Abstract
The bioactive iridoid component catalpol was successfully separated by high-speed countercurrent chromatography with high purity from the partially purified crude extract of Rehmannia glutinosa. A polar two-phase solvent system composed of ethyl acetate–n-butanol–water (2:1:3, v/v/v) was selected by thin-layer chromatography and run on a preparative scale where the lower aqueous phase was used as the mobile phase with a head-to-tail elution mode. A 105 mg quantity of the partially purified sample containing 39.2% catalpol was loaded on a 270-mL capacity high-speed countercurrent separation column, yielding 35 mg of catalpol at 95.6% purity. The chemical structure of catalpol was determined by comparison with the high-performance liquid chromatography retention time of standard substance as well as the 1H NMR spectrum.
Introduction
Dihuang, Rehmanniae Radix, is the fresh or dry roots of Rehmannia glutinosa Libosch., which belongs to the family of Scrophulariaceae. It is officially listed in the Chinese Pharmacopoeia as a traditional herbal drug to be used in fresh or dried form or after processing (1). Modern pharmacological studies have demonstrated that R. glutinosa possesses not only very comprehensive pharmacological actions on the blood system, endocrine system, immune system, cardiovascular system and the nervous system, but also it provides wide clinical applications, and mainly used for hemostasis, anti-tumor treatment, immune-enhancement, anti-hypertension, anti-diabetes, treatment for concretion in the urinary tract and ulcerative stomatitis, etc. (2). The main bioactive constituents of R. glutinosa were found to be iridoid glycosides, in which catalpol was the most important active constituent and it was used for quality control marker for this medicinal plant in the Chinese Pharmacopoeia. Content of this component in the plant is believed to be over 0.2% (weight percentage). Pharmacological studies showed that catalpol could improve the memory of animal models (3) and could ameliorate β-amyloid-induced degeneration of cholinergic neurons (4). It has also been reported to induce neuronal differentiation in PC12 cells through activation of the intracellular signal transduction pathway (5) and to attenuate apoptosis induced by H2O2 in PC12 cells in vitro (6). The antioxidant property of catalpol is also well documented (7). Other literatures demonstrated the protective effect of catalpol on mesencephalic neurons; it not only serves as a free radical scavenger but also prevents mitochondrial dysfunction and subsequent lipid peroxidation. Therefore, catalpol is probably a candidate chemical for the treatment of oxidative stress-induced neurodegenerative disease (8). The chemical structure of catalpol is shown in Figure 1.
Figure 1.
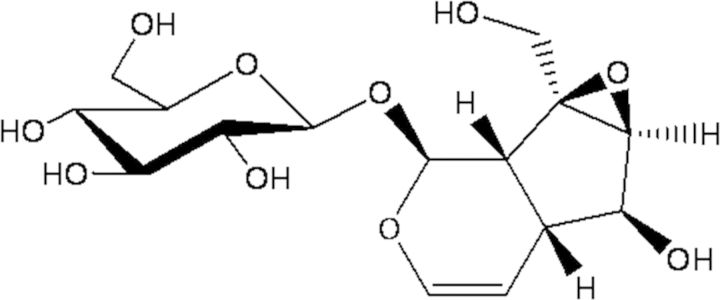
Chemical structure of catalpol.
In view of these pharmacological effects, development of an efficient method for the preparative separation and purification of catalpol from natural sources is warranted. It was reported that more than 33 iridoid monomers (which are the most important components in the herb) as well as monoterpenes and glycosides have been separated and identified (2), such as dihydrocatalpol, danmelittoside, acetylcatalpol, leonuride, aucubin, melittoside, rehmaglutin A, B, C, D, cerebroside glutinoside, rehmannioside A, B, C, D, rehmapicroside, purpureaside C, echinacoside, cistanoside A, F, jionoside A1, B1, Jioglutoside A, B, geniposide, etc. The chemical structures of most of the iridoid monomers are found to be very similar with catalpol. Therefore, it is difficult to separate catalpol with a high purity from the plant. Traditionally, catalpol was purified from the crude extract by repeated column chromatography and crystallizations, which are tedious and often require several steps (9). In recent years, study on the separation and purification technology of catalpol from the crude extract of R. glutinosa Libosch. by various macroporous adsorption resins was reported (10), in which the environment-friendly solvent ethanol and water were used to separate catalpol. However, no catalpol with high purity (≥90%) could be obtained by macroporous column due to its low separation efficiency. Further silica column chromatography or crystallization was necessary for obtaining catalpol with high purity.
