Abstract
Learning of a complex olfactory discrimination (OD) task results in acquisition of rule learning after prolonged training. Previously, we demonstrated enhanced synaptic connectivity between the piriform cortex (PC) and its ascending and descending inputs from the olfactory bulb (OB) and orbitofrontal cortex (OFC) following OD rule learning. Here, using recordings of evoked field postsynaptic potentials in behaving animals, we examined the dynamics by which these synaptic pathways are modified during rule acquisition. We show profound differences in synaptic connectivity modulation between the 2 input sources. During rule acquisition, the ascending synaptic connectivity from the OB to the anterior and posterior PC is simultaneously enhanced. Furthermore, post-training stimulation of the OB enhanced learning rate dramatically. In sharp contrast, the synaptic input in the descending pathway from the OFC was significantly reduced until training completion. Once rule learning was established, the strength of synaptic connectivity in the 2 pathways resumed its pretraining values. We suggest that acquisition of olfactory rule learning requires a transient enhancement of ascending inputs to the PC, synchronized with a parallel decrease in the descending inputs. This combined short-lived modulation enables the PC network to reorganize in a manner that enables it to first acquire and then maintain the rule.
Keywords: field potentials recordings, olfactory bulb, olfactory rule learning, orbitofrontal cortex, piriform cortex
Introduction
The ability to extract generalizable rules from specific experiences is a fundamental attribute of higher cognitive functioning (Pinker 1991; Penn and Povinelli 2007). Rats trained in a particularly difficult olfactory discrimination (OD) task demonstrate a dramatic increase in their capability to acquire memories of new odors, termed “rule learning,” or “learning set” (Saar et al. 1998; Slotnick et al. 2000). At the cellular level, rule learning results in a global change in the 3 components controlling the intrinsic PC circuit: intrinsic neuronal excitability (Saar et al. 1998; Saar and Barkai 2009), excitatory synaptic inputs (Saar et al. 1999, 2002, 2012; Knafo et al. 2001, 2005; Cohen et al. 2008), and synaptic inhibition (Brosh and Barkai 2009; Saar et al. 2012). These modifications have 2 major common traits:
They are widespread throughout the piriform cortex (PC) network. Both physiological and morphological modifications are found in most of the studied neurons (Saar et al. 1998, 1999, 2002; Saar and Barkai 2003; Knafo et al. 2005).
The time course in which these modifications appear and disappear is strongly correlated with the time course in which the skill is acquired and decays (Saar et al. 1998, 1999; Quinlan et al. 2004; Knafo et al. 2005). However, memories for specific odors outlast these modifications by far. Thus, the identified modifications are related to rule learning (learning how to learn), rather than to long-term memory for the specific odors for which the rats are trained (Barkai 2005).
Olfactory learning also induces changes in encoding of the learned odor in the olfactory bulb (OB) (Freeman and Schneider 1982; Wilson and Sullivan 1994; Mandairon et al. 2006; Doucette and Restrepo 2008) and the orbitofrontal cortex (OFC; Schoenbaum et al. 1998, 1999, 2003). In particular, we have reported (Cohen et al. 2008) an enhancement of reciprocal connectivity between the PC and its ascending input from the OB and descending input from the OFC 3 days after learning.
The inputs from the OB are nontopographically spread across the entire surface of the PC. Individual pyramidal cells have widespread axonal arbors that extend over nearly the full length of the cerebral hemisphere (Johnson et al. 2000; Franks et al. 2011). Accordingly, odor responses are highly distributed, and responses are rather sparse (Illig and Haberly 2003; Rennaker et al. 2007; Poo and Isaacson 2009; Stettler and Axel 2009). It has been hypothesized that the PC carries out functions that have traditionally defined association cortex—it detects and learns correlations between olfactory gestalts and a large repertoire of behavioral, cognitive, and contextual information through reciprocal connections other brain areas, such as OFC (Haberly 2001; Gottfried and Zelano 2011; Wilson and Sullivan 2011).
Single units in anterior PC rapidly learn to synthesize co-occurring odorant features into odor objects, distinct from their components (Wilson 2000; Kadohisa and Wilson 2006). These cortical changes allow enhanced behavioral discriminability of familiar and learned odors (Fletcher and Wilson 2002; Chapuis and Wilson 2011). Disruption of the synaptic plasticity within the PC (Patil et al. 1998) impairs both learned cortical processing of odor objects (Wilson 2001; Linster et al. 2009) and olfactory perceptual learning (Fletcher and Wilson 2002). Furthermore, there are significant anatomical and physiological differences between the anterior and posterior PC (Litaudon et al. 1997, 2003; Gottfried et al. 2006; Kadohisa and Wilson 2006). Importantly, the OFC is most strongly and reciprocally connected with the anterior PC (Illig 2005).
Rule learning is a dynamic process, perhaps comparable to opening of a sensitive period. That is, it takes several days of training to emerge, a period of heightened learning is then maintained for several days, and then the system can revert back to normality. The present study is aimed to reveal how the PC network and interactions between the PC and its external inputs are modified as rule learning develops.
Materials and Methods
Behavioral Procedure
Age-matched adult Sprague Dawley male rats (12–16 weeks of age) were used. Before training, they were maintained on a 23.5-h water-deprivation schedule, with food available ad libitum. OD training protocol was performed with commercial odors that are used regularly in the cosmetics and food industry. Olfactory training consisted of 20 trials per day for each rat as described previously (Saar et al. 1999) (Fig. 1A). Briefly, in each trial, the rat had to choose between 2 odors (positive and negative cues) presented simultaneously. Rats designated to the trained group were rewarded with water after choosing the positive cue. The criterion for learning was at least 80% positive-cue choices in the last 10 trials of a training day. Rats in the pseudo-trained group were rewarded in a random manner in 50% of the trials, regardless of the odor they chose.
Figure 1.
