Abstract
Consolidation of motor skills after training can occur in a time- or sleep-dependent fashion. Recent studies revealed time-dependent consolidation as a common feature of visuomotor tasks. We have previously shown that anodal transcranial direct current stimulation (tDCS) in combination with repeated motor training benefits consolidation by the induction of offline skill gains in a complex visuomotor task, preventing the regular occurrence of skill loss between days. Here, we asked 2 questions: What is the time course of consolidation between days for this task and do exogenously induced offline gains develop as a function of time or overnight sleep? We found that both the development of offline skill loss in sham-stimulated subjects and offline skill gains induced by anodal tDCS critically depend on the passage of time after training, but not on overnight sleep. These findings support the view that tDCS interacts directly with the physiological consolidation process. However, in a control experiment, anodal tDCS applied after the training did not induce skill gains, implying that coapplication of tDCS and training is required to induce offline skill gains, pointing to the initiation of consolidation already during training.
Keywords: cortical excitability, motor skill learning, noninvasive brain stimulation, warm-up decrement
Introduction
Motor skills are crucial for our everyday life, from human relations to occupations to sports. Thus, strategies to improve motor skill learning are of great general and clinical interest. Losing a motor skill, for example, due to brain injury or neurological disease, significantly reduces autonomy and quality of life and requires intense, expensive neurorehabilitation. Motor skills are acquired through training and rely on effective consolidation, that is, stabilization or enhancement of the motor memory obtained during training (Robertson, Pascual-Leone, Miall 2004; Dayan and Cohen 2011). Consolidation depends on several factors, such as the task utilized, the instruction given to the subject, and the type of motor memory (Robertson, Pascual-Leone, Miall 2004). While explicit sequential finger-tapping tasks in most cases show skill gains after a posttraining period that includes sleep (Walker et al. 2002; Korman et al. 2003; Fischer et al. 2005; Debas et al. 2010), consolidation of implicit motor sequence learning (as offline gains) depends on the passage of time, regardless of wakefulness or sleep (Robertson, Pascual-Leone, Press 2004; Song et al. 2007; Hotermans et al. 2008). Similar to implicit sequence learning, consolidation of visuomotor adaptation tasks is time-dependent (Criscimagna-Hemminger and Shadmehr 2008; Doyon et al. 2009; Debas et al. 2010). A consolidation time window of approximately 6 h has been defined using interference studies, in which learning of a second task was used to disturb the consolidation of the first task (Brashers-Krug et al. 1996; Krakauer et al. 2005; Krakauer and Shadmehr 2006). In brief, when the time interval between the training of task A and task B was extended, the interference effect of task B became smaller and finally disappeared, suggesting a time-dependent consolidation of task A.
Research on the consolidation of continuous skill tasks is relatively sparse. For a visuospatial finger-tracking skill task in which a position had to be accurately tracked at fixed movement speed of the target, similar offline gains were found after a 12-h period independent of wakefulness or sleep, also supporting time-dependent consolidation (Borich and Kimberley 2011). In the present study, we use a previously characterized complex, continuous sequential visual isometric pinch force skill task (SVIPT, Camus et al. 2009; Reis et al. 2009; Fritsch et al. 2010), for which skill is acquired by shifting the speed–accuracy–tradeoff function (in other words both speed and accuracy are dependent variables which can improve). For this task's consolidation, the default mode seems to be memory stabilization, with a slight offline decrease in skill between training days. Previously, we have interpreted this skill loss as a warm-up decrement. The warm-up decrement has been reported for several continuous motor skill tasks (Adams 1952; Catalano 1978; Anshel 1995; Etnyre and Poindexter 1995; Stratton et al. 2010). There has been a debate to which extent this behavioral phenomenon represents a “loss of activity set” (in factors not directly related to the motor memory itself, e.g. vision, posture, and attention) or forgetting of the motor skill memory (Adams 1961; Nacson and Schmidt 1971; Schmidt and Lee 2005; Stratton et al. 2010). Since continuous skill tasks can show large warm-up decrements, despite skill being generally retained over long periods of time, the loss of activity-set hypothesis is currently favored (Adams 1952, 1961; Schmidt and Lee 2005; Stratton et al. 2010). This is supported by the fact that reinstating the set has a time scale faster than that of motor memory stabilization, in other words only a few trials are needed to reestablish motor skill at previous levels (Schmidt and Lee 2005; Stratton et al. 2010).
In the last decades, transcranial direct current stimulation (tDCS) has been frequently used to transiently modulate cortical excitability (Nitsche and Paulus 2000; Nitsche et al. 2008). Anodal tDCS can promote different aspects of motor learning (Reis et al. 2009; Galea et al. 2011; Kantak et al. 2012), which may translate into stable performance improvements for several months (Reis et al. 2009). We have previously shown that anodal tDCS applied to the motor cortex during 5 days of training of the SVIPT significantly improves total learning. This effect was due to the induction of positive offline skill gains between sessions in contrast to the offline skill loss observed in sham-stimulated subjects. However, since we did not perform a within-day retest, the physiological course of the consolidation of the SVIPT and the point of interaction with tDCS remained unclear.
Understanding the interaction of exogenously applied brain stimulation and the endogenous learning process induced by training is of high relevance to gain further insights into the consolidation process itself, to test for the applicability of noninvasive brain stimulation to improve motor deficits, and to predict effects in different subject populations, for example, elderly and/or neurological patients. Hence, in the present study, we asked 2 questions: What is the physiological time course of consolidation between days for this task, and do exogenously (tDCS) induced offline gains depend on the passage of time or overnight sleep. We predicted that the SVIPT is consolidated in a time-dependent fashion, in accordance with other visuomotor tasks. Furthermore, given the well-described outlasting excitability increase attributed to anodal tDCS (Nitsche and Paulus 2001) and the long term potentiation induction by anodal DCS combined with a second input (mimicking training) in vitro (Fritsch et al. 2010), we expected training combined with anodal tDCS to promote offline gains at some time after training, in the absence of sleep.
