Abstract
Background
Fibromyalgia, the classic non-inflammatory pain syndrome, has been associated with chronic inflammatory makers which are linked with increased morbidity and mortality. We tested the primary hypothesis that patients with fibromyalgia undergoing hospital procedures have a high risk of cardiovascular complications. Our secondary goals were to evaluate the association of fibromyalgia with: (i) in-hospital thromboembolic events, (ii) in-hospital mortality, and (iii) in-hospital microvascular complications.
Methods
We obtained 21.78 million discharge records from 2009 to 2010 from the US Agency for Healthcare Research and Quality censuses across the seven states. We matched fibromyalgia records and compared records with controls based on age, gender, state of discharge, principal procedure, and a propensity score developed from the set of diagnosis-related predictors. A multivariable logistic regression was used to compare matched fibromyalgia patients and controls on the primary and secondary outcomes.
Results
We matched 89 589 pairs for a total sample size of 179 178 discharge records. The adjusted odds ratio for in-hospital cardiovascular complications was 1.04 [99% confidence interval (CI): 0.90–1.19, P=0.51], for thromboembolic events was 1.03 (99% CI: 0.93–1.15, P=0.46), for in-hospital mortality was 0.81 (99% CI: 0.73–0.89, P<0.001), and for microvascular complications was 0.96 (99% CI: 0.88, 1.04, P=0.18). Two separate sensitivity analyses produced results similar to that of the primary analysis for all three complication outcomes.
Conclusions
We found no evidence that the diagnosis of fibromyalgia increased the risk of in-hospital complications. Fibromyalgia seems to be associated with a reduction in in-hospital mortality, but this requires confirmation with a large prospective controlled study.
Keywords: anaesthesia, general; chronic pain; complications, death; risk; surgery, non-cardiac
Editor's key points.
Fibromyalgia has features that may increase perioperative risk.
This retrospective review could not identify an association between fibromyalgia and major complications.
There was an unexpected reduction in mortality in those with fibromyalgia.
A diagnosis of fibromyalgia may lead to treatments that otherwise reduce perioperative risk, but an alternative explanation is unmeasured or residual confounding.
Fibromyalgia is a debilitating pain syndrome characterized by widespread generalized pain, affecting 2% of the US population and more than 3% of women totalling to ∼5 million adult cases.1 Although the term fibromyalgia was first introduced in 1976,2 the condition is not new. Clinical manifestations of muscular pain were recognized as early as 1592, and identified by the term muscular rheumatism; there have since been numerous refinements in nomenclature as our understanding of this entity evolved.3
Fibromyalgia is classically described as a non-inflammatory pain syndrome, but increased concentrations of inflammatory markers including C-reactive protein,4 interleukin-10, interleukin-8, and tumour necrosis factor-α5 challenge this notion. Chronic inflammation, even at low levels, is associated with heart disease.6 Autonomic dysfunction is common in patients with fibromyalgia,7 and includes postural orthostatic tachycardia syndrome8 and abnormal heart rate responses during and after exercise.9 Moreover, impaired baroreceptor reflexes in fibromyalgia reduce responsiveness to changes in arterial pressure.10 During episodes of acute stress, hypotension and hypertension may remain unchecked, either of which may lead to cardiac ischaemia by decreased coronary perfusion.
Chronic autonomic dysfunction, including poor cardiac rate control,9,11 is associated with increased risk of coronary artery disease possibly mediated by endothelial dysfunction.12,13 There is evidence to suggest increased arterial wall stiffness and endothelial dysfunction in patients with fibromyalgia, leading to impaired endothelium-mediated vasodilation, thus compromising blood flow.14,15 Endothelial dysfunction, aside from being a risk for cardiac disease, is also linked to thromboembolic events. It is thus not surprising that hypertension, dyslipidaemia, and coronary atherosclerosis are highly associated with fibromyalgia.16,17 Autonomic nervous system hyperactivity and impaired endothelial functions as seen in fibromyalgia may also be associated with end-organ damage to sensitive organs such as the kidney.18 This concept is supported by a study that noted microvascular changes and decreased functioning of the smaller vessels in patients with fibromyalgia.19
There are common underlying processes between fibromyalgia and myocardial infarction, thromboembolic events, impaired microcirculation, all of which can lead to increased mortality, but the clinical relationships have yet to be evaluated. Myocardial infarction risk appears to be increased in patients with various rheumatological diseases, notably systemic lupus erythematosus, which shares clinical features with fibromyalgia.20 However, the association between fibromyalgia and cardiovascular complications has yet to be established.