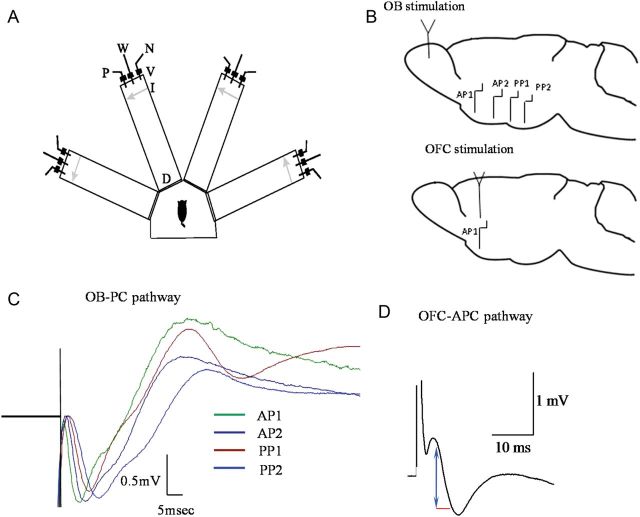
Olfactory discrimination training-apparatus and in vivo fPSPs recordings. (A) Schematic description of the 4-arm maze. Protocols for trained and pseudo-trained rats are similar: an electronic “start” command opens randomly 2 of 8 valves (V), releasing a positive-cue odor (P) into one of the arms and a negative-cue odor (N) into another. Eight seconds later, the 2 corresponding guillotine doors (D) are lifted to allow the rat to enter the selected arms. Upon reaching the far end of an arm (90 cm long), the rat body interrupts an infrared beam (I, arrow) and a drop of drinking water is released from a water hose (W) into a small drinking well (for a trained rat—only if the arm contains the positive-cue odor, for pseudo-trained rat—randomly). A trial ends when the rat interrupts a beam, or in 10 s, if no beam is interrupted. A fan is operated for 15 s between trials, to remove odors. (B) Top: Location of stimulating electrode in the OB and of the 4 recording electrodes in the PC. To record responses to ascending input stimulation, 2 recording electrodes are located in layer Ia of the anterior PC (APC1 and APC2) and 2 in layer Ia of the posterior PC (PPC1 and PPC2). Bottom: Location of stimulating electrode in the OFC and of the recording electrode in the anterior PC. To record responses to descending input stimulation, one recording electrode is located in layer Ib/II of the anterior PC (APC). (C) Simultaneous recordings the PC in response to stimulation of the ascending pathway for the OB. (D) Typical responses recorded in layer Ib of the anterior PC in response to stimuli applied in the descending pathway form the OFC (see Cohen et al. 2008 for detailed description of location of stimulating and recording electrodes). Arrow in blue and line red show amplitude measurement of 10%–90% and time distance between the 2 points, used to measure the fPSP slope.
When reaching the criterion for rule learning, training was continued with the same pair of odors, except for the last day of training, in which all rats were trained with a second pair of odors.
Overall, 7 groups were examined: 5 trained groups and 2 pseudo-trained groups. In the OB-PC pathway, group 1, group 2, and group 3 received electrical stimulation 15 min, 1 h, and 2 h, respectively, after daily training. Group 4 is a control-trained group in which the same set of electrodes was implanted, but no stimulation was applied. Group 5 is the trained group for the OFC-PC pathway in which post-training stimulation in the OFC was applied daily 15 min after each training session, and groups 6 and 7 are the pseudo-trained groups for each pathway which received stimulation 15 min after daily training (Table 1).
Table 1.
Summary of training protocols
| Procedure | Pathway | Description | |
|---|---|---|---|
| Group 1 | Trained | OB-PC | 15 min. Post-training daily stimulation |
| Group 2 | Trained | OB-PC | 1 h. Post-training daily stimulation |
| Group 3 | Trained | OB-PC | 2 h. Post-training daily stimulation |
| Group 4 | Trained | OB-PC | Implanted group, without stimulation |
| Group 5 | Trained | OFC-PC | 15 min. Post-training daily stimulation |
| Group 6 | Pseudo-trained | OB-PC | 15 min. Post-training daily stimulation |
| Group 7 | Pseudo-trained | OFC-PC | 15 min. Post-training daily stimulation |
Note: Stimulating and recording protocols of the groups used in the study.
Group 1: n = 7; Group 2: n = 4; Group 3: n = 4; Group 4: n = 6; Group 5: n = 6; Group 6: n = 6; Group7: n = 4.
Surgery and Recording
Rats were anesthetized with ketamine (ketalar, SC 0.75 mg/kg) and medetomidine (dormitor, SC 0.5 mg/kg) and were placed in a stereotaxic frame with body temperature maintained at 37 ± 0.5°C. The procedure was performed in a strict accordance with the Haifa University regulations and guidelines of the National Institutes of Health.
Recordings in the OB-PC Pathway
Small holes were drilled in the skull at various stereotaxic coordinates for the placement of stimulating and recording electrodes in the brain. Both stimulating and recording electrodes consisted of stainless steel wire, 175 μm, insulated with Teflon. The bipolar stimulating electrode consisted of a pair of twisted stainless steel wires. A skull screw was used as reference.
For stimulating the ascending pathway, a stimulating electrode was chronically implanted into the left OB (7.9 mm anterior to bregma; 1.1 mm lateral; 2 mm below the surface of the bulb). Four monopolar recording electrodes were chronically implanted ipsilaterally in the PC (Fig. 1B): 2 electrodes into the anterior PC (APC1: 3.2 mm anterior, 3.5–4.2 mm lateral relative to Bregma; APC2: 1.2 mm, 5 mm lateral relative to Bregma), and 2 electrodes into the posterior PC (PPC1: 0.8–1.8 mm posterior, 6 mm lateral relative to Bregma; PPC2: 2.8–3.8 mm posterior, 6–7 mm lateral relative to Bregma). Accurate positioning of recording electrode depth was achieved using the field potential profile evoked in the distal apical dendrites in response to electrical stimulation of OB, during the surgery. In APC1, APC2, PPC1, and PPC2, recording electrode tips were positioned in the superficial cortical area of layer 1 where the field potential signal (Ketchum and Haberly 1993) presented large stable amplitude of a negative monosynaptic response, which corresponded to the approximate depths of 5–5.4, 6.5–7.5, 7.5–8.2, and 7.6–9 mm, respectively. As expected, the delay of the synaptic responses in the ascending pathway was increased with increasing distance from the OB (Fig. 1C). Field postsynaptic potential (fPSP) slope was calculated using 10–90% of the response from baseline to peak amplitude.
Recordings in the OFC-PC Pathway
For stimulating the descending pathway, a stimulating electrode was chronically implanted into the Ventralis lateralis pars oralis of the left OFC, which has strong reciprocal connections with the APC (Illig 2005). Electrode position was 3.2 mm anterior to bregma, 2.2 mm lateral, and 4–4.5 mm below the pial surface. One monopolar recording electrode was chronically implanted in the anterior PC (3.7 mm anterior to bregma; 3.3–3.6 mm lateral; 5–5.4 mm below the pial surface) (Fig. 1B). Accurate positioning of recording electrode depth was achieved using the field potential profile evoked in the proximal dendrites of pyramidal neurons dendrites in response to electrical stimulation of OFC, during the surgery. Recording electrode tip was positioned in the superficial cortical area of layer 1b/II where the field potential signal (Cohen et al. 2008) presented large stable amplitude of a negative monosynaptic response.