High-speed countercurrent chromatography (HSCCC) belongs to liquid–liquid partition chromatography with high separation efficiency (11). HSCCC uses no solid support, so that the irreversible adsorption of solutes on the solid stationary phase and artifact formation can be eliminated. This technique has maximum high sample loading capacity with an excellent sample recovery and can be employed for preparative-scale separation in a completely straightforward manner. Furthermore, it permits introduction of crude samples directly into the hollow column. In recent years, HSCCC has been widely used for separation and purification of chemical constituents from various natural products (11). In our previous studies, the bioactive iridoid component harpagoside was successfully separated from the crude extract of Scrophularia ningpoensis Hemsley by one-step purification using the HSCCC technique (12). However, HSCCC usually needs to be combined with various column chromatography techniques in order to obtain the iridoid components with high purity because most of iridoids are high polarity and coexisted with other constituents with very similar chemical structures. Other literatures are available about separation and purification of iridoid components from natural products by the countercurrent chromatography technique (13–18). This paper reports the successful preparative separation and purification of the main iridoid component catalpol from R. glutinosa by the combination of silica gel chromatography and HSCCC.
Experimental
Apparatus
A Model TBE-300A HSCCC (Shanghai Tauto Biotechnique, Shanghai, China) was used in this study. It was equipped with a set of three preparative multilayer coils (270 mL, wound with 1.6 mm i.d. PTFE tubing). The β values of this column range from 0.46 to 0.73 (β = r/R, R = 6.5 cm, where r is the distance from the coil to the holder shaft, and R, the revolution radius or the distance between the holder shaft and central axis of the centrifuge). The revolution speed of the apparatus can be regulated with a speed controller in the range between 0 and 1,000 rpm. The columns of HSCCC were installed in a vessel that maintained temperature at 30°C by a Model SDC-6 constant-temperature controller (Ningbo Scientz Biotechnology Co. Ltd., Ningbo, China). The solvent was pumped into the column with a Model NS-1007 constant-flow pump (Beijing Shengyitong Technique Co. Ltd., Beijing, China). Continuous monitoring of the effluent was achieved with a Model UV-II detector Monitor (Shanghai Institute of Biochemistry of Academy of Science, Shanghai, China). A manual sample injection valve with a 20 mL loop (Shanghai Tauto Biotechnique, Shanghai, China) was used to introduce the sample into the column, and a Sepu3010 workstation (Hangzhou PuHui Technology, Hangzhou, China) was employed to record the chromatogram. Eluate from the HSCCC column was collected with a Model BSZ-100 fraction collector (Shanghai Huxi Tech, Shanghai, China) at 3 mL for each fraction.
The high-performance liquid chromatography (HPLC) instrument used was a CLASS-VP Ver.6.1 system (Shimadzu Corporation, Kyoto Japan) comprised a Shimadzu SPD10Avp UV detector, a Shimadzu LC-10ATvp Multisolvent Delivery System, a Shimadzu SCL-10Avp controller, a Shimadzu LC pump and a CLASS-VP Ver.6.1 workstation.
Reagents and materials
All organic solvents used for HSCCC were of analytical grade and purchased from Hangzhou HuiPu Chemical Factory (Hangzhou, China). Acetonitrile used for HPLC analysis was of chromatographic grade. Rehmannia glutinosa was purchased from Huadong Drugstore (Hangzhou, China).