Materials and Methods
Subjects
One hundred and fifteen healthy subjects between 18 and 53 years were invited to participate in this study. A lack of acute or chronic neurological or psychiatric disease, sleep disorder, or any severe medical condition, and lack of drug or alcohol intake (except tobacco and caffeine) in the 24 h prior to or at the time of testing, was required. None of the participants had taken part in tDCS experiments before, and all of them were naïve to the motor task utilized. All subjects gave written informed consent to participate in the study according to the Declaration of Helsinki. The study was approved by the Ethics Committee of the University of Freiburg. Ten percent of the participants were tested by the same investigator (J.R.) under an IRB approved protocol at the NINDS, NIH before transferring the project to Freiburg.
Study Design and Motor Task
All subjects practiced the SVIPT (Reis et al. 2009) with their right, dominant hand over 3 consecutive days for approximately 40 min per day (160 trials/day, separated into 5 blocks, Fig. 1). In brief, subjects squeezed a force transducer between thumb and the index finger of the right hand to control a horizontal screen cursor movement (more force = more rightward movement). Upon presentation of a GO signal, subjects had to squeeze and release the force transducer to move the cursor as quickly and as accurately as possible back and forth between a start position (fingers relaxed) and a numbered order of gates (Home-1–Home-2–Home-3–Home-4–Home-5). Transduction of pinch force into cursor movement was logarithmic, with the maximum rightward movement set to 35–45% of maximum force.
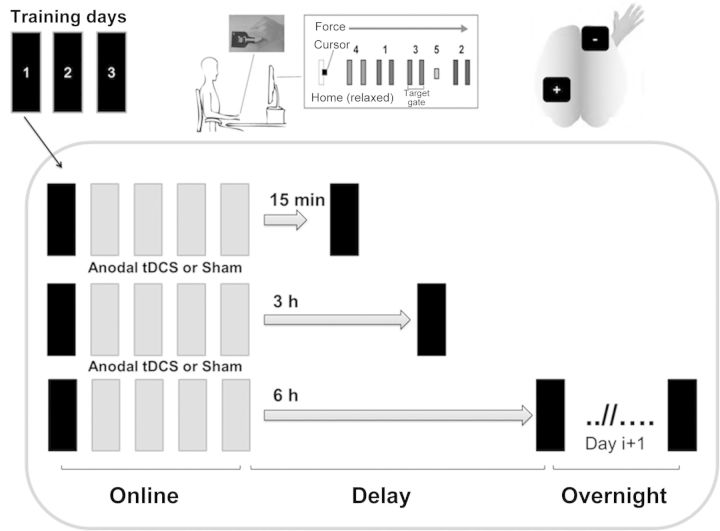
Figure 1.
Schematic overview of the study design and measurement of subcomponents of learning. Three training days are depicted (upper, left corner). Subjects practice the SVIPT (upper, middle). For tDCS, a classical montage was chosen with the anode over the left M1 and the cathode on the right supraorbital area (upper, right corner). Every training day consisted of 5 training blocks and a retest block at the end of the delay period. The first training block per day and the retest block were always tested in the absence of sham or anodal tDCS.
Time of training (between 8 AM and 2 PM) and retest, training environment, and investigator were kept constant for individuals in all sessions. After the first training block per day, anodal tDCS or sham tDCS was applied for 20 min to the left motor cortex (M1) contralateral to the training hand in a double-blind fashion. Allocation to a stimulation condition (sham/anodal tDCS) followed a fully balanced randomization list prepared prior to the experiment. The rationale to start stimulation after block 1 was to keep the first training block free of online stimulation effects for the analysis of overnight skill gains. Subjects continued training during tDCS. Given that every training block took approximately 4 min, and blocks were separated by a 90-s break to avoid fatigue, training outlasted stimulation duration for approximately 2 min. To assess the effect of tDCS applied during training on the subsequent consolidation process, a within-day delayed retest (40 trials, no tDCS) was completed every day, in the absence of sleep. Subjects were retested either 15 min, 3 h, or 6 h after training, resulting in 6 separate groups (“sham 15 min, anodal 15 min, sham 3 h, anodal 3 h, sham 6 h, and anodal 6 h”; see Table 1). Randomization into a particular retest group (15 min, 3 h, and 6 h) was prearranged with the participants for logistical reasons. Motor skill changes occurring in the delay period (no sleep) were then compared with motor skill changes occurring overnight (including night sleep). During the retest period, all subjects remained awake (resting, or doing simple activities of daily living such as reading, eating, listening to lectures, lab work etc.).
Table 1.
Demographics
| Sham 15 min | Anodal 15 min | Sham 3 h | Anodal 3 h | Sham 6 h | Anodal 6 h | Anodal after | P (group) | |
|---|---|---|---|---|---|---|---|---|
| No. of subjects | 17 | 17 | 17 | 17 | 16 | 16 | 11 | |
| Age (years) | 27.2 ± 2 | 27.1 ± 1 | 29.2 ± 2 | 28.1 ± 2 | 30.5 ± 3 | 28.4 ± 3 | 29.6 ± 2 | 0.829 |
| Gender (M:F) | 9:8 | 7:10 | 7:10 | 7:10 | 7:9 | 7:9 | 5:6 | 0.994 |
| Handedness (laterality) | 93.5 ± 3 | 94.4 ± 3 | 93.2 ± 3 | 93.2 ± 4 | 91.3 ± 3 | 97.5 ± 2 | n.a. | 0.624 |
| Average sleep (h) | 7.2 | 7.2 | 7.1 | 7.1 | 7.0 | 7.3 | 7.2 | 0.948 |
| Baseline skill | −1.6 | −1.7 | −1.8 | −1.8 | −1.8 | −1.9 | −1.6 | 0.932 |
Note: Data are given as group mean (±SEM). Baseline skill is a negative unit-less measure, calculated by the mathematical model introduced in Reis et al. (2009).