Our primary goal was thus to evaluate the association between fibromyalgia and cardiovascular complications in the perioperative period. Specifically, we tested the primary hypothesis that patients with fibromyalgia have a higher risk of postoperative myocardial infarctions. Our secondary goals were to evaluate the association of fibromyalgia with: (i) in-hospital thromboembolic events characterized by deep vein thrombosis, pulmonary embolism, pulmonary infarction, transient ischaemic attack, and stroke; (ii) in-hospital mortality; and (iii) microvascular complications characterized by impaired renal function and impaired wound healing.
Methods
Under authorization by the US Agency for Healthcare Research and Quality, censuses of inpatient hospital discharge data across the following seven states were obtained: Arizona, California, Florida, Iowa, Maryland, Michigan, and New Jersey.21 A total of 21.78 million discharge records from 2009 to 2010 were reviewed. Discharge data included basic patient characteristics such as age and gender, diagnosis codes with present-on-admission (POA) indicators, and procedure codes. All diagnosis and procedure codes were based on the International Classification of Diseases and Injuries, Version 9, Clinical Modification coding system.
We excluded medical visits (as defined by zero procedures performed) and visits associated with patients aged <40 yr. Fibromyalgia was identified using the POA diagnosis code 729.1 (myalgia and myositis, unspecified). We considered year of discharge, state of discharge, gender, age, principal procedure code, and all POA diagnosis codes (excluding that specified above for fibromyalgia) as potential confounding variables.
Diagnosis and procedure codes are hierarchical in nature. Each diagnosis code is represented by a maximum of five digits and each procedure code is represented by a maximum of four digits. Truncating trailing digits thus results in an aggregation of detailed diagnoses (or procedures) to a more general diagnosis (or procedure) class. For example, the diagnosis code 550.03 represents bilateral recurrent inguinal hernia with gangrene, while 550.0 represents all inguinal hernias with gangrene (unilateral, bilateral, or unspecified), and 550 represents all inguinal hernias. Five-digit diagnosis codes are thus more sparsely represented than three-digit codes—some to the extent that they can potentially introduce stability issues relating to small cell sizes during estimation of regression models. Therefore, in encoding baseline diagnosis-related predictors for analysis, we aggregated POA diagnoses if they were represented by fewer than 50 000 patients (which represented 0.56% of the patients meeting study inclusion criteria). Coupled with an assumed fibromyalgia incidence of ∼1%, this implied a minimum cell size of about 500 discharges for any predictor in our propensity model. Diagnoses not meeting the minimum cell size criterion were truncated down to a minimum of three digits, and three-digit diagnosis codes still not meeting the criterion were removed. Likewise, patients' primary procedures were aggregated from four digits to a minimum of two digits.
Cardiovascular, thromboembolic, and microcirculatory complication outcomes were encoded from the diagnosis codes not recorded as POA. In-hospital mortality was available as a binary indicator variable in the discharge record. Neither mortality nor any of the complication outcomes were mutually exclusive; therefore, a record may have more than one outcome. However, multiple events in the same outcome category would only result in one outcome.
Statistical analysis
We matched fibromyalgia discharges to a single control discharge before modelling. Matches were based on age, gender, state of discharge, (aggregated) principal procedure, and a propensity score developed from the set of aggregated POA diagnosis-related predictors. Propensity scores (i.e. the estimated probability of having fibromyalgia based on patients' other POA diagnoses) were estimated using elastic net logistic regression.22,23
Elastic net logistic regression is a ‘shrinkage’ methodology, wherein the overall size of fitted model coefficients is purposely biased towards zero in order to maximize predictive accuracy in external samples. In short, coefficients pertaining to highly correlated diagnoses tend to be averaged together, while coefficients pertaining to irrelevant diagnoses are ‘shrunk’ entirely to zero, effectively removing the diagnosis from the model altogether. The R statistical software (The R Foundation for Statistical Computing, Vienna, Austria) package ‘glmnet’ was used to fit the propensity model from the aggregated POA diagnosis-related predictors.24
Successful matched pairs were restricted to those with common gender, common state of discharge, common principal procedure, a difference in age of <5 yr, and a difference in diagnosis-based propensity score of <0.01. We used a greedy distance-based algorithm for the matching;25 that is, observations were randomly ordered and for each successive fibromyalgia discharge in the data set, the nearest qualifying discharge without fibromyalgia according to age and propensity score was selected as the matched control.
