] [Google Scholar]
- 13.Lee TC, Frenette C, Jayaraman D, et al. Antibiotic self-stewardship: trainee-led structured antibiotic time-outs to improve antimicrobial use. Ann Intern Med 2014; 161:S53–8. [DOI] [PubMed] [Google Scholar]
- 14.Li J-S, Zhang X-G, Wang H-Q, et al. The meaningful use of EMR in Chinese hospitals: a case study on curbing antibiotic use. J Med Syst 2013; 37:9937. [DOI] [PubMed] [Google Scholar]
- 15.McGarry M, Alvarez K, Kannan S, et al. Antimicrobial stewardship on the hospitalist service: skin and soft tissue infections [abstract]. J Hosp Med 2012; 7(suppl 2):55.21954169 [Google Scholar]
- 16.Kisuule F, Wright S, Barreto J, Zenilman J. Improving antibiotic utilization among hospitalists: a pilot academic detailing project with a public health approach. J Hosp Med 2008; 3:64–70. [DOI] [PubMed] [Google Scholar]
- 17.Rohde JM, Jacobsen D, Rosenberg DJ. Role of the hospitalist in antimicrobial stewardship: a review of work completed and description of a multisite collaborative. Clin Ther 2013; 35:751–7. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Buul LW, Sikkens JJ, van Agtmael MA, et al. Participatory action research in antimicrobial stewardship: a novel approach to improving antimicrobial prescribing in hospitals and long-term care facilities. J Antimicrob Chemother 2014; 69:1734–41. [DOI] [PubMed] [Google Scholar]
- 20.Avorn J, Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacological basis of therapeutics. Ann Intern Med 2000; 133:128–35. [DOI] [PubMed] [Google Scholar]
- 21.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013; 309:2345–52. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz DN, Abiad H, DeMarais PL, et al. An educational intervention to improve antimicrobial use in a hospital-based long-term care facility. J Amer Geriatr Soc 2007; 55:1236–42. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao CJ, Hing E. Use and characteristics of electronic health record systems among office-based physician practices: United States, 2001–2012. Available at: http://www.cdc.gov/nchs/data/databriefs/db111.htm Accessed 18 June 2014. [PubMed]
- 24.Mathematica Policy Research, Harvard School of Public Health, Robert Wood Johnson Foundation. Health information technology in the United States 2013: better information systems for better care. DesRoches CM, Painter MW, Jha AK, eds. Princeton, NJ: Robert Wood Johnson Foundation, 2013. Available at: http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf406758 Accessed 18 June 2014. [Google Scholar]
- 25.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338:232–8. [DOI] [PubMed] [Google Scholar]
- 26.Hermsen ED, VanSchooneveld TC, Sayles H, Rupp ME. Implementation of a clinical decision support system for antimicrobial stewardship. Infect Control Hosp Epidemiol 2012; 33:412–5. [DOI] [PubMed] [Google Scholar]
- 27.Kullar R, Goff DA, Schulz LT, et al. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis 2013; 57:1005–13. [DOI] [PubMed] [Google Scholar]
- 28.Pogue JM, Potoski BA, Postelnick M, et al. Bringing the “power” to Cerner's PowerChart for antimicrobial stewardship. Clin Infect Dis 2014; 59:416–24. [DOI] [PubMed] [Google Scholar]
- 29.Moody J, Cosgrove SE, Olmsted R, et al. Antimicrobial stewardship: a collaborative partnership between infection preventionists and health care epidemiologists. Am J Infect Control 2012; 40:94–5. [DOI] [PubMed] [Google Scholar]
- 30.Edwards R, Drumright L, Kiernan M, Holmes A. Covering more territory to fight resistance: considering nurses’ role in antimicrobial stewardship. J Infect Prev 2011; 12:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]