Preparation of partially purified crude sample
A 200 g amount of R. glutinosa was extracted with 75% ethanol under refluxing twice (1 +1 h). The ethanol extracts were combined and concentrated to dryness under reduced pressure. Then the crude extract was redissolved in water and extracted with ethyl acetate and n-butanol subsequently. The n-butanol extract was concentrated to dryness under reduced pressure, yielding 5.3 g of crude extract. This n-butanol extract was further subjected to silica gel column chromatography (200 g of silica gel H, 100–200 mesh, Qingdao Haiyang Chemica, Qingdao, China) eluted successively with dichloromethane–methanol (4:1, v/v) to obtain 105 mg of mixtures mainly containing catalpol, and this partially purified sample was directly subjected to HSCCC separation.
Selection of two-phase solvent system
Selection of two-phase solvent system for separation of catalpol by HSCCC was conducted by thin-layer chromatography (TLC) on normal-phase silica gel plates GF254 (Qingdao Haiyang Chemical, Qingdao, China). The crude extract was spotted on silica gel TLC plates and developed with n-butanol:glacial acetic acid:water (7:1:2, v/v/v). The visual detections were done by concentrated sulfuric acid vapor. The experimental results showed that the Rf value of catalpol spot on the TLC plate was about 0.51. The evaluation of partition behavior of catalpol in the two-phase solvent system was performed as follows: small amount of crude extract was added into the two mutually equilibrated solvent phases (2 mL each) in a stoppered test tube (10 ×120 mm), followed by thorough stirring with a vortex mixer to equilibrate the contents. After settling, the upper and lower phases were analyzed by TLC to observe the spots of catalpol in each phase. The biphasic solvent system that gave the roughly identical distribution behavior of catalpol in both phases were selected and tested by HSCCC.
Preparation of two-phase solvent system and sample solution
The selected solvent system was thoroughly equilibrated in a separation funnel by repeating vigorously shaking under the same temperature as in an HSCCC column. The two phases were separated shortly prior to use. The upper phase was used as the stationary phase, and the lower phase, as the mobile phase. The sample solution was prepared by dissolving the crude sample in the mixture of lower phase and upper phase (1:1, v/v) of the solvent system used for HSCCC separation.
HSCCC separation procedure
The multilayer-coiled column was first entirely filled with the upper phase as a stationary phase. The lower aqueous mobile phase was then pumped into the head end of the column inlet at a flow-rate of 1.0 mL min−1, while the apparatus was run at a revolution speed of 800 rpm. After hydrodynamic equilibrium was reached, as indicated by a clear mobile phase eluting at the tail outlet, the sample solution (105 mg dissolved in 10 mL mixture solution of lower phase and upper phase (1:1, v/v) of the solvent system) was injected through the sample port. The effluent from the tail end of the column was continuously monitored with a UV detector at 254 nm. Peak fraction was manually collected according to the elution profile and analyzed by HPLC. After the separation was completed, retention of the stationary phase was measured by collecting the column contents by forcing them out of the column with pressurized air.
HPLC analysis and identification of HSCCC peak fractions
The partially purified n-butanol extract mainly containing catalpol and its HSCCC peak fractions were analyzed by HPLC. The analyses were performed with YMC-Pack ODS-A column, 5 μm particle size of the packing material, 250 × 4.6 mm i.d. (YMC Co., Ltd., Kyoto, Japan). The mobile phase composed of acetonitrile–0.1% phosphoric acid aqueous solution (1:99, v/v) was eluted at a flow-rate of 0.6 mL/min, and the effluent was monitored by a UV detector at 210 nm. Routine sample calculations were made by comparison of the peak area with that of the standard.
Identification of HSCCC peak fractions was carried out by 1H NMR and comparison with the HPLC retention time of the standard compound. 1H NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer (Bruker Corporation, Switzerland) with tetramethylsilane as an internal standard.