After matching, the balance between fibromyalgia and normal controls on the aforementioned baseline variables (including all diagnosis-related predictors) was assessed using standard univariable summary statistics (means and standard deviations, medians and quartiles, or proportions, as appropriate) and standardized difference scores (defined as the difference in means, mean rankings, or proportions divided by a combined estimate of standard deviation). Any baseline variable exhibiting a standardized difference score >0.05 in absolute value was used for adjustment within our final models comparing matched patients on outcomes.
For the final models, multivariable logistic regression was used to compare matched fibromyalgia patients and controls on risk of in-hospital cardiovascular complications, thromboembolic complications, microcirculatory complications, and mortality (after adjustment for any imbalanced baseline variables according to the definition above). Adjusted odds ratios (ORs) and 99% confidence intervals (CIs) for each outcome were derived from these models. Respective hypotheses of independent association between fibromyalgia and each outcome were evaluated using model-based Wald tests with type I error rate (α) fixed at 0.01.
To evaluate the dependence of the observed relationship on diagnoses, we conducted post hoc sensitivity analyses. We utilized a smaller absolute standardized difference criterion of 0.02 in order to include more variables for adjustment. An additional sensitivity analysis was performed that excluded all baseline diagnoses (but did adjust for state of admission, gender, age, and procedure) using all 8.33 million discharge records.
Results
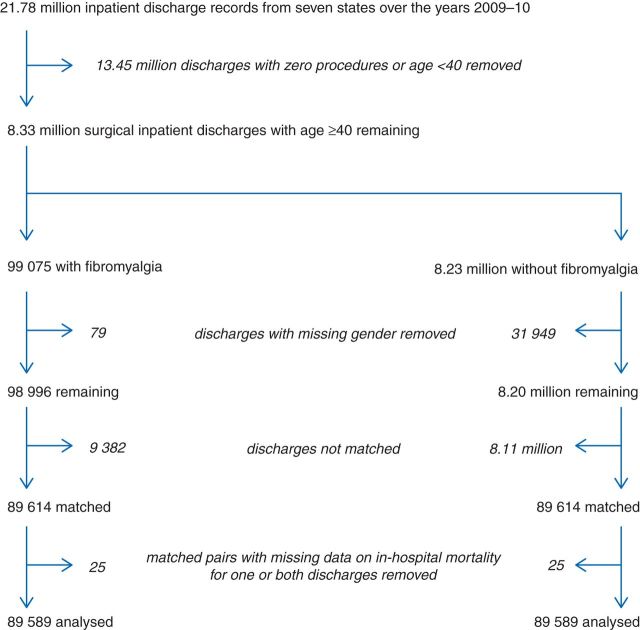
Of the 21.78 million discharges included in the statewide censuses, 8.33 million met study inclusion criteria (Fig. 1). There were a total of 82.94 million POA diagnosis codes in the data set, for an average of 10.0 per discharge [median (quartiles) number of diagnoses: 7 (3, 11)]. Overall, 99 075 discharges (1.19%) contained a POA diagnosis code for fibromyalgia. By gender, this incidence was estimated at 10 026/3.92 million (0.26%) for males and at 88 970/4.38 million (2.03%) for females. Among patients discharged in 2009, the incidence was 46 964/4.16 million (1.13%), while in 2010, it was 52 111/4.18 million (1.25%). State-level discharge summaries are given in Table 1.
Fig 1.
Study flow diagram.
Table 1.