Following electrode implantation, craniotomy holes were then filled with a small amount of dental cement and the electrode assembly was secured firmly to screws previously inserted in the skull. Surgical wounds were sutured, and antibiotic was injected during and after surgery to prevent infection.
Stimulating and Recording Protocol During OD Learning
Recordings began at least 2 weeks after the surgery to allow positioning and stabilization of the electrodes in the PC and OB areas. Animals were habituated to a recording box for a minimum 2 days. In vivo baseline recordings were made 2 days and 1 day before the learning, for 10–15 min. Measurements were made of the slope of fPSP using averages of 10 successive responses to a given stimulation intensity (1 mA) applied at 0.1 Hz. The overall average of responses during the 10- to 15-min recording was always equal to the average of any 10 consecutive responses. In vivo recordings were performed throughout the learning period (6–11 days), 15 min after each session was ended, throughout the training period.
Input–output (I–O) functions for stimulus intensity versus fPSP magnitude were recorded in response to increasing intensities of stimulation from 0.3 to 1 mA applied at 0.1 Hz. I–O curves were constructed by using averages of 10 successive responses to a given stimulation intensity versus the entire range of intensities sampled. Only those experiments having stable baselines and stable I–O curves over multiple trials were included in the analysis.
Evoked responses were digitized (10 kHz) and analyzed using the Cambridge Electronic Design 1401+ and its Spike2 software. Off-line measurements were made during the surgery of the slope of fPSP using averages of 5 successive responses to a given stimulation intensity applied at 0.1 Hz. Test stimuli (monopolar pulses, 100 μs duration) were delivered at 0.1 Hz. For responses in both pathways, fPSP slope was calculated using 10–90% of the response from fPSP onset to peak amplitude (Fig. 1D). As can be seen in the example recordings in Figures 3–6, fPSP enhancement was usually not accompanied by changes in the latency to amplitude.
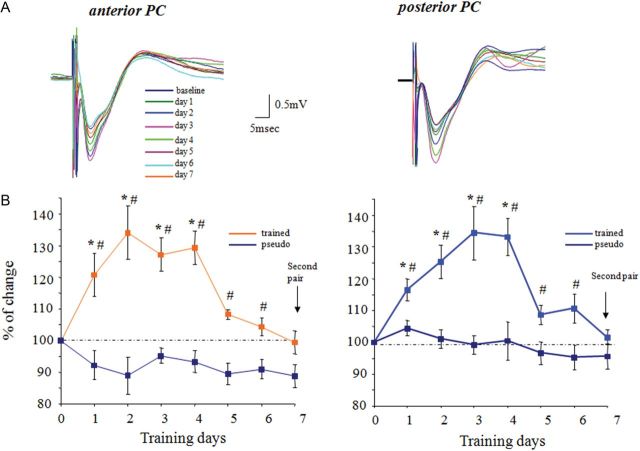
Figure 3.
Rule learning-induced enhancement of synaptic transmission in the ascending pathway is similar for the APC and PPC. (A) Examples of synaptic response evoked each day after training in response to stimulation at intensity of 1 mA in the APC and PPC from the same rat. In both recording sites, the maximal response occurs at the third day during learning. (B) Time course and enhancement of the synaptic response evoked by stimulating the ascending pathway is similar throughout the piriform cortex. For each rat, responses recorded with the 2 electrodes in the APC were averaged to calculate the APC response, and responses recorded with the 2 electrodes in the PPC were averaged to calculate the PPC response. Recordings from 5 rats were averaged for each group. Values represent mean ± SE. *P < 0.05 between a particular training day and the baseline recoding in trained rat prior to training. #P < 0.05 between trained and pseudo-trained rats.
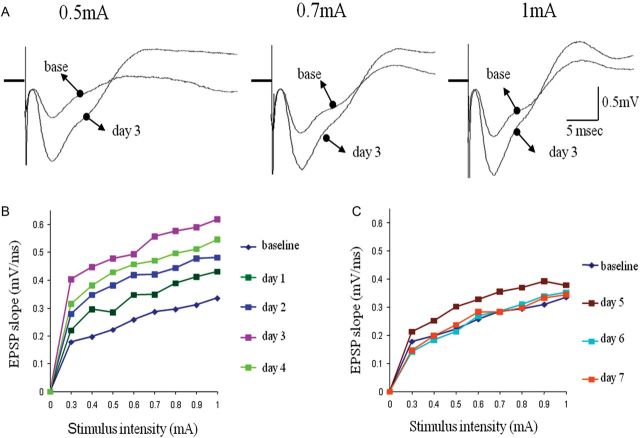
Figure 6.
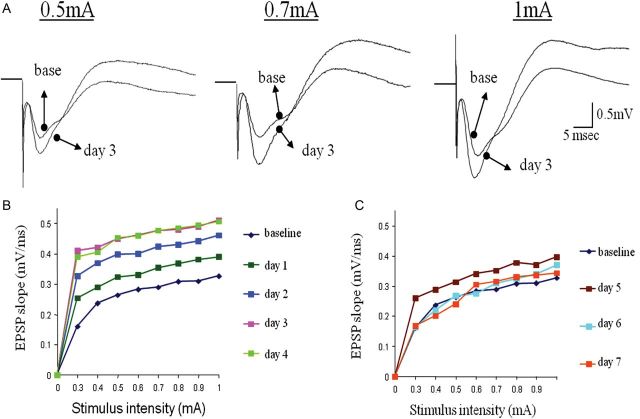
Transient enhancement synaptic connectivity from the olfactory bulb to the posterior PC during rule learning. (A) Examples of responses to OB stimulation in the posterior PC before learning (base) and 3 days during learning. Enhanced synaptic responses are evident for all 3 shown stimulation intensities. (B) Example of analysis of the input–output function of the synaptic response in the ascending input to APC on the first 4 days of training. Here too, the strength of synaptic response increases each day for all stimulus intensities until it reaches its peak on the fourth day of training, before rule learning is completed (see Fig. 2). (C) Example of analysis of the input–output function of the synaptic response in the ascending input to APC starting 5 days of training. Here too, the strength of synaptic response first decreases slightly between the fifth and sixth day of training, and then resumes its pretraining value (base). Notably, although training on the seventh day was performed with a pair of new odors, the synaptic did not potentiate again.
Notably, each rat was stimulated either in the ascending or the descending fibers. Thus, the comparison between the effects of the 2 stimulation sites was made between the 2 experimental groups.