Results
Selection of suitable two-phase solvent system of HSCCC
The typical polar biphasic solvent systems, including n-butanol–water (1:1, v/v), ethyl acetate–n-butanol–water (2:3:5, 3:2:5, 2:1:3, v/v/v), n-butanol–acetic acid–water (4:1:5, v/v/v), ethyl acetate–ethanol–water (5:1:5, 10:1:10, 25:1:25, v/v/v) and ethyl acetate–iso-propanol–water (3:2:5, v/v/v), were investigated by TLC. The results showed that a large amount of catalpol were partitioned into the aqueous phase for most of the above solvent systems because of its high polarity. Fortunately, the solvent system ethyl acetate–n-butanol–water (2:1:3, v/v/v) provided a visible partition behavior for catalpol in upper organic phase. Thus, the solvent system ethyl acetate–n-butanol–water (2:1:3, v/v/v) was selected. HSCCC experiments demonstrated that catalpol could be separated from the partially purified crude extract.
Separation of catalpol by HSCCC
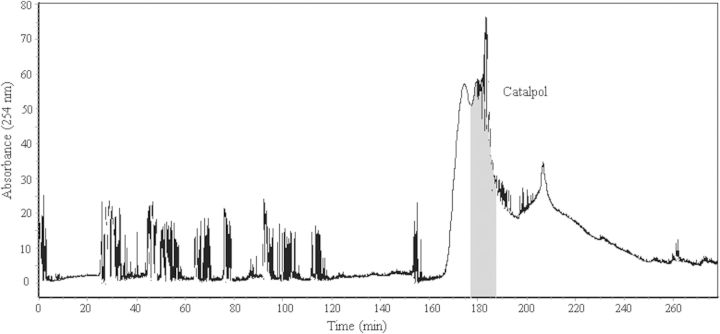
The partially purified crude sample was dissolved in 10 mL mixture of upper phase and lower phase (1:1, v/v) of the solvent system used for HSCCC separation. A 105 mg quantity of partially purified n-butanol extract was separated by HSCCC, which yielded 35 mg of catalpol with 95.6% purity. The retention of the stationary phase was 55%, and the total separation time was 260 min. Figure 2 shows the separation result obtained from 105 mg of the partially purified n-butanol extract of R. glutinosa by preparative HSCCC.
Figure 2.
Chromatogram for separation of catalpol from the partially purified crude sample of R. glutinosa by HSCCC. Solvent system: ethyl acetate–n-butanol–water (2:1:3, v/v/v); stationary phase: upper organic phase; mobile phase: lower aqueous phase; flow-rate: 1.0 mL min−1; revolution speed: 800 rpm; sample: 105 mg dissolved in 10 mL mixture solution of lower phase and upper phase (1:1, v/v) of the solvent system and retention of the stationary phase: 55%.
HPLC analysis of the crude sample and HSCCC peak fractions
The partially purified n-butanol extract of R. glutinosa was analyzed by HPLC and the result indicated that under UV 254 nm the crude contained several compounds among which catalpol represented 39.2% of the total. After HSCCC separation, the fractions containing catalpol were collected. The analysis indicated that the fractions contained 35 mg of catalpol at over 95.6% purity, as determined by HPLC (Figure 3). The structural identification of catalpol was carried out by 1H NMR spectra and comparison with the HPLC retention time of the standard compound.
Figure 3.
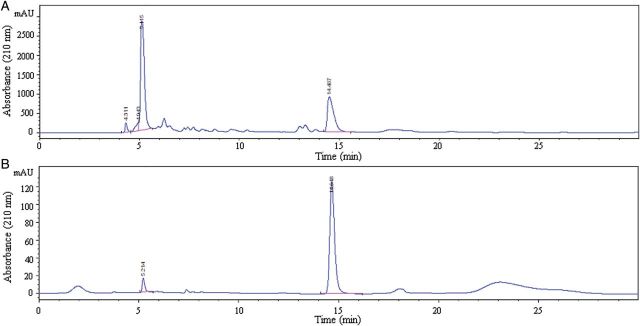
Results of HPLC analysis of the partially purified crude sample of R. glutinosa and its HSCCC fraction. Column: YMC-Pack ODS-A column, 5 μm particle size of the packing material, 250 × 4.6 mm i.d.; mobile phase: acetonitrile–0.1% phosphoric acid aqueous solution (1:99, v/v); flow-rate: 0.6 mL min−1 and UV detector: 210 nm. (A) The partially purified crude sample and (B) HSCCC fraction containing catalpol.