Summary of discharges meeting study inclusion/exclusion criteria by state of discharge
| State | Met inclusion criteria (n) | Sample (%) | Diagnosis of fibromyalgia [n (%)] | Fibromyalgia population (%) |
|---|---|---|---|---|
| Arizona | 585 893 | 7.0 | 10 619 (1.8) | 10.7 |
| California | 2 886 641 | 34.6 | 30 238 (1.0) | 30.5 |
| Florida | 2 014 279 | 24.2 | 25 601 (1.3) | 25.8 |
| Iowa | 259 268 | 3.1 | 3889 (1.5) | 3.9 |
| Maryland | 593 418 | 7.1 | 7135 (1.2) | 7.2 |
| Michigan | 1 071 634 | 12.9 | 15 137 (1.4) | 15.3 |
| New Jersey | 922 330 | 11.1 | 6456 (0.7) | 6.5 |
| Total | 8 333 463 | 100.0 | 99 075 (1.2) | 100.0 |
Aggregation of the POA diagnosis codes resulted in 466 distinct diagnosis-related covariates (each with >50 000 discharges represented). Among the 82.94 million total POA diagnosis codes, 75.06 million (90.5%) were successfully mapped. The propensity score model based on these diagnosis-related covariates discriminated between normal and fibromyalgia discharges moderately well, with a C-statistic (area under the receiver operating characteristic curve) of 0.83.
Before matching, 32 028 discharges (0.38%) were removed due to lack of gender information (79 of which had a POA fibromyalgia code). The matching procedure yielded successful controls for 89 614 of the remaining 98 996 fibromyalgia discharges (90.5%). Twenty-five matched pairs had at least one discharge with missing data on in-hospital mortality; these were removed from further consideration and therefore our final matched sample contained 89 589 matched pairs (i.e. a total sample size of n=179 178). Balance was excellent on all preoperative variables (Table 2 and Supplementary Appendix)—only two of the 466 diagnosis-related predictors (600.00 hyperplasia of prostate and 427.31 atrial fibrillation) and none of the other preoperative characteristics displayed a standardized difference score of >0.05 between the groups.
Table 2.
Balance of select preoperative characteristics among matched fibromyalgia and control discharges. A complete listing of all 466 baseline diagnosis-related characteristics is provided in the Supplementary Appendix. *ASD, absolute standardized difference score. The ASD is equal to the difference in means, mean rankings, or proportions divided by a combined estimate of standard deviation. Any baseline variable exhibiting a standardized difference score >0.05 in the absolute value was used for adjustment within our final models comparing matched patients on outcomes
| Control (n=89 589) | Fibromyalgia (n=89 589) | ASD* | |
|---|---|---|---|
| Patient characteristic and surgical characteristics | |||
| Surgery in 2010 (vs 2009) | 51.28 | 52.32 | 0.021 |
| Age (yr) | 61 (40–100) | 61 (40–100) | 0.021 |
| Female gender | 89.8 | 89.8 | 0.000 |
| State | |||
| Arizona | 10.3 | 10.3 | 0.000 |
| California | 31.4 | 31.4 | |
| Florida | 26.2 | 26.2 | |
| Iowa | 3.7 | 3.7 | |
| Maryland | 6.9 | 6.9 | |
| Michigan | 15.1 | 15.1 | |
| New Jersey | 6.3 | 6.3 | |
| Top 15 diagnosis-related characteristics (ordered by descending ASD) | |||
| 600.00 Hypertrophy of prostate without urinary obstruction and other lower urinary tract symptoms (LUTS), benign | 0.5 | 1.0 | 0.062 |
| 427.31 Atrial fibrillation | 6.0 | 7.3 | 0.051 |
| 338.29 Chronic pain, other | 2.6 | 1.9 | 0.047 |
| 338.4 Chronic pain syndrome | 4.5 | 5.5 | 0.046 |
| 414.01 Coronary atherosclerosis, native coronary artery | 14.2 | 15.8 | 0.045 |
| V45.81 Aortocoronary bypass, status post | 2.3 | 2.9 | 0.042 |
| 414.00 Coronary atherosclerosis, unspecified type of vessel, native or graft | 2.8 | 3.5 | 0.041 |
| 244.9 Hypothyroidism, unspecified | 22.1 | 20.4 | 0.041 |
| 493.90 Asthma, without status asthmaticus | 11.0 | 9.8 | 0.040 |
| 715.90 Osteoarthrosis, unspecified | 10.0 | 11.1 | 0.038 |
| V45.82 Percutaneous transluminal coronary angioplasty, status post | 4.1 | 4.9 | 0.036 |
| 278.01 Morbid obesity | 10.1 | 9.1 | 0.034 |
| V88.01 Acquired absence of both the cervix and uterus | 3.4 | 2.8 | 0.032 |
| 274.9 Gout, unspecified | 1.3 | 1.7 | 0.031 |
| 278.00 Obesity, unspecified | 12.4 | 11.4 | 0.030 |
Seven hundred and one (0.78%) of the matched fibromyalgia discharges and 669 (0.75%) of the matched controls had associated in-hospital cardiovascular complications (Table 3). Based on the multivariable logistic regression model for this outcome, the adjusted OR was 1.04 (99% CI: 0.90, 1.19), which was not statistically significant (P=0.51, Wald's test).