Histology
After termination of the electrophysiological recordings, lesions were made by passing anodal currents (10 mA for 3 s) through the stimulating electrode and recording electrodes. The brains were removed and frozen with dry ice. Brain slices of 60 μm were cut using a cryostat. The electrode tract and lesion locations were identifiable under a light microscope.
Statistical Analysis
For the behavior analyses, between-groups comparison was done using repeated-measures ANOVA for all the groups (control and trained groups), and post hoc Fisher tests were then applied to compare the groups (control and trained groups). Student's t-test was also used to compare the groups where appropriate.
For the recording analyses in the OB-PC pathway, we used the averages of 2 electrodes for each individual (N = 5 for each group). We used 2-way repeated-measures ANOVA to test the effect of the days as within-subject factor and of the group as between-subject factor. We used “Huynh-Feldt correction” when required.
In the OFC-PC pathway, we used 2-way repeated-measures ANOVA to test the effect of the days as within-subject factor and of the group as between-subject factor.
Results
Effect of Electrical Stimulation on Learning
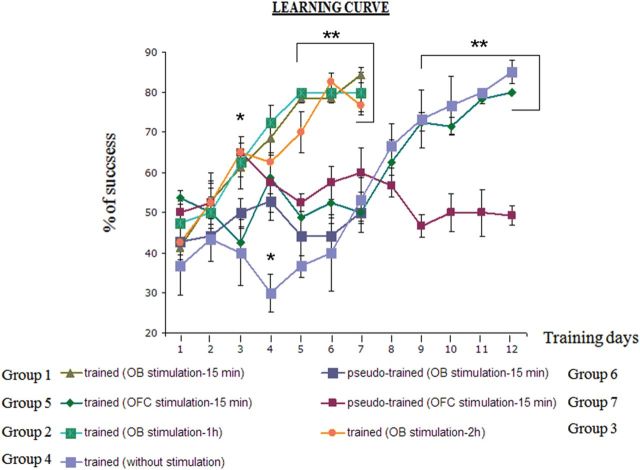
As previously shown, OD learning requires 7–8 days of training on average for the first pair of odors and only 1 day for a subsequent new pair of unfamiliar odors (Saar et al. 1998, 1999; Saar and Barkai 2003), indicating that rule learning has been completed. In the present study, all rats trained with a second pair of odors reached the criterion for learning within 1 day of training. Electrical stimulation, aimed to monitor learning-induced modulation of the evoked field potentials amplitudes, was applied daily after each training session. Daily post-training stimulation of the OB enhanced the rate of OD learning dramatically. Such enhancement was apparent when stimulation was applied at 15 min (Group 1, n = 7 rats), 1 h (Group 2, n = 4), or 2 h (Group 3, n = 4) after training. Rats that received such daily stimulation after each training session completed rule learning within 4–5 days (Fig. 2). Rats in which the same set of electrodes was implanted, but no stimulation was applied (Group 4, n = 6) and rats in which post-training stimulation in the OFC was applied daily 15 min after each training session (Group 5, n = 6) required 10 days of training to reach the rule-learning criterion (Fig. 2), a significantly longer time period. A repeated-measures ANOVA across the first 7 days of training and including all 7 conditioning groups showed a significant main effect of training day (F6,180 = 12.94, P < 0.001), a significant main effect of group (F6,180 = 17.41, P < 0.001) and a significant group × day interaction (F36,180 = 2.99, P < 0.001). Post hoc Fisher tests revealed a significant difference between the OB-stimulated groups (groups 1, 2 and 3) and all other groups on days 5, 6, and 7. A second repeated-measures ANOVA was performed on the 3 groups trained for 12 days. There was a significant main effect of day (F11,132 = 11.02, P < 0.001) and a significant group × day interaction (F22,132 = 5.85, P < 0.001). Post hoc tests revealed a significant difference between the OFC-stimulated (Group 5) and pseudo-conditioned group (Group 7, n = 4) and between the No-stim group (Group 4) and the pseudo-conditioned group (Group 7), but no difference between the OFC stimulated (Group 5) and NO-Stim group (Group 4) on days 9–12. Notably, OFC stimulation had no detectable effect on learning rate.
Figure 2.
Learning curves of the different experimental groups. Learning curve for trained and pseudo-trained rats expressed as correct choices in the last 10 trials of the day. The learning curve shows a gradual improvement in performance in all groups of trained rats and no learning in pseudo-trained rats, which show no preference for any of the odors during the entire training period. Notably, when daily stimulation was applied to the OB, learning was completed within 5 days, while rats stimulated at the OFC show no advantage in learning over naive rats implemented with stimulating electrodes but not stimulated. Values represent mean ± SE. *P < 0.05. **P < <0.05. Numbers of rats are noted in the text.
Transient Potentiation of Synaptic Responses Evoked in the Ascending Pathway During Learning
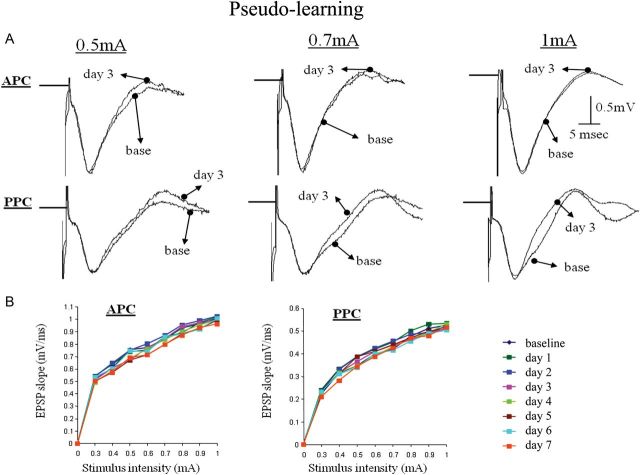
During learning, synaptic responses evoked by stimulating the OB each day 15 min after the training session showed a stable response throughout the training period (7 days) in the PC of pseudo-trained rats, with a small nonsignificant tendency toward reduction as training proceeded (Figs 3B and 4). These data indicate that without learning, the ascending evoked synaptic response maintains stability for long periods of time and is not modified by the electrical stimulation itself. Thus, the protocol in this study is appropriate for the examination of learning-induced synaptic modifications during learning.
Figure 4.
Synaptic connectivity in ascending pathway is not modified in pseudo-trained rats. (A) Examples of responses to OB stimulation in the anterior and posterior PC before learning (base) and 3 days during learning. Traces for 3 stimulation intensities are shown. (B) Example of analysis of the input–output function of the synaptic response in the ascending input to APC and PPC. The strength of synaptic response remained stable for all stimulus intensities throughout the training period.