Discussion
The most important step for successful separation of the target compound by HSCCC is the selection of the suitable two-phase solvent system which should meet the following requirements: (1) no decomposition or denaturation of the sample; (2) sufficient sample solubility; (3) suitable partition coefficient (K) values of the target compound (i.e., usually between 0.2 and 5) and (4) satisfactory retention of the stationary phase (the settling time of the solvent system should be ≤30 s) (11). Catalpol is a highly polar compound and freely dissolves in water. Several typical biphasic solvent systems for separation of component with high polarity are available, such as n-butanol–water, ethyl acetate–n-butanol–water, n-butanol–acetic acid–water, ethyl acetate–ethanol–water and ethyl acetate–iso-propanol–water (12, 14, 16). On the other hand, aqueous–aqueous biphasic solvent system, such as polyethylene glycol (PEG)–dibasic potassium phosphate and PEG-dextran, could be used for the separation of highly polar compound. But it proved to be very difficult for recovery of sample from the fraction containing polymers after separation by HSCCC. Recently, an organic-high ionic strength aqueous solvent system was reported for separation of highly polar compounds (19), in which a total of 21 of solvent systems composed of n-butanol–ethanol–saturated ammonium sulfate–water at various volume ratios are introduced. Because the target component was a highly polar compound, we have tried to use the biphasic solvent system n-butanol–ethanol–saturated ammonium sulfate–water (0:1:1:1, v/v/v/v). Although TLC analysis showed that this solvent system gave almost identical distribution behavior between the upper and lower phases for catalpol, no successful HSCCC separation was obtained. Furthermore, high concentration of ammonium sulfate in either of the two phases made it very difficult to recover the sample from the fractions. As a result, several typical polar biphasic solvent systems, as described above, were investigated by the TLC method. The results showed that ethyl acetate–n-butanol–water (2:1:3, v/v/v) was suitable for separation of catalpol from the partially purified crude extract.
On the other hand, it is worthy of being pointed out here that silica gel TLC could be used to select a suitable solvent system for separation of target components by countercurrent chromatography, which was a very simple and reliable method for screening the biphasic solvent system. However, the TLC method has been always overlooked as for selecting the optimum solvent systems because HPLC is more convenient or preferred during selection of biphasic solvent system for HSCCC. As a matter of fact, TLC could be more widely used for various kinds of chemical constituents in the natural products than HPLC due to its highly versatile visual detection method.
Conclusion
In this work, preparative purification of bioactive component catalpol from the partially purified crude extract of R. glutinosa by HSCCC was established. It was also difficult to separate catalpol with high purity from n-butanol extract by HSCCC in one step because there were lots of constituents with quite similar chemical structures coexisted in this plant. But it was quite efficient to separate the component with high purity when silica gel column chromatography combined with HSCCC technique was employed. Modern HSCCC methods provide us a powerful separation way for isolation and purification of bioactive components from natural products.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 21105090), Department of Education of Zhejiang Province of China (pd2013031) and Innovative Program for the Undergraduates of Zhejiang University of Technology (2013059). S.Q. Tong thanks Personnel Department of Zhejiang University of Technology for providing the visiting scholar program (2011).