Table 3.
Summary of outcomes among n=179 178 matched discharges (89 589 in each group). *ORs adjusted for hyperplasia of prostate (600.00) and atrial fibrillation (427.31); †The Type I error rate (alpha) for all tests and confidence intervals (CIs) was 0.01. All other baseline variables exhibited a standardized difference score <0.05 in absolute value after matching
| Control [n (%)] | Fibromyalgia [n (%)] | Adjusted OR (99% CI)*,† | P-value† | |
|---|---|---|---|---|
| Cardiovascular outcomes | ||||
| 997.1 Cardiac arrest/insufficiency during or resulting from a procedure | 344 (0.38) | 351 (0.39) | ||
| 411.81 Acute coronary occlusion without myocardial infarction | 4 (0) | 2 (0) | ||
| 518.4 Pulmonary oedema, postoperative | 49 (0.05) | 65 (0.07) | ||
| 410 Myocardial infarction | 319 (0.36) | 324 (0.36) | ||
| Any of the above cardiovascular complications | 669 (0.75) | 701 (0.78) | 1.04 (0.90, 1.19) | 0.51 |
| Thromboembolic outcomes | ||||
| 453.4 Venous embolism and thrombosis of unspecified deep vessels of lower extremity | 211 (0.24) | 191 (0.21) | ||
| 451.83 Venous embolism and thrombosis of unspecified deep vessels of upper extremities | 2 (0) | 4 (0) | ||
| 453 Other venous embolism and thrombosis | 443 (0.49) | 423 (0.47) | ||
| V12.51 Pulmonary embolism | 294 (0.33) | 376 (0.42) | ||
| 415.1 Pulmonary embolism and infarction | 185 (0.21) | 166 (0.19) | ||
| 997.02 Postoperative stroke | 44 (0.05) | 47 (0.05) | ||
| 435 Temporary ischaemic attack | 45 (0.05) | 41 (0.05) | ||
| 434 Occlusion of cerebral arteries | 168 (0.19) | 156 (0.17) | ||
| 997.2 Phlebitis or thrombophlebitis during or resulting from a procedure | 77 (0.09) | 70 (0.08) | ||
| 997.7 Vascular complication of surgical and medical care | 11 (0.01) | 14 (0.02) | ||
| Any of the above thromboembolic complications | 1130 (1.26) | 1178 (1.31) | 1.03 (0.93, 1.15) | 0.46 |
| Microcirculatory outcomes | ||||
| 998.3 Dehiscence of operation wound | 139 (0.16) | 90 (0.1) | ||
| 997.5 Renal insufficiency/failure (acute) specified as due to procedure | 216 (0.24) | 204 (0.23) | ||
| 584.9 Acute kidney injury | 1484 (1.66) | 1482 (1.65) | ||
| 593.9 Acute renal disease | 209 (0.23) | 189 (0.21) | ||
| Any of the above microcirculatory complications | 1976 (2.21) | 1923 (2.15) | 0.96 (0.88, 1.04) | 0.18 |
| In-hospital mortality | 1446 (1.61) | 1200 (1.34) | 0.81 (0.73, 0.89) | <0.001 |
For the secondary outcomes, we found no difference in the odds of in-hospital thromboembolic complications [adjusted OR 1.03 (0.93, 1.15); P=0.46] or in the odds of in-hospital microcirculatory complications [OR 0.96 (0.88, 1.04); P=0.18]. On the other hand, fibromyalgia was associated with reduced risk of in-hospital mortality. Mortality was recorded for 1200 (1.34%) of the matched fibromyalgia discharges and 1446 (1.61%) of the controls. The adjusted OR was 0.81 (0.73, 0.89), which was statistically significant (P<0.001).