In a “control experiment”, anodal tDCS was applied immediately “after” completion of the 15-min retest, to test whether simultaneous application of tDCS during training was a requirement for the occurrence of delayed offline gains or whether a posttraining excitability increase alone would be sufficient to boost consolidation. Results of this group (“anodal after”) were compared with that of the sham–tDCS group retested after 15 min every day (“sham 15 min”).
Transcranial Direct Current Stimulation
First, the left M1 hand area (“hot spot” for the first dorsal interosseus muscle) was localized in all subjects with transcranial magnetic stimulation using a Magstim 200 stimulator and a 7-cm figure-of-eight coil (Magstim, Dyfed, UK). tDCS was applied in a double-blind fashion using a Neuroconn DC-Stimulator plus (Neuroconn, Ilmenau, Germany) connected to 2 carbon electrodes covered by a 16-cm2 (4 × 4 cm) sponge soaked in saline solution. Anodal tDCS was delivered at a current density of 0.062 mA/cm2 (equal to 1 mA/16 cm2) to the left primary motor cortex. The cathode was placed over the contralateral supraorbital area. A ramp-up and -down period of 15 s was used at the beginning and end of tDCS to avoid discomfort or phosphenes. In the sham tDCS condition, the current was ramped up then down over 30 s (Gandiga et al. 2006). Blinding of subjects and investigator was managed through a preprogrammed “study mode” of the tDCS device (a numeric code is entered for each subject, and no stimulation details are presented).
Screening and Assessment Instruments
The Edinburgh Handedness Inventory was used to assess hand dominance (Oldfield 1971). Only right-handed participants with clear laterality (laterality index >70) were selected for participation. All participants were screened for contraindications to noninvasive brain stimulation techniques using the Transcranial Magnetic Stimulation Adult Safety Screen (Keel et al. 2001) with extra questions for tDCS, for example, about skin lesions.
Before and after the motor training course subjects completed the positive and negative affect scale (PANAS, Watson et al. 1988) in order to assess any effects of training/stimulation on mood or motivation or vice versa. Before each session on every day, participants rated their mental fitness on a visual analog scale (VAS) from 1 to 10 (1 was defined as “perfectly mentally fit” and 10 was “extremely mentally unfit/tired”) and reported their sleep duration the previous night. Subjects were also asked whether any side effects were experienced.
Subcomponents of Skill Learning
To calculate the temporal subcomponents of learning, we assessed skill differences at various time points (Fig. 1). This enabled us to break down the 3-day learning curve into online effects (within session), delayed offline effects (within the delay period), and overnight offline effects (including sleep). We avoid using the term “offline learning” in the remainder of the manuscript since this term has been attributed to both the process of consolidation and a positive direction of induced skill changes.
Online effects were defined as the sum of differences between the first and last training blocks of days 1, 2, and 3 [(Day1Block5 − Day1Block1) + (Day2Block5 − Day2Block1) + (Day3Block5 − Day3Block1)]. Delayed offline effects were defined as the sum of the differences between the retest block (block 6) and the last training block of days 1, 2, and 3 [(Day1Block6 − Day1Block5) + (Day2Block6 − Day2Block5) + (Day3Block6 − Day3Block5)]. Overnight offline effects were defined as the sum of the differences between the first block of days 2 and 3 and the last block of the previous day [(Day2Block1 − Day1Block6) + (Day3Block1 − Day2Block6)]. Total learning was defined as the sum of online and delayed and overnight offline effects, which are mathematically the same as taking the skill difference between the last retest block of day 3 and the baseline block on day 1 (Day3Block6 − Day1Block1).
Data Handling and Statistical Analysis
Raw data analysis (analysis of movement times and errors per trial) was semi-automated using a Matlab programming script (ForceDataViewer® by Ethan Buch, see Reis et al. 2009), which allows for unbiased analysis. The investigator responsible for data processing and analysis (determination of the skill measure) was blinded for the type of stimulation, but not for the delay interval. Baseline skill (Day1Block1) was compared between all experimental groups using a 1-way analysis of variance (ANOVA). We did not expect the retest delay interval to influence online effects or total learning per se. Therefore, online effects and total learning were assessed with “motor skill change” as the dependent variable and a single factor “stimulation type” (sham vs. anodal tDCS) as the independent variable. A post hoc analysis was performed in the groups retested at the same time point. Since motor skill was sampled at different retest time points depending on groups, separate ANOVAs were then performed for the 2 groups retested at the same time point for delayed offline and overnight offline effects. Motor skill change was used as the dependent variable and the factor group (sham, anodal tDCS) as the independent variable. Post hoc pair-wise t-tests were used whenever appropriate. We did not correct for family-wise error rates as the comparison between stimulations is exploratory (procedure recommended in Bender and Lange 2001). ANOVAs for demographical data (handedness, age, and average sleep duration) were performed with group as a factor. The Kruskal–Wallis test for independent samples was used to test for binary differences between each group on gender. Repeated-measures ANOVAs with factors “group” (6 groups) and “time” were used to assess changes in sleep (daily), positive and negative PANAS scores (pre and post 3 days of training), and mental fitness VAS scores (daily). The level of significance was set to P < 0.05 for all tests.
Results
Demographics
A total of 115 healthy subjects were enrolled in this study. None of the subjects had contraindications for noninvasive brain stimulation techniques. One person withdrew participation after day 1 due to a slight headache after TMS/tDCS. One participant reported tinnitus and a mild headache during anodal tDCS, but completed the experiment. Three participants were excluded on day 1, because they did not follow the instructions to perform the motor task. All exclusions and side effects occurred in subjects tested in Freiburg. One hundred and nine participants (mean age = 28.5, SEM = 0.7 years, 61 females) completed the study. All were right handed as assessed by the Edinburgh Handedness Inventory (mean score = 94.0, SEM = 1.0). No significant differences between groups were found in ANOVAs with age, handedness, or average sleep as a dependent variable (Table 1); the Kruskal–Wallis analysis showed no significant difference between groups for gender (Table 1). Repeated-measures ANOVA showed a significant effect of time on negative PANAS scores (P = 0.001), with subjects showing a lower score (less depressed/negative) at the end of the training. However, there was no significant effect of group or group × time. There were no significant effects of time, group, or group × time on sleep, mental fitness VAS scores, and positive PANAS scores (Table 2). In addition, an ANOVA on baseline scores (Day1Block1) showed no significant differences between experimental groups (P = 0.932; Table 1).