In contrast, OD learning resulted in a transient increase in the ascending pathway-evoked response throughout the PC. In the anterior PC, a potentiation of the OB fPSP was observed during the first 4 days of learning (i.e., before rule learning completion, see Fig. 2 for OB stimulation). Two-way repeated-measures ANOVA showed significant differences between days (F3.6, 29.2 = 9.52, P < 0.001) and between groups (F1.8 = 47.77, P < 0.001) and, in addition, a significant interaction between day and group (F3.6, 29.2 = 6.84, P = 0.001). The trained group showed a pattern of increase in the first 2 days (max = 134 ± 6.38) and sharp decrease of ∼20% in the fifth day of the experiment (Figs 3 and 5). The pattern of changes between days in the slope of fPSPs (Fig. 3A) was significantly different among the 2 groups.
Figure 5.
Transient enhancement synaptic connectivity from the olfactory bulb to the anterior PC during rule learning. (A) Examples of responses to OB stimulation in the anterior PC before learning (base) and 3 days during learning. Enhanced synaptic responses are evident for all 3 shown stimulation intensities. (B) Example of analysis of the input–output function of the synaptic response in the ascending input to APC on the first 4 days of training. The strength of synaptic response increases each day for all stimulus intensities until it reaches its peak on the third and fourth day of training, before rule learning is completed (see Fig. 2). (C) Example of analysis of the input–output function of the synaptic response in the ascending input to APC starting 5 days of training. The strength of synaptic response first decreases slightly between the fifth and sixth day of training, and then resumes its pretraining value (base). Notably, although training on the seventh day was performed with a pair of new odors, the synaptic response did not potentiate again.
Similar results were obtained in the posterior PC, 2-way repeated-measures ANOVA showed significant differences between days (F6,48 = 7.82, P < 0.001) and between groups (F1.8 = 194.19, P < 0.001) and, in addition, a significant interaction between day and group (F6,48 = 4.49, P = 0.001). Here too, enhanced fPSP response was detected in the trained group only (n = 5 rats) during rule learning. The averaged fPSP increased in the first 4 days (max = 134.4 ± 7.44) and sharp decrease of ∼25% in the fifth day of the experiment (Figs 3 and 6).
Thus, a strong correlation was observed between the learning stage and the dynamics of change in the field potentials' slope evoked by OB stimulation; a significant, uniform increase in slopes of the ascending pathway-evoked fPSP was observed throughout the acquisition phase of the rule. This increase was followed by return of the amplitude to the baseline on the day after, when training was continued with a new pair of odors.
Learning-Related Transient Depression in the Descending Pathway
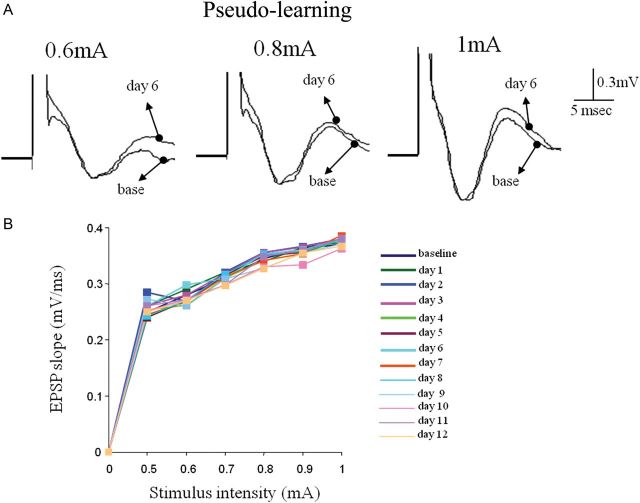
During learning, synaptic responses evoked by stimulating the OFC each day 15 min after the training session showed a stable response throughout the training period (7 days) in the PC of pseudo-trained rats (Fig. 7). These data indicate that without learning, the descending pathway-evoked synaptic response also maintains stability for long periods of times.
Figure 7.
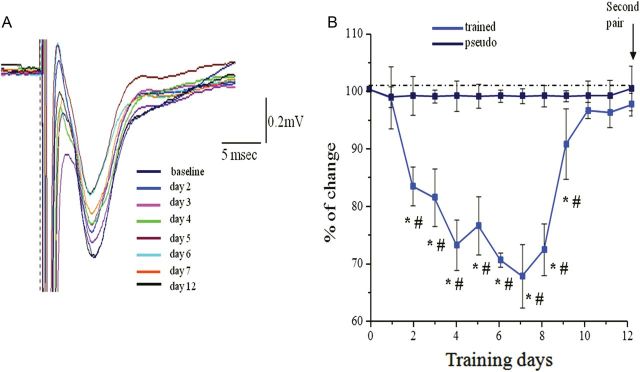
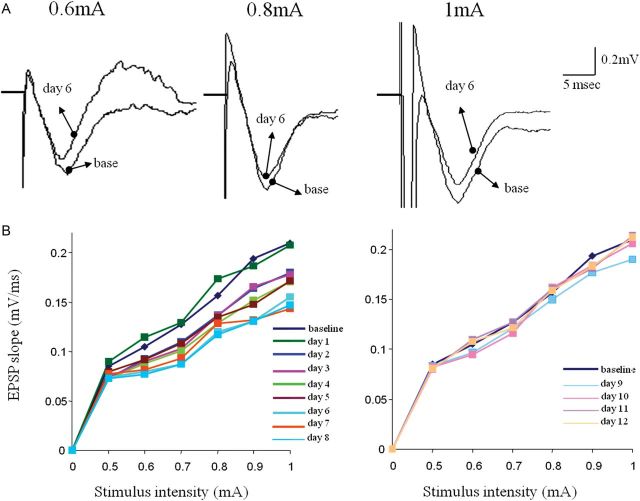
Time course of reduced transmission in the descending pathway. (A) Examples of synaptic response evoked each day after training in response to stimulation at intensity of 1 mA in the APC. In this example, the maximal response occurs at the fifth and sixth day during learning. (B) Time course of reduction in the synaptic response evoked by stimulating the descending. For each rat, responses recorded with one electrode in the APC. Recordings from 5 rats were averaged for each group. Values represent mean ± SE. *P < 0.05 between a particular training day and the baseline recoding in trained rat prior to training. #P < 0.05 between trained and pseudo-trained rats. Note that the averaged value resumes its initial value on the ninth day of training, somewhat before when rule learning is completed.