Our first sensitivity analysis—which used a smaller absolute standardized difference cut-off of 0.02 for deciding which baseline variables were included for adjustment in our multivariable models—identified 50 baseline covariates. Adjusting for these variables, results were nearly identical to those obtained in our primary analysis. For instance, adjusted OR estimates for cardiovascular complications and mortality were 1.02 (0.89, 1.18) and 0.81 (0.73, 0.90), respectively.
Our second sensitivity analysis—which used all 8.33 million patients and did not adjust for other diagnoses—produced results that were largely similar to that of the primary analysis for all three complication outcomes (Table 4). For mortality, the adjusted OR estimate was 0.56 (0.52, 0.61), which was lower than the adjusted OR obtained in the primary matched analysis.
Table 4.
Summary of outcomes among n=8.33 million patients not adjusted for other diagnoses
| Adjusted OR (99% CI) | P-value | |
|---|---|---|
| Cardiovascular outcomes | 0.91 (0.82, 0.99) | 0.006 |
| Thromboembolic outcomes | 0.99 (0.92, 1.07) | 0.74 |
| Microcirculatory outcomes | 0.93 (0.88, 0.98) | 0.001 |
| In-hospital mortality | 0.56 (0.52, 0.61) | <0.001 |
Discussion
In contrast to our expectations, the incidence of postoperative myocardial infarction and other cardiovascular events in fibromyalgia patients was similar to other patients. This result is surprising given a previous study which found that the physical health status of fibromyalgia patients was worse than patients with a history of hypertension, acute myocardial infarction, diabetes, or congestive heart failure.26 Furthermore, a previous prospective cohort study in the non-operative period found a trend towards increased cardiac deaths in 761 widespread chronic pain patients, although it did not reach significance.27 Our study of 89 589 fibromyalgia patients in the perioperative period still failed to show an increase in cardiovascular events.
There was also no statistically significant difference in thromboembolic events in patients with and without fibromyalgia. Lack of increased thromboembolic events may be due to the fact that patients with and without fibromyalgia were matched for potential confounders including a history of coronary angioplasty and cardiac bypass—both of which are of vascular origin and mediators of thrombosis. To the extent that thromboembolic events are co-linear (i.e. occur in the same patients) with vascular diseases, a true increase in thromboembolic events in fibromyalgia patients may have been ‘adjusted away’ in our multivariable analysis.
Another surprising result is that mortality was significantly lower, by 19%, in patients with the diagnosis of fibromyalgia. This perioperative result contrasts with a previous study which reported comparable long-term mortality in fibromyalgia patients, but increased death from accident or suicide (neither of which contributed in our postoperative patients).27 A potential explanation is that patients with fibromyalgia are disproportionately given protective treatments that reduce mortality,28 including statins and antihypertensive medications, and perhaps life-style modifications. An alternative explanation is that fibromyalgia, restricted to the subgroup population in the perioperative period, induces a collider conditioning effect resulting in a protective association through selection bias.29
Another potential explanation is that our retrospective analysis depends critically on diagnostic billing codes because we matched patients with and without fibromyalgia for concurrent conditions. A potential difficulty is that fibromyalgia is difficult to diagnose; for example, the average time from initial physician presentation to diagnosis was 2.3 yr after seeing over three separate physicians.30 When disability is involved, it takes an average of 4 yr and visits to six different physicians to establish the diagnosis.31 Furthermore, patients with the condition require considerable medical care. Both factors augment the chances of these patients carrying many diagnoses, and therefore many diagnostic codes.