Table 2.
Psychophysical assessment
| Sham 15 m | Anodal 15 m | Sham 3 h | Anodal 3 h | Sham 6 h | Anodal 6 h | Anodal after | P (time) | P (group) | P (interact.) | |
|---|---|---|---|---|---|---|---|---|---|---|
| PANAS + pre | 33.1 | 36.5 | 33.6 | 33.6 | 33.7 | 35.1 | 34.9 | 0.189 | 0.793 | 0.402 |
| PANAS + post | 33.1 | 32.9 | 33.5 | 33.6 | 33.2 | 35.9 | 34.0 | |||
| PANAS − pre | 15.3 | 16.3 | 15.5 | 13.4 | 17.3 | 15.6 | 16.2 | 0.001 | 0.223 | 0.362 |
| PANAS − post | 14.2 | 14.1 | 13.8 | 12.8 | 17.3 | 13.4 | 14.0 | |||
| Sleep pre day 1 | 7.1 | 6.8 | 6.9 | 6.8 | 7.0 | 7.2 | 7.7 | 0.531 | 0.892 | 0.328 |
| Sleep pre day 2 | 6.9 | 7.4 | 7.1 | 6.9 | 7.4 | 7.2 | 7.0 | |||
| Sleep pre day 3 | 7.1 | 6.7 | 7.5 | 6.8 | 7.2 | 6.7 | 6.8 | |||
| Mental fitness day 1 | 3.2 | 3.6 | 3.3 | 3.1 | 3.4 | 2.9 | 2.7 | 0.473 | 0.313 | 0.579 |
| Mental fitness day 2 | 3.3 | 3.5 | 3.4 | 3.2 | 2.6 | 2.6 | 2.8 | |||
| Mental fitness day 3 | 3.7 | 3.7 | 3.2 | 3.6 | 3.1 | 2.9 | 2.3 |
Bold values indicates level of significance for all tests was set to P < 0.05.
Total Learning (Days 1–3)
As expected, all subjects showed an increase in total skill over the 3 days of visuomotor training. When grouped over all retesting intervals, subjects who received anodal tDCS showed significantly greater skill improvements compared with sham-stimulated subjects (1-way ANOVA, factor stimulation: P < 0.0001). P-values for group-wise comparisons (15 min, 3 h, and 6 h retest groups) on total learning were P = 0.046, 0.009, and 0.008, respectively, for sham versus anodal tDCS.
Online Effects (Within Session)
The ANOVA with factor STIMULATION (sham versus anodal tDCS) as the independent variable revealed no significant difference in online effects (P = 0.514). P-values for group-wise comparisons (15 min, 3 h, and 6 h retest groups) of online effects were P = 0.32, 0.39, and 0.12, respectively, for sham versus anodal tDCS. Visual inspection of Figure 2 suggests that there was a small positive effect of anodal tDCS on online effects during the first session compared with sham tDCS, as has been described in previous studies (Nitsche et al. 2003; Antal et al. 2004). However, this difference was not statistically significant when tested for all groups [ANOVA with factor STIMULATION (sham vs. anodal tDCS), P = 0.16].
Figure 2.
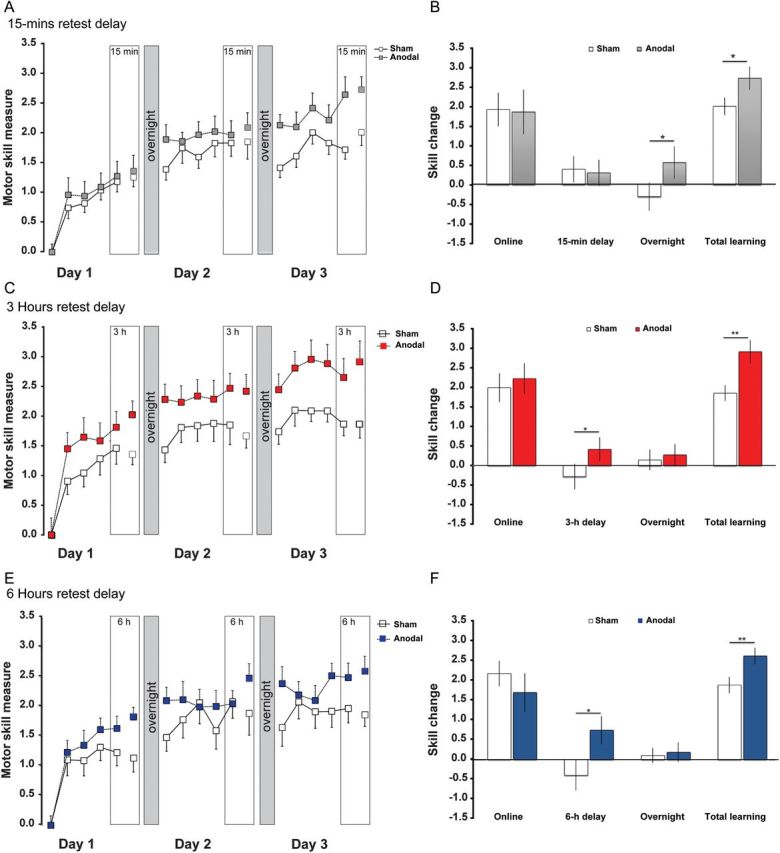
Learning curves and separated subcomponents of learning. (A) Increase in motor skill over 3 consecutive days in the groups retested every day after a 15-min interval. Note the offline loss in skill in the overnight interval in the sham group (white blocks) compared with the increase in skill in the tDCS-stimulated group (gray blocks). (B) The analysis of subcomponents illustrates no significant change of skill in the 15-min retest interval, but a clear difference in overnight offline skill, contributing to the greater total learning in tDCS-stimulated subjects. (C) Increase in motor skill over 3 consecutive days in the groups retested every day after a 3-h interval. Note the offline loss in skill in the 3-h interval in the sham group (white blocks) compared with the increase in skill in the tDCS-stimulated group (blue blocks). (D) The analysis of subcomponents illustrates a significant change of skill in the 3-h retest interval, contributing to the greater total learning in tDCS-stimulated subjects. There was no difference in overnight offline skill. (E) Increase in motor skill over 3 consecutive days in the groups retested every day after a 6-h interval. Note the offline loss in skill in the 6-h interval in the sham group (white blocks) compared with the increase in skill in the tDCS-stimulated group (red blocks). (F) The analysis of subcomponents illustrates a significant change of skill in the 6-h retest interval, contributing to the greater total learning in tDCS-stimulated subjects. There was no difference in overnight offline skill. All data are presented as mean ± SEM. Level of significance: *P < 0.05, **P < 0.01.