References
- 1.China Pharmacopoeia Committee (ed). Pharmacopoeia of the People's Republic of China (The First Division of 2010 Edition). China Medical Science Press, Beijing, (2010), pp. 115–116. [Google Scholar]
- 2.Zhang R.-X., Li M.-X., Zheng P.J.; Rehmannia glutinosa: review of botany, chemistry and pharmacology; Journal of Ethnopharmacology, (2008); 117: 199–214. [DOI] [PubMed] [Google Scholar]
- 3.Wang J.H., Sun Q.X., Xia Z.Q., Hu Y.E.; Regulatory effect of catalpol from Radix Rehmanniae on M2 receptor density in M2 receptor transfected CHO cells; Chinese Pharmacological Bulletin, (2006); 22: 1462–1466. [Google Scholar]
- 4.Wang Z., Liu Q., Zhang R., Liu S., Xia Z., Hu Y.; Catalpol ameliorates beta amyloid-induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors; Neuroscience, (2009); 163: 1363–1372. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki M., Chiba K., Mohri T.; Neuritogenic effect of natural iridoid compounds on PC12 cells and its possible relation to signaling protein kinases; Biological & Pharmaceutical Bulletin, (1996); 19: 791–795. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B., Liu J.H., Bao Y.M., An L.J.; Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade; Toxicon, (2004); 43: 53–59. [DOI] [PubMed] [Google Scholar]
- 7.Li D.Q., Duan Y.L., Bao Y.M., Liu C.P., Liu Y., An L.J.; Neuroprotection of catalpol in transient global ischemia in gerbils; Neuroscience Research, (2004); 50: 169–177. [DOI] [PubMed] [Google Scholar]
- 8.Tian Y.-Y., Jiang B., An L.-J., Bao Y.M.; Neuroprotective effect of catalpol against MPP+-induced oxidative stress in mesencephalic neurons; European Journal of Pharmacology, (2007); 568: 142–148. [DOI] [PubMed] [Google Scholar]
- 9.Li G.S., Wang H.S., Du H.Q., Tu W.Q.; Separation and preparation of active component of Radix Rehmanniae catalpol by liquid chromatography; Chinese Traditional Patent Medicine, (1998); 20: 36–28. [Google Scholar]
- 10.Zhang X.-L., Wang J.-Y., Jiang B., Chang Y., An L.J.; Study on the separation and purification technology of catalpol from Rehmannia by macroporous adsorption resins; China Biotechnology, (2008); 28: 65–69. [Google Scholar]
- 11.Ito Y.; Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography; Journal of Chromatography A, (2005); 1065: 145–168. [DOI] [PubMed] [Google Scholar]
- 12.Tong S.Q., Yan J.Z., Lou J.Z.; Preparative isolation and purification of harpagoside from Scrophularia ningpoensis Hemsley by high-speed countercurrent chromatography; Phytochemical Analysis, (2006); 17: 406–408. [DOI] [PubMed] [Google Scholar]
- 13.Maurya A., Dwivedi G.R., Darokar M.P., Srivastava S.K.; Preparative isolation of bioenhancer loganetin from sf Alstonia scholaris by fast centrifugal partition chromatography; Separation Science and Technology, (2014); 49: 773–777. [Google Scholar]
- 14.Yue H.-L., Zhao X.-H., Wang Q.-L., Tao Y.-D.; Separation and purification of water-soluble iridoid glucosides by high speed counter-current chromatography combined with macroporous resin column separation; Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, (2013); 936: 57–62. [DOI] [PubMed] [Google Scholar]
- 15.Liang J.R., He J., Zhu S., Zhao W.N., Zhang Y.M., Ito Y., et al. Preparation of main iridoid glycosides in Fructus corni by macroporous resin column chromatography and countercurrent chromatography; Journal of Liquid Chromatography & Related Technologies, (2013); 36: 983–999. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C.Y., Kim J.W.; Preparative isolation and purification of geniposide from Gardenia fruits by centrifugal partition chromatography; Phytochemical Analysis, (2007); 18: 115–117. [DOI] [PubMed] [Google Scholar]
- 17.Leitao G.G., Andre de Souza P., Moraes A.A., Brown L.; Step-gradient CCC separation of phenylpropanoid and iridoid glycosides from roots of Stachytarpheta cayennensis (Rich.) Vahl; Journal of Liquid Chromatography & Related Technologies, (2005); 28: 2053–2060. [Google Scholar]
- 18.Chaudhuri R.K., Salama O., Sticher O.; Preparative separation of diastereoisomeric iridoid glucosides by droplet counter-current chromatography; Planta Medica, (1980); 40: 164–167. [Google Scholar]
- 19.Zeng Y., Liu G., Ma Y., Chen X.Y., Ito Y.; Organic-high ionic strength aqueous solvent systems for spiral counter-current chromatography: graphic optimization of partition coefficient; Journal of Liquid Chromatography & Related Technologies, (2013); 36: 504–512. [PMC free article] [PubMed] [Google Scholar]