In sharp contrast to synaptic enhancement observed in the ascending pathway, OD learning resulted with a transient substantial decrease in synaptic response in the PC evoked by stimulating the descending pathway. Decreased fPSP was observed during the first 10 days of learning (i.e., before rule learning completion, see Fig. 2 for OFC stimulation).
Two-way repeated-measures ANOVA showed significant differences between days (F11, 77 = 5.31, P < 0.001*) and between groups (F1.7 = 69.73, P < 0.001) and in addition a significant interaction between day and group (F11, 77 = 5.91, P < 0.001) (Fig. 7). The pattern of changes between days, in the slope of fPSPs (Fig. 9), was significantly different among the 2 groups. The pseudo-trained group showed no changes between days (Figs 7 and 8) while the trained group showed a pattern of decrease, up to 32%, in the fPSP slope, which returned to pretraining values as rule learning was completed the (Figs 7 and 9).
Figure 9.
Transient decrease in synaptic connectivity from the orbitofrontal to the anterior PC during rule learning. (A) Examples of responses to OFC stimulation in the anterior PC before learning (base) and 6 days during learning. Reduced synaptic responses are evident for all 3 shown stimulation intensities. (B) Example of analysis of the input–output function of the synaptic response in the descending input to APC on the first 8 days of training. The strength of synaptic response decreased each day for all stimulus intensities until it reaches its lowest peak on the sixth day of training, before rule learning is completed (see Fig. 7). (C) Example of analysis of the input–output function of the synaptic response in the ascending input to APC starting 9 days of training. The strength of synaptic response remains slightly decreases during the ninth day of training, and then resumes its pretraining value (base). Notably, although training on the 12th day was performed with a pair of new odors, the synaptic was not reduced again.
Figure 8.
Synaptic connectivity in descending pathway is not modified in pseudo-trained rats. (A) Examples of responses to OFC stimulation in the anterior PC before learning (base) and 3 days during learning. Traces for 3 stimulation intensities are shown. (B) Example of analysis of the input–output function of the synaptic response in the descending input to APC. The strength of synaptic response remained stable for all stimulus intensities throughout the training period.
Thus, a strong correlation was observed also between the learning stage and the dynamics of change in the field potentials' slopes evoke by OFC stimulation. Moreover, the transient change in the OFC-evoked response mirrored that evoked by OB simulation; OB-evoked fPSP was enhanced during rule learning, while OFC-evoked fPSP was reduced during learning. This difference was maintained throughout the learning period although the time course of learning was strongly affected by the daily OB stimulation.
Discussion
Odor memory displays higher order characteristics, such as object-oriented perception (Gottfried 2010; Yeshurun and Sobel 2010; Wilson and Sullivan 2011), pattern completion (Barnes et al. 2008; Chapuis and Wilson 2011), transitive inference (Dusek and Eichenbaum 1997), and rule learning (Saar et al. 1998; Slotnick et al. 2000). While some of these apparently higher order phenomena derive from computations within the olfactory system itself, others emerge due to interactions with, or within, other systems. In particular, key roles have been suggested for the OB (Wilson and Sullivan 1994; Linster et al. 2001; Fletcher and Wilson 2003; Churchwell et al. 2009), the entorhinal cortex (Xu and Wilson 2012), and the OFC (Roesch et al. 2007; MacDonald 2008; Churchwell et al. 2009). Indeed, we showed previously that both the ascending and descending synaptic inputs to the PC, from the OB and OFC are enhanced 3 days after learning (Cohen et al. 2008). Here, we examined the dynamics of changes in synaptic strength in the 2 pathways during the prolonged process of rule learning. We found profound differences between the effects of OB and OFC input to the PC, which suggest they have different roles in rule learning.
In particular, enhanced responses to afferent stimuli have been detected in the PC following OB stimulation. Several lines of evidence suggest that enhanced amplitude of evoked field potentials represents the cellular and network processes underlying rule learning:
Enhanced evoked field potentials occur during rule learning only; once rule learning is completed, the field potentials resume their prelearning values. Further training with a new pair of odors does not result in changes in the field potentials' amplitude.
Such enhanced field potentials occur simultaneously throughout the PC, anterior as well as posterior (Fig. 3).
Such enhancement is pathway specific; it occurs only after OB stimulation.
Enhanced field potentials are detected only in rats that learn the rule. Exposure to the odors and olfactory maze (pseudo-trained group) does not result in such changes.
Electrical Stimulation of OB Enhances Rule Learning
Daily electrical stimulation applied to the OB for up to 2 h after training reduced by half the number of trials required acquiring the rule (Fig. 2). However, the electrical stimulation did not modify the synaptic responses in the control groups, indicating that such changes in evoked synaptic responses are learning dependent.
Notably, OFC stimulation had no detectable effect on learning rate (Fig. 2). Thus, given that both the OB and OFC project to the PC, these results suggest that the enhancement in learning rate may not be due to stimulation induced activation of the PC itself. Rather, the results suggest that the OB-PC interaction pathway has a key role during the course of OD rule learning. Interestingly, enhanced response to OB stimulation is observed as soon as after the first day of training, when performance is still at chance level. Furthermore, the level of OB activity may affect odor processing (Mandairon et al. 2006) and/or memory consolidation and thus the rate of odor learning. The processes taking place in the first relay of the olfactory system during the 2 first hours postlearning have already been suggested to support olfactory memory consolidation (Mouly et al. 1990, 1993). Olfactory learning-related activity (Martin et al. 2007; Kay and Beshel 2010) and experience-induced plasticity have been shown to occur also within the OB (Wilson and Sullivan 1994; Linster et al. 2001; Ravel et al. 2003; Doucette et al. 2011; Fletcher 2012). The OB has been suggested to have a specific role in enhancing olfactory discrimination and learning (Shimshek et al. 2005; Escanilla et al. 2010). Learning-induced changes within the PC seem to not only modify the responses of the cortical neuron directly but also shape subsequent mitral and tufted cell-response patterns (Manabe et al. 2011; Markopoulos et al. 2012). Understanding of how OB stimulation influences these properties may give insight into the neurobiology of odor memory and perception.
Several previous studies have shown that brain stimulation can enhance learning. For example, deep brain stimulation of the entorhinal cortex improved spatial learning in adult mice (Stone et al. 2011). A recent study in epileptic patients showed that stimulation of the entorhinal region enhanced memory of spatial information when applied during learning (Suthana et al. 2012). Here, we suggest that low-frequency stimulation applied to the OB during rule learning enhanced the rate of the learning. Several delays were examined but future work would be important to check if such a facilitation of learning would still have been observed after longer delays.