Delayed Offline Effects (Delayed Retest Interval)
When retesting 15 min after the end of training, both sham- and anodal DCS-stimulated subjects showed small continued improvements in motor skill, which were not significantly different between groups (P = 0.47). In contrast, “delayed” offline gains were detectable in the anodal tDCS groups at 3 and 6 h after training, while a loss of motor skill occurred in the corresponding sham groups. One-way ANOVA indicated a significant difference for the factor STIMULATION at 3 h (P = 0.037) and at 6 h (P = 0.016). As can be seen in Figure 2, the amount of skill gain with tDCS and skill loss with sham tDCS was almost identical in the 2 subexperiments, suggesting that consolidation of tDCS-enhanced motor skills expresses behaviorally after 15 min and as early as 3 h after the end of training and remains stable afterwards. These results were also found, when removing the warm-up decrement (by excluding the first 5 trials of the delayed retest block; Supplementary Fig. 1A); statistically this approach resulted in a close to significant difference at 3 h (P = 0.051) and a significant difference at 6 h (P = 0.039, Supplementary Fig. 1B). It should be noted that this result was weaker, because anodal tDCS had an additional positive effect already in these first few trials and, therefore, probably on the warm-up decrement itself (Supplementary Fig. 2A).
Overnight Offline Effects
In the 15-min retest group, in which offline gains were not yet detectable early on, “overnight” offline gains were found in subjects stimulated with anodal tDCS, while sham-stimulated subjects showed a skill loss (P = 0.024), similar to our previous results (Reis et al. 2009). However, no significant differences were found in the groups retested 3 or 6 h after training when tested for overnight offline effects (P = 0.48 and 0.40, respectively). It should be noted that in these groups (3 and 6 h) skill remained stable in sham-stimulated as well as anodal-stimulated subjects overnight, suggesting a beneficial effect of the retest on subsequent overnight consolidation (Fig. 2D,E). When removing the warm-up decrement by the same approach as described above, we observed identical results. Statistically, this resulted in a close to significant difference in the 15-min retest group (P = 0.056) and no differences at 3 and 6 h (Supplementary Fig. 1B). In accordance, the weaker effect in the 15-min retest group can be explained by an additional small effect of tDCS on the overnight warm-up decrement (Supplementary Fig. 2B).
In summary, these results suggest that offline gains induced by tDCS in this particular task occur in a time-dependent, but not sleep-dependent, fashion. Moreover, offline gains in the anodal tDCS group are not simply explained by the prevention of warm-up decrement in the first few trials.
Control Experiment: Anodal tDCS After Training
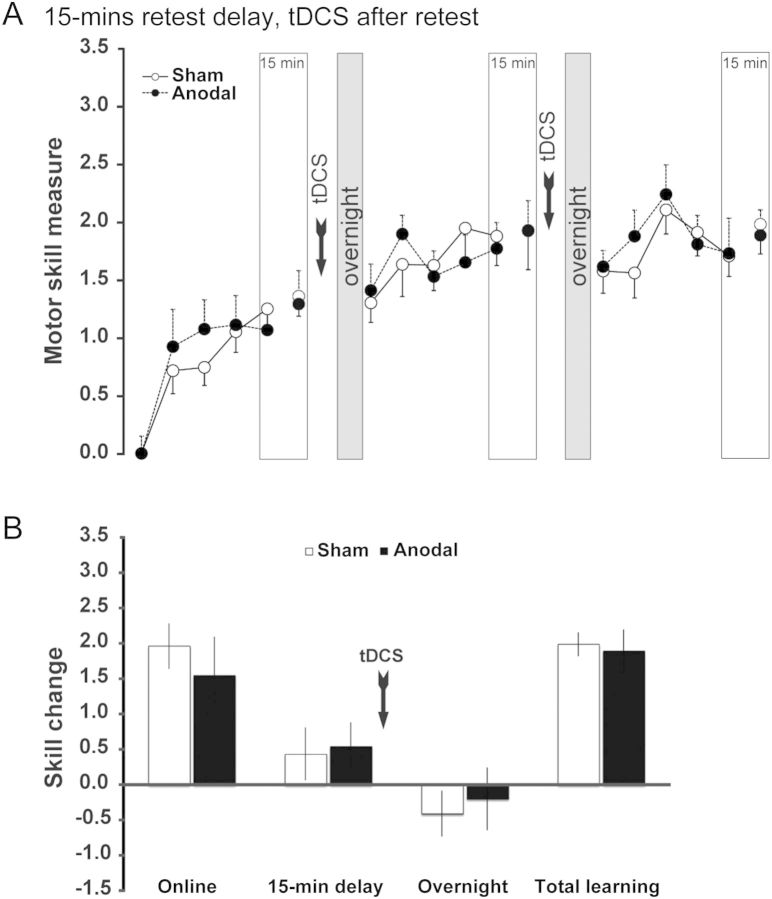
Anodal tDCS-induced offline gains were detectable in a critical time period after the end of training, but not present immediately after the end of training. This raises the important question whether the outlasting excitability increase induced by anodal tDCS (measured as increased motor evoked potentials (MEPs) for ∼2 h after 13 min of stimulation in the absence of training, Nitsche and Paulus 2001; measured as reduction of short intracortical inhibition for at least 45 min after 20 min of stimulation in combination with training, own unpublished data) falling into the critical time period is beneficial for the development of offline gains. On the other hand, the induction of offline gains could also depend on the simultaneous application of anodal tDCS and training, which would support the view that other mechanisms already initiated during training are a requirement for subsequent offline gains or there may be aspects of consolidation already evolving during training. To address this question, a separate group of subjects was stimulated with anodal tDCS after the 15-min retest interval instead of during training. In this way, an excitability increase in M1 was induced in the critical time period mentioned above, but without any effect of tDCS during training. Anodal tDCS applied 15 min after the end of training had virtually no effect on total learning (P = 0.39) or any of the temporal subcomponents compared with sham stimulation (online: P = 0.15, delay: P = 0.28, overnight: P = 0.39, Fig. 3).