The consequence is that patients with fibromyalgia may carry more diagnostic codes than comparably sick patients without fibromyalgia. This means that after propensity matching (which is largely based on diagnostic codes), fibromyalgia patients identified as being at comparable risk may actually be healthier. That healthier patients have reduced mortality is natural, and would result in an apparent (but false) protective effect of fibromyalgia.
To address this concern, we conducted an important sensitivity analysis which did not adjust for comorbidities, thus removing the possible influence of over-diagnosis leading to decreased mortality. The result of this analysis indicated an even stronger association between fibromyalgia and decreased mortality, the opposite of what would be expected if the outcome was attributed to the directional bias of over-diagnosis. Over-diagnosis of potential confounding diagnoses thus does not explain our results.
There was a change in the diagnostic criteria for fibromyalgia in 201032 and our sample epoch, 2009–2010, included part of this transition. The new diagnostic criteria extend beyond multiple pain locations and incorporate cognitive and somatic symptoms. Our propensity-matched analysis included year of surgery, but we do not know the extent to which each criterion was applied to individual patients. As in any retrospective analysis, a major concern is that unknown—and therefore unadjusted—factors could substantively influence our conclusions.
Our study is limited by the data elements available in the large data set. The methods of anaesthesia were not available, nor were intraoperative variables, which limit our ability to properly adjust for them. General anaesthesia, blood loss, arterial pressure, heart rate, administered medications, among other unreported factors, possibly could have profoundly impacted the results of this study. Diagnosis codes were dichotomous variables; therefore, we can only adjust for the presence of a disease, but not the severity. Confounding may have also influenced the results; for instance, patients with fibromyalgia may have received more opioids masking the signs of myocardial infarction and decreasing its detection.
Our study is strengthened by the inclusion of more than 89 000 matched pairs across seven states broadly representing the US population. We used a novel approach in adjusting for patient's diagnoses, namely deriving aggregated diagnosis-related predictors based on a minimum cell size criterion and developing a diagnosis-based propensity score for fibromyalgia, resulting in a comprehensive diagnosis adjustment. We adjusted for confounding variables by strictly matching on procedure, gender, state of discharge, age (to within 5 yr), and concurrent diagnoses.
Some might argue that fibromyalgia is a controversial construct given its continual evolution, and that the full scope and implications of the syndrome have yet to be elucidated. Nonetheless, we found no evidence that the diagnosis of fibromyalgia increased the risk of in-hospital complications and, surprisingly, fibromyalgia may be associated with a reduction in in-hospital mortality. The decreased mortality in our hypothesis generating study should be confirmed by further investigations.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Authors' contributions
B.D.H.: study design, data analysis, first draft of paper, critical revision, and final approval of manuscript. J.E.D.: study design, data retrieval, data analysis, first draft of paper, and final approval of manuscript. H.S.: study design, first draft of paper, and final approval of manuscript. P.C.: study design, first draft of paper, and final approval of manuscript. L.S: study design, first draft of paper, critical revision, and final approval of manuscript. D.I.S.: study design, data analysis, first draft of paper, critical revision, and final approval of manuscript. A.T.: study design, data analysis, first draft of paper, critical revision, and final approval of manuscript.
Declaration of interest
None declared.
Funding
This work was supported by internal department funds. J.E.D.'s effort was supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Financial support for all other authors of this investigator initiated work was solely funded by the Outcomes Research Department and the Anesthesiology Institute at the Cleveland Clinic Foundation.
Supplementary Material
Acknowledgements
The authors would like to thank the US Agency for Healthcare Research and Quality for allowing access to the inpatient hospital discharge censuses data. We would also like to sincerely thank Tanya Smith for her editorial assistance.