Temporal and Spatial Properties of Enhanced OB-PC Synaptic Connectivity
Interestingly, enhancement in OB-PC pathway is evident only while the rat is acquiring the rule. Once rule learning is completed, the synaptic response resumes its control value. We observed a significant increase and then decrease in the slope of the fPSP in trained rats throughout a wide range of stimulus intensities during learning. Such a uniform enhancement that persists with increasing stimulus intensity (which presumably increases the number of activated ascending fibers) suggests that learning-induced enhancement in synaptic connectivity occurs in most of the synapses activated in this pathway.
Rule learning-related transient enhancement was observed in both the anterior and posterior PC regardless of the significant anatomical and physiological differences between the anterior and posterior PC. As one moves from anterior to posterior PC, these differences include more diffuse afferent termination zones, an expansion of association fibers relative to afferent fibers (Neville and Haberly 2004), and stronger input from nonolfactory areas such as amygdala (Majak et al. 2004). Associated with these anatomical changes, important information processing and experience-dependent differences emerge in the posterior PC, including coding of odorant similarities or odor qualities (Litaudon et al. 2003; Gottfried et al. 2006; Kadohisa and Wilson 2006), and encoding of nonolfactory stimulus associations with odors (Chabaud et al. 2000; Moriceau and Sullivan 2006; Calu et al. 2007).
Taken together, our data suggest that during rule learning the PC undergoes a widespread transient change, in which the cortical network is physiologically and anatomical reorganized to enable rule-learning acquisition and maintenance.
Transient Decrease of Synaptic Strength in the OFC-PC Pathway
We previously described enhanced synaptic connectivity in the descending pathway to the PC from the OFC after OD rule learning (Cohen et al. 2008). The short latency of the evoked fPSP in the OFC-APC pathway and its stability suggests that the connection is monosynaptic. Thus, learning-induced modifications in synaptic strength should occur at synaptic connections onto layer II/Ib pyramidal neurons.
Here, we show a large, transient decrease in this descending pathway during rule learning. We observed a significant decrease and then return to control values, in the descending pathway throughout a wide range of stimulus intensities. Such a uniform difference between groups that persists with increasing stimulus intensity, that presumably increases the number of activated descending fibers, suggests that learning-induced depression in synaptic connectivity occurs in most of the synapses activated in this pathway. Here too, once rule learning was complete, training with a new pair of odors did not induce any modifications of the synaptic response.
This finding strongly indicates that the partial shutdown of the OFC-PC pathway accompanies the process of rule learning. Interestingly, what may be presented as the mirror phenomenon was detected in the human cortex; a week of odor deprivation resulted in reduced odor-activation of the PC, but enhanced activation of the OFC (Wu et al. 2012). This effect was also transient and reversed when odor deprivation period ended. Thus, while a strong correlation was observed between rule learning and transient reduction in the OFC-PC connectivity, the full significance of such physiological manifestation to complex learning remains to be determined.
System Activity During Rule Learning
During OD rule learning rats develop a strategy for performing the task skillfully (Saar et al. 1998). The development of a strategy temporally corresponds to the enhancement of the ascending inputs and the reduction of the descending inputs. Such a strong transient reduction in the weight of the descending synaptic pathway may be explained by several possibilities:
First, rule-learning acquisition might be dependent on bottom-up inputs (and perhaps reciprocal inputs) rather than top-down inputs. The input from the OB to the PC reflects the identity of the odor and the inputs from the OFC to the PC are assumed to reflect the reward value of specific trained odors (Schoenbaum and Eichenbaum 1995). As the rule-learning phase is related to the ability to enter into a learning mode (learning how to learn), rather than a long-term memory for the specific trained odors, descending information transmitted in the OFC-PC pathway might not be crucial for the acquisition phase. Second, a temporary decrease in strength of OFC-PC pathway may serve as to counterbalance for over excitation in the PC during learning (such that is induced by enhanced OB-PC connectivity). It should be noted that a similar enhancement in simple odor learning has been observed after lesions of the entorhinal cortex (Wirth et al. 1998) further suggesting that some situations are enhanced by reduced top-down interference. Finally, ascending inputs from the PC terminate in the distal parts of the apical dendrites and descending inputs terminate on the proximal dendrites. Thus, the proximal inputs must be shut down to allow the distal inputs from the OB to reach the pyramidal cell bodies without being modified by proximal inputs from another source.
Notably, in a previous study (Cohen et al. 2008), we found that OD learning results in a postlearning enhancement of the OB-PC- and OFC-PC-evoked synaptic responses 3 days after learning (Cohen et al. 2008). The important difference between the previous and the present studies is that here we examined these pathways during the learning. We show that the transient reduction in the OFC-evoked fPSP is apparent only during learning; the synaptic response resumes its pretraining value with rule-learning completion. Thus, we suggest that postlearning mechanisms are involved to maintain long-term modifications more efficiently by enhancement of both pathways after the learning.
To summarize, our data suggest that complex olfactory learning is not simply divided between different stations of the olfactory pathways, but requires a combined and correlated activation of the different brain regions, such as the OB, the PC, and the OFC. In addition, this multifaceted activation is different in the “acquisition” of the rule learning and in the modulation of specific trained odors following the learning. The process is dynamic, comparable to opening of a sensitive period in which a period of heightened learning is then opened and maintained for several days, and then the system activity can revert back to normality.
Funding
This work was supported by grant 698/11 from the Israel Science Foundation to E.B.
Notes
Conflict of Interest: None declared.