Figure 3.
Control experiment: Learning curves and separated subcomponents of learning. (A) Increase in motor skill over 3 consecutive days in the groups retested every day after a 15-min interval but with anodal tDCS applied after the retest. There was an offline loss in skill in the overnight interval in the sham group (white blocks), but also in the tDCS-stimulated group (black blocks). (B) The analysis of learning subcomponents illustrates no significant differences between sham- and tDCS-stimulated subjects, when tDCS is administered in the consolidation period, immediately after the 15-min retest.
Discussion
Here, we show for the first time the physiological between-day time course of the consolidation of a visuomotor skill task over multiple days: Consolidation of the task critically depends on the passage of time after training, but not on overnight sleep. Furthermore, anodal tDCS applied to M1 during training strengthens this physiological process and prevents the skill loss after the end of training. When anodal tDCS is administered after training, no offline skill gains are induced, suggesting that simultaneous application of tDCS and training is a requirement to obtain offline skill gains. Hence, from a mechanistic point of view, the trigger to strengthen the consolidation of motor skills by tDCS is already set during training.
Physiological Time Course of Consolidation of the SVIPT (Sham Groups)
Participants practicing the complex, continuous SVIPT develop a fine motor skill over several days of training. When behaviorally tested on the day following training consolidation of the SVIPT is associated with stable (first night) or slightly declined skill (multiple nights) at the beginning of the retest after a delay period (Fig. 2A,B in the present study; Reis et al. 2009; Cantarero et al. 2013). When training is resumed, skill continues to increase to levels exceeding that of the previous day, arguing against forgetting of the skill. Accordingly, learning curves of motor skills often follow a power law (Adams 1961; Stratton et al. 2010). As can be seen in Figure 2 in our present study, in the “sham stimulation groups” the skill loss in the consolidation period was not present 15 min after the end of training (Fig. 2B), but it occurred 3 h after the end of training (Fig. 2D). Its magnitude was similarly expressed after 6 h (Fig. 2F) and overnight (Fig. 2B; although not tested in the same individuals). Hence, these skill changes after training are most likely time-dependent. Since participants never return to naïve skill levels, it is conceivable that motor memory of the skill acquired in the SVIPT is stabilized as a function of time, independent of overnight sleep, as has been shown for several other (visuo)motor tasks (Brashers-Krug et al. 1996; Robertson, Pascual-Leone, Miall 2004; Doyon et al. 2009; Debas et al. 2010). Accordingly, overnight maintenance of skill was visible in the 3 and 6 h retest groups (Fig. 2D,F). Strong support for a time-dependent consolidation of the SVIPT is given by a recent study using a behavioral interference paradigm (Cantarero et al. 2013): Subjects practiced version A of the SVIPT followed by an equal amount of training a version B. Training of version B interfered with the consolidation of the SVIPT (measured as reduced retention of skill A on the following day) when trained approximately 30 min after version A; however, the interference effect disappeared when version B was trained 6 h later. In a different study using a fixed retest window of 12 h and no interference task, time-dependent consolidation of a visuospatial finger-tracking skill task was found, as both the passage of time and overnight sleep induced similar amounts of offline skill gains (Borich and Kimberley 2011).
Our subanalysis of the first few trials per block in the delay blocks and on the first block of subsequent days allowed us to distinguish a warm-up decrement from consolidation itself. A warm-up decrement contributed to the time-dependent skill loss in the sham groups as previously proposed (Reis et al. 2009), but it did not fully explain it. In fact, warm-up decrement was present in all experimental groups as soon as there was a break between training and the delayed retest (Supplementary Fig. 2). In contrast, comparing the 3 sham groups, consolidation was affected differently at the individual delay intervals. Hence, the occurrence of warm-up decrement in this task is in favor of the activity-set hypothesis (see Introduction and Adams 1961; Nacson and Schmidt 1971; Schmidt and Lee 2005; Stratton et al. 2010). Unless the warm-up decrement for the SVIPT is much more pronounced than expected (>5 trials needed to reinstate, so that it would not have been completely removed by our additional analysis), we will thus consider the small skill loss in the consolidation phase a characteristic feature of motor memory stabilization of the SVIPT.
Anodal tDCS Improves Total Learning
To date, several research groups have confirmed a beneficial effect of anodal tDCS on motor skill learning, although most of the studies assess only the initial component of a skill learning curve on the first training day (Nitsche et al. 2003; Antal et al. 2004; Stagg et al. 2011; Kantak et al. 2012). There is, however, evidence that repeated sessions of combined tDCS and training lead to greater overall learning in healthy subjects (Reis et al. 2009; Zimerman et al. 2013), which we also observed in our present study. Total learning was significantly improved in anodal tDCS-stimulated subjects compared with sham-stimulated subjects (who also showed improvement), emphasizing the cumulative effect of tDCS and training on motor skill gains over time.