References
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hench PK. Twenty-second rheumatism review. Review of the American and English literature for the years 1973 and 1974. Arthritis Rheum. 1976;19:973–1223. [PubMed] [Google Scholar]
- 3.Inanici F, Yunus MB. History of fibromyalgia: past to present. Curr Pain Headache Rep. 2004;8:369–78. doi: 10.1007/s11916-996-0010-6. [DOI] [PubMed] [Google Scholar]
- 4.Lund Haheim L, Nafstad P, Olsen I, Schwarze P, Ronningen KS. C-reactive protein variations for different chronic somatic disorders. Scand J Public Health. 2009;37:640–6. doi: 10.1177/1403494809104358. [DOI] [PubMed] [Google Scholar]
- 5.Bazzichi L, Rossi A, Massimetti G, et al. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin Exp Rheumatol. 2007;25:225–30. [PubMed] [Google Scholar]
- 6.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Br Med J. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buskila D. Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res Ther. 2009;11:242. doi: 10.1186/ar2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staud R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep. 2008;10:463–6. doi: 10.1007/s11926-008-0076-8. [DOI] [PubMed] [Google Scholar]
- 9.da Cunha Ribeiro RP, Roschel H, Artioli GG, et al. Cardiac autonomic impairment and chronotropic incompetence in fibromyalgia. Arthritis Res Ther. 2011;13:R190. doi: 10.1186/ar3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes del Paso GA, Garrido S, Pulgar A, Duschek S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res. 2011;70:125–34. doi: 10.1016/j.jpsychores.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 12.Strawn WB, Bondjers G, Kaplan JR, et al. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–9. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- 13.Manuck SB, Kaplan JR, Adams MR, Clarkson TB. Effects of stress and the sympathetic nervous system on coronary artery atherosclerosis in the cynomolgus macaque. Am Heart J. 1988;116:328–33. doi: 10.1016/0002-8703(88)90110-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Cho KI, Kim SM, Lee HG, Kim TI. Arterial stiffness in female patients with fibromyalgia and its relationship to chronic emotional and physical stress. Korean Circ J. 2011;41:596–602. doi: 10.4070/kcj.2011.41.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho KI, Lee JH, Kim SM, Lee HG, Kim TI. Assessment of endothelial function in patients with fibromyalgia—cardiac ultrasound study. Clin Rheumatol. 2011;30:647–54. doi: 10.1007/s10067-010-1599-8. [DOI] [PubMed] [Google Scholar]
- 16.Haviland MG, Banta JE, Przekop P. Fibromyalgia: prevalence, course, and co-morbidities in hospitalized patients in the United States, 1999–2007. Clin Exp Rheumatol. 2011;29:S79–87. [PubMed] [Google Scholar]
- 17.Ablin JN, Beilinson N, Aloush V, Elkayam O, Finkelstein A. Association between fibromyalgia and coronary heart disease and coronary catheterization. Clin Cardiol. 2009;32:E7–11. doi: 10.1002/clc.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–76. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 19.Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209–16. doi: 10.1186/ar1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 21.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2013. February. Available from www.hcup-us.ahrq.gov/sidoverview.jsp . [PubMed] [Google Scholar]
- 22.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005;67:301–20. [Google Scholar]
- 23.Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd Edn. New York, NY: Springer; 2009. [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Sekhon JS. Multivariate and propensity score matching software with automated balance optimization: the matching package for R. J Stat Softw. 2011;42:1–52. [Google Scholar]
- 26.Hoffman DL, Dukes EM. The health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12. Int J Clin Pract. 2008;62:115–26. doi: 10.1111/j.1742-1241.2007.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe F, Hassett AL, Walitt B, Michaud K. Mortality in fibromyalgia: a study of 8,186 patients over thirty-five years. Arthritis Care Res. 2011;63:94–101. doi: 10.1002/acr.20301. [DOI] [PubMed] [Google Scholar]
- 28.Scherrer JF, Garfield LD, Lustman PJ, et al. Antidepressant drug compliance: reduced risk of MI and mortality in depressed patients. Am J Med. 2011;124:318–24. doi: 10.1016/j.amjmed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Banack HR, Kaufman JS. The ‘obesity paradox’ explained. Epidemiology. 2013;24:461–2. doi: 10.1097/EDE.0b013e31828c776c. [DOI] [PubMed] [Google Scholar]
- 30.Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. doi: 10.1186/1472-6963-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzcharles MA, Ste-Marie PA, Shir Y. A medicolegal analysis of worker appeals for fibromyalgia as a compensable condition following workplace soft-tissue injury. J Rheumatol. 2013;40:323–8. doi: 10.3899/jrheum.121062. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.