References
- Barkai E. Dynamics of learning-induced cellular modifications in the cortex. Biol Cybern. 2005;92(6):360–366. doi: 10.1007/s00422-005-0564-0. [DOI] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh I, Barkai E. Learning-induced enhancement of feedback inhibitory synaptic transmission. Learn Mem. 2009;16(7):413–416. doi: 10.1101/lm.1430809. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in posterior piriform cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17(6):1342–1349. doi: 10.1093/cercor/bhl045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud P, Ravel N, Wilson DA, Mouly AM, Vigouroux M, Farget V, Gervais Exposure to behaviourally relevant odour reveals differential characteristics in rat central olfactory pathways as studied through oscillatory activities. Chem Senses. 2000;25:561–573. doi: 10.1093/chemse/25.5.561. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat Neurosci. 2011;15(1):155–161. doi: 10.1038/nn.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Maroun M, Barkai E. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28(26):6664–6669. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Station. Neuron. 2011;69(6):1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol. 2008;6(10):e258. doi: 10.1371/journal.pbio.0060258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanilla O, Arrellanos A, Karnow A, Ennis M, Linster C. Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory blubber. Eur J Neurosci. 2010;32:458–468. doi: 10.1111/j.1460-9568.2010.07297.x. [DOI] [PubMed] [Google Scholar]
- Fletcher ML. Olfactory aversive conditioning alters olfactory bulb mitral/tufted cell glomerular odor responses. Front Syst Neurosci. 2012;6:16. doi: 10.3389/fnsys.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22(2):RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Olfactory bulb mitral/tufted cell plasticity: odorant- specific tuning reflects prior odorant exposure. J Neurosci. 2003;23:6946–6955. doi: 10.1523/JNEUROSCI.23-17-06946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Schneider W. Changes in spatial patterns of rabbit olfactory EEG with conditioning to odors. Psychophysiol. 1982;19:44–56. doi: 10.1111/j.1469-8986.1982.tb02598.x. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11(9):628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 2006;49:467–479. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zelano C. The value of identity: olfactory notes on orbitofrontal cortex function. Ann NY Acad Sci. 2011;1239:138–148. doi: 10.1111/j.1749-6632.2011.06268.x. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Illig KR. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 2005;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci USA. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 2010;104:829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Membrane currents evoked by afferent fiber stimulation in rat piriform cortex. I. Current source-density analysis. J Neurophysiol. 1993;69:248–260. doi: 10.1152/jn.1993.69.1.248. [DOI] [PubMed] [Google Scholar]
- Knafo S, Grossman Y, Barkai E, Benshalom G. Olfactory learning is associated with increased spine density along apical dendrites of pyramidal neurons in the rat piriform cortex. Eur J Neurosci. 2001;13:633–638. doi: 10.1046/j.1460-9568.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- Knafo S, Libersat F, Barkai E. Olfactory learning-induced morphological modifications in single dendritic spines of young rats. Eur J Neurosci. 2005;21(8):2217–2226. doi: 10.1111/j.1460-9568.2005.04041.x. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Menon AV, Singh CY, Wilson DA. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn Mem. 2009;16:452–459. doi: 10.1101/lm.1403509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- Litaudon P, Mouly AM, Sullivan R, Gervais R, Cattarelli M. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur J Neurosci. 1997;9:1593–1602. doi: 10.1111/j.1460-9568.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- MacDonald KB. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol Rev. 2008;115:1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- Majak K, Rönkkö S, Kemppainen S, Pitkänen A. Projections from the amygdaloid complex to the piriform cortex: a PHA-L study in the rat. J Comp Neurol. 2004;476:414–428. doi: 10.1002/cne.20233. [DOI] [PubMed] [Google Scholar]
- Manabe H, Kusumoto-Yoshida I, Ota K, Mori M. Olfactory cortex generates synchronized top-down inputs to the olfactory bulb during slow-wave sleep. J Neurosci. 2011;31:8123–8133. doi: 10.1523/JNEUROSCI.6578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24:3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76:1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Gervais R, Holley A. Evidence for the involvement of the rat olfactory bulb in processes supporting long- term olfactory memory. Eur J Neurosci. 1990;2(11):978–984. doi: 10.1111/j.1460-9568.1990.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Kindermann U, Gervais R, Holley A. Involvement of the olfactory bulb consolidation processes associated with long- term olfactory memory in rats. Behav Neurosci. 1993;107(3):451–457. doi: 10.1037//0735-7044.107.3.451. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Olfactory cortex. New York: Oxford University Press; 2004. [Google Scholar]
- Patil MM, Linster C, Lubenov E, Hasselmo ME. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. J Neurophysiol. 1998;80:2467–2474. doi: 10.1152/jn.1998.80.5.2467. [DOI] [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. Casual cognition in human and non-human animals: a comparative, critical review. Ann Rev Psych. 2007;58:97–118. doi: 10.1146/annurev.psych.58.110405.085555. [DOI] [PubMed] [Google Scholar]
- Pinker S. Rules of language. Science. 1991;253:530–535. doi: 10.1126/science.1857983. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan E, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Ravel N, Chabaud P, Martin C, Gaveau V, Hugues E, Tallon-Baudry C, Bertrand O, Gervais R. Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60–90 Hz) and beta (15–40 Hz) bands in the rat olfactory bulb. Eur J Neurosci. 2003;17:350–358. doi: 10.1046/j.1460-9568.2003.02445.x. [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-lasting maintenance of learning-induced enhanced neuronal excitability: mechanisms and functional significance. Mol Neurobiol. 2009;39:171–177. doi: 10.1007/s12035-009-8060-5. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. Review. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol. 2002;107:1222–1229. doi: 10.1152/jn.2002.87.5.2358. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. J Neurosci. 1999;19:8616–8622. doi: 10.1523/JNEUROSCI.19-19-08616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Reuveni I, Barkai E. Mechanisms underlying rule learning-induced enhancement of excitatory and inhibitory synaptic transmission. J Neurophysiol. 2012;87:2358–2363. doi: 10.1152/jn.00356.2011. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Bus T, Kim J, Mihaljevic A, Mack V, Seeburg PH, Sprengel R, Schaefe AT. Enhanced odor discrimination and impaired olfactory memory by spatially controlled switch of AMPA receptors. PLoS Biol. 2005;3(11):e354. doi: 10.1371/journal.pbio.0030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Hanford L, Hodos W. Can rats acquire an olfactory learning set? J Exp Psychol: Anim Behav Process. 2000;26:399–415. doi: 10.1037//0097-7403.26.4.399. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus. 2011;21:1348–1362. doi: 10.1002/hipo.20845. [DOI] [PubMed] [Google Scholar]
- Suthana N, Haneef Z, Stern J, Mukamel R, Behnke E, Knowlton B, Fried I. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Odor specificity of habituation in the rat anterior piriform cortex. J Neurophysiol. 2000;83:139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Objects. Neuron. 2011;72(4):506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth S, Ferry B, Di Scala G. Facilitation of olfactory recognition by lateral entorhinal cortex lesion in rats. Behav Brain Res. 1998;91:49–59. doi: 10.1016/s0166-4328(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Wu KN, Tan BK, Howard JD, Conley DB, Gottfried JA. Olfactory input is critical for sustaining odor quality codes in human orbitofrontal cortex. Nat Neurosci. 2012;15:1313–1319. doi: 10.1038/nn.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wilson DA. Odor-evoked activity in the mouse lateral entorhinal cortex. Neuroscience. 2012;223:12–20. doi: 10.1016/j.neuroscience.2012.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 2010;61:219–241. doi: 10.1146/annurev.psych.60.110707.163639. C1–C5. [DOI] [PubMed] [Google Scholar]