Anodal tDCS Strengthens the Time-Dependent Consolidation of the SVIPT
Online Effects
There was a small (nonsignificant) effect of anodal tDCS on online effects in the first session in all 3 experimental groups. This is in line with single-session studies (Nitsche et al. 2003; Antal et al. 2004; Stagg et al. 2011; Kantak et al. 2012) and our previous multisession study (Reis et al. 2009). Likewise, over 3 consecutive training days, we did not observe significant differences in online effects between anodal tDCS and sham-stimulated subjects (Fig. 2B,D,F). This difference between positive online effects on day 1 and lack of effect over consecutive days raises the hypothesis that the efficacy of tDCS on existing synaptic connections may be more pronounced early during training, while such effects may diminish over repeated sessions (Rioult-Pedotti et al. 1998; Rosenkranz et al. 2007). Alternatively, it may be that the effects of tDCS appear more prominent during early phases of learning, because this is the time when the largest and fastest skill gains are made; this could make that the effects of tDCS appear more pronounced during early learning purely due to affecting the learning curve during its steepest period. It is also possible that the skill loss in the sham groups creates larger online skill gains in these subjects compared with the anodal groups, which then mask the tDCS effect (in other words since anodal tDCS groups do not show the skill loss, the “room for improvement within session” may be smaller).
Offline Effects
Extending our earlier study (Reis et al. 2009), we found major differences between tDCS- and sham-stimulated subjects in the consolidation period between sessions. While there were no differences between tDCS and sham at the 15-min retest interval, tDCS-stimulated subjects expressed positive cumulative offline gains over night (Fig. 2B). In contrast, when retested before night time, positive cumulative offline gains in the tDCS groups were observed at the 3- and 6-h retest intervals with no further gains overnight (Fig. 2D,F). Separating offline effects into effects on a potential warm-up decrement and effects on consolidation revealed a profound effect on the latter (Supplementary Fig. 1), although anodal tDCS did also slightly reduce the warm-up decrement over daytime (Supplementary Fig. 2). These data confirm a time-dependent development of offline gains in the first few hours after training in the anodal tDCS groups, independent of sleep. To our knowledge, this is the first demonstration of the between-day time course of consolidation over multiple days in the absence and presence of tDCS. Aside from our previous study (Reis et al. 2009), tDCS studies in the context of motor learning rarely reported data on consolidation, or collected data over multiple sessions. There is some evidence that anodal tDCS applied to M1 during a “single” practice session leads to a stronger motor memory for thumb abduction tested 10 min after the end of practice (Galea and Celnik 2009), a better retention of the acquired skill in a visuomotor adaptation paradigm when retested during immediate deadaptation (Hunter et al. 2009; Galea et al. 2011) and better offline stabilization at 24 h following practice of the serial reaction time task (Kantak et al. 2012) in healthy young subjects. In above-mentioned studies and in our own experiments, anodal tDCS was applied during training, but positive aftereffects were induced in the period following training. In contrast, elderly subjects exposed to anodal tDCS applied to M1 during training of a finger-tapping sequence showed significantly better online skill acquisition than sham-stimulated subjects, but without any additional effect of tDCS on consolidation (Zimerman et al. 2013).
Up to this point, we conclude that our visuomotor task (SVIPT) undergoes time-dependent consolidation, and that tDCS directly interacts with this physiological process. The lack of sole sleep-dependent skill gains in the anodal tDCS groups also supports the view that interplay of training-related and tDCS-related plasticity takes place early after the end of training.
Anodal tDCS after Training Does not Influence the Consolidation Process
Anodal tDCS-induced offline gains were detectable when tested later than 15 min after the end of training. This raises the important question whether the outlasting excitability increase induced by anodal tDCS (measured as increased MEPs for ∼2 h after 13 min of stimulation in the absence of training, Nitsche and Paulus 2001; measured as reduction of short intracortical inhibition for at least 45 min after 20 min of stimulation in combination with training, own unpublished data) is beneficial for the development of offline gains. Alternatively, the induction of offline gains could depend on the simultaneous application of anodal tDCS and training, which would then support the view that mechanisms already initiated by tDCS during training are a requirement for subsequent offline gains. Strikingly, in our control experiment, anodal tDCS applied 15 min after the end of training was not sufficient to induce offline gains. In accordance, anodal tDCS applied a few minutes after training of version A and before training of version B in the interference paradigm described above did not affect retention of skill A on day 2 (Cantarero et al. 2013). Contrary to these findings, Tecchio et al. (2010) found an increase in performance on the serial reaction time task when tDCS was applied after training. However, this study differs from our study with regard to the retest interval, because they retested performance immediately after tDCS during the consolidation phase, while we and Cantarero et al. (2013) tested consolidation between days. In Tecchio's study, direct effects of an excitability increase induced by tDCS may affect performance during the retest without any effect on consolidation per se. In our study, the lack of the effect of tDCS when applied in the consolidation phase could reflect interference with ongoing consolidation by tDCS when applied immediately after the 15-min retest. However, no detrimental effect of tDCS on motor skills was observed in this experiment and in Cantarero et al.'s study (2013).
In summary, we favor the view that the coapplication of tDCS and training is needed to develop consecutive offline gains. This is in line with previous experiments in which only the coapplication induced behavioral improvements in humans (Kuo et al. 2008; Reis et al. 2009; Fritsch et al. 2010; Stagg et al. 2011; Kantak et al. 2012; Vollmann et al. 2012; Zimerman et al. 2012). In accordance, long-term plasticity indicated by a prolonged increase in synaptic strength in brain slices can only be induced if DCS is applied together with a second weak synaptic input (Fritsch et al. 2010). The application of DCS alone to M1 slices did not result in changes of synaptic strength. Moreover, neurotransmitters (N-methyl-d-aspartic acid and γ-aminobutyric acid) and neurotrophic factors play a role in mediating the neuroplastic effect of DCS (Fritsch et al. 2010; Stagg and Nitsche 2011). It is therefore conceivable that activation of such signaling cascades during training could interact downstream with the consolidation process, assessable as skill improvements over time. While the sole increase in excitability induced by anodal tDCS [e.g. increased neuronal firing rates, which is likely chaotic (Bindman and Lippold 1964)] may not be sufficient to induce long-term changes, an additional synaptic activation (by costimulation or motor training) may lead to synapse specificity as a source for long-term plasticity: Synapses within the motor cortex are functionally potentiated and structurally stabilized during motor training (Rioult-Pedotti et al. 1998, 2000; Xu et al. 2009), a process that may be catalyzed when anodal tDCS is applied to a cortical region involved in this process.
Limitations
We have shown that the SVIPT is consolidated in a time-dependent fashion, and that anodal tDCS directly interacts with this process. We have not specifically tested in this study whether some manipulation during sleep (e.g. sleep disruption/deprivation or even stimulation approaches) would alter the consolidation pattern of the motor task used here. Moreover, anodal tDCS applied to M1 after training had no effect on consolidation. It is possible though that stimulation of M1 or other cortical regions (e.g. premotor cortex) may exert different effects when applied during sleep (for example, see Nitsche et al. 2010). This is subject to future investigations. Finally, due to the lack of focality of tDCS, it is possible that cortical areas adjacent to or connected with M1 (the area that was targeted) contribute to the behavioral effects observed in this study.
Conclusion
Our data add to the understanding of mechanisms by which tDCS interacts with motor skill learning. Strengthening consolidation may condense training time courses, which could be crucial in case of relearning after brain damage, for example, stroke.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by a personal grant of the Alexander-von-Humboldt-Foundation and the NINDS Intramural Program (J.R. and L.G.C.) as well as the Neurex Support Program for welcome/coming back of researchers (J.R. and B.F.).
Supplementary Material
Notes
We thank Frank Huethe and Gerd Strohmeier for technical support with the setup of the visuomotor task and tDCS. Conflict of Interest: None declared.
References
- Adams JA. The second facet of forgetting: a review of warmup decrement. Psychol Bull. 1961;58:257–273. doi: 10.1037/h0044798. [DOI] [PubMed] [Google Scholar]
- Adams JA. Warm-up decrement in performance on the pursuit-rotor. Am J Psychol. 1952;65:404–414. [PubMed] [Google Scholar]
- Anshel MH. Examining warm-up decrement as a function of interpolated open and closed motor tasks: implications for practice strategies. J Sports Sci. 1995;13:247–256. doi: 10.1080/02640419508732234. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann KP, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci. 2004;19:2888–2892. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich MR, Kimberley TJ. Both sleep and wakefulness support consolidation of continuous, goal-directed, visuomotor skill. Exp Brain Res. 2011;214:619–630. doi: 10.1007/s00221-011-2863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009;120:1859–1865. doi: 10.1016/j.clinph.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Tang B, O'Malley R, Salas R, Celnik P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J Neurosci. 2013;33:4634–4641. doi: 10.1523/JNEUROSCI.4706-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano JF. The effect of rest following massed practice of continuous and discrete motor tasks. J Mot Behav. 1978;10:63–67. doi: 10.1080/00222895.1978.10735136. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci. 2008;28:9610–9618. doi: 10.1523/JNEUROSCI.3071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Korman M, Morin A, Dostie V, Hadj Tahar A, Benali H, Karni A, Ungerleider LG, Carrier J. Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp Brain Res. 2009;195:15–26. doi: 10.1007/s00221-009-1748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnyre BR, Poindexter HB. Characteristics of motor performance, learning, warm-up decrement, and reminiscence during a balancing task. Percept Mot Skills. 1995;80:1027–1030. doi: 10.2466/pms.1995.80.3.1027. [DOI] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Celnik PA. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, Orban de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21(8):1761–1770. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, De Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol. 2009;587:2949–2961. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning—evidence for competition between implicit and explicit human motor memory systems. Eur J Neurosci. 2012;36:2710–2715. doi: 10.1111/j.1460-9568.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- Keel J, Smith M, Wassermann E. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1–2. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA. 2003;100:12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-F, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia. 2008;46:2122–2128. doi: 10.1016/j.neuropsychologia.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Nacson J, Schmidt R. The activity set hypothesis for warm-up decrement. J Motor Behav. 1971;3:1–15. doi: 10.1080/00222895.1971.10734887. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jakoubkova M, Thirugnanasambandam N, Schmalfuss L, Hullemann S, Sonka K, Paulus W, Trenkwalder C, Happe S. Contribution of the premotor cortex to consolidation of motor sequence learning in humans during sleep. J Neurophysiol. 2010;104:2603–2614. doi: 10.1152/jn.00611.2010. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007;27:12058–12066. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor control and learning: a behavioral emphasis. 2nd ed. Champaign, Illinois: Human Kinetics; 2005. [Google Scholar]
- Song S, Howard JH, Howard DV. Sleep does not benefit probabilistic motor sequence learning. J Neurosci. 2007;27:12475–12483. doi: 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49:800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stratton SM, Liu YT, Hong SL, Mayer-kress G, Newell KM. 2010. Snoddy 1926 revisited: time scales of motor learning. J Mot Behav. 37–41.
- Tecchio F, Zappasodi F, Assenza G, Tombini M, Vollaro S, Barbati G, Rossini PM. Anodal transcranial direct current stimulation enhances procedural consolidation. J Neurophysiol. 2010;104:1134–1140. doi: 10.1152/jn.00661.2009. [DOI] [PubMed] [Google Scholar]
- Vollmann H, Conde V, Sewerin S, Taubert M, Sehm B, Witte OW, Villringer A, Ragert P. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimulation. 2012;6:101–107. doi: 10.1016/j.brs.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig Ja, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73(1):10–15. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.