In this randomized clinical trial in nursing homes, manual tooth/gum brushing plus 0.12% chlorhexidine oral rinse, twice per day, plus upright positioning during feeding, did not significantly reduce the incidence of a first radiographically confirmed pneumonia compared with usual care.
Keywords: pneumonia, nursing homes, chlorhexidine, prevention, oral care
Abstract
Background. Pneumonia remains an important public health problem among elderly nursing home residents. This clinical trial sought to determine if a multicomponent intervention protocol, including manual tooth/gum brushing plus 0.12% chlorhexidine oral rinse, twice per day, plus upright positioning during feeding, could reduce the incidence of radiographically documented pneumonia among nursing home residents, compared with usual care.
Methods. This cluster-randomized clinical trial was conducted in 36 nursing homes in Connecticut. Eligible residents >65 years with at least 1 of 2 modifiable risk factors for pneumonia (ie, impaired oral hygiene, swallowing difficulty) were enrolled. Nursing homes were randomized to the multicomponent intervention protocol or usual care. Participants were followed for up to 2.5 years for development of the primary outcome, a radiographically documented pneumonia, and secondary outcome, a lower respiratory tract infection (LRTI) without radiographic documentation.
Results. A total of 834 participants were enrolled: 434 to intervention and 400 to usual care. The trial was terminated for futility. The number of participants in the intervention vs control arms with first pneumonia was 119 (27.4%) vs 94 (23.5%), respectively, and with first LRTI, 125 (28.8%) vs 100 (25.0%), respectively. In a multivariable Cox regression model, the hazard ratio in the intervention vs control arms, respectively, was 1.12 (95% confidence interval [CI], .84–1.50; P = .44) for first pneumonia and 1.07 (95% CI, .79–1.46, P = .65) for first LRTI.
Conclusions. The multicomponent intervention protocol did not significantly reduce the incidence of first radiographically confirmed pneumonia or LRTI compared with usual care in nursing home residents.
Clinical Trials Registration. NCT00975780.
(See the Editorial Commentary by Mody on pages 858–9.)
Pneumonia remains a global health problem, and it is particularly burdensome among elderly nursing home residents, among whom it is an important cause of functional decline, death, and healthcare costs [1–3]. Interventions targeted to risk factors for aspiration (eg, impaired oral hygiene, swallowing difficulty) prevent pneumonia, including in older adults, in several healthcare settings [4]. A clinical trial in Japan reported that enhanced oral care (ie, oral brushing after meals and weekly dental professional care) among nursing home residents reduced the incidence of pneumonia from 19% with usual care to 11% using enhanced oral care [5]. In acute healthcare settings, clinical trials showed that topical oral chlorhexidine reduced nosocomial lower respiratory tract infection (LRTI) after cardiac surgery, and semirecumbant positioning reduced the incidence of nosocomial LRTI [6, 7]. Nonetheless, recent meta-analyses have challenged the value of enhanced oral care in preventing pneumonia among mechanically ventilated patients apart from cardiac surgery patients with short duration of mechanical ventilation [8, 9].
In a pilot study of nursing home residents, individual intervention components (ie, topical oral chlorhexidine, oral brushing, and upright feeding positioning) were feasible to administer, adhered to by staff, and effective in improving oral hygiene and swallowing over a 3-month period [10]. This clinical trial sought to determine whether the incidence of radiographically documented pneumonia was reduced among residents of nursing homes that adopted a multicomponent intervention protocol, compared with residents of homes that continued usual care.
METHODS
Study Design and Oversight
The study targeted nursing home facilities within a 60-mile radius of New Haven, Connecticut that housed at least 90 residents. The design was a cluster-randomized trial in which participants were followed for up to 2.5 years. The Yale University Human Investigation Committee, and the administrative leadership at all homes, approved the study. Nursing home administrators signed letters of agreement to participate, and participants or their surrogates provided written consent. An independent data safety and monitoring board (DSMB) monitored the study, which was registered at ClinicalTrials.gov (NCT00975780).
Participants
Study personnel established whether residents at each participating nursing home met eligibility criteria and had capacity for informed consent. Both sexes and all races were included if they were long-term-care residents age >65 years, resided at the nursing home for at least 1 month, and had at least 1 of 2 modifiable risk factors for pneumonia (ie, impaired oral hygiene, swallowing difficulty). Exclusion criteria included (1) housing for short-term rehabilitation; (2) presence of a gastric (including percutaneous endoscopic gastrostomy or nasogastric tube) or jejunostomy tube; (3) presence of a tracheostomy; (4) life expectancy <3 months; (5) current use of chlorhexidine; (6) pneumonia within the previous 6 weeks; (7) previous enrollment in the study; (8) unwillingness to give informed consent (from residents or designated surrogates); (9) non–English speaking; or (10) inappropriateness for the study in the opinion of nursing home administration.
Residents were screened for impaired oral hygiene and swallowing difficulty, and following written consent they underwent an oral examination to determine their oral plaque score. Impaired oral hygiene was considered present if (1) the staff who cared for the resident identified him/her as having impaired oral hygiene; (2) dental care was triggered for the resident as identified in the medical record; or (3) the oral plaque score was ≥1. Swallowing difficulty was considered present if staff identified him/her as having an episode of cough during swallowing at least once per week. Residents with a plaque score of ≥1 were eligible for enrollment (Supplementary Appendix). For decisionally impaired residents, surrogate consent was obtained, and verbal assent was sought from the resident if they were cognitively capable of understanding study participation.
Substudy Participants
A random sample of approximately 20% of participants had longitudinal evaluations targeted at baseline, 3 months, and every 6 months after enrollment of oral plaque scores and cough during swallowing (Supplementary Appendix).
Randomization and Interventions
The 36 participating homes were stratified into 2 groups by the number of minutes that nursing aides spent with residents per day as reported on http://www.medicare.gov/nursinghomecompare, because nursing staff are responsible for oral care and feeding within each home. Homes with ≥140 aide minutes per day were considered “high stratum”; homes with <140 aide minutes per day were considered “low stratum.” Homes were randomized within each stratum using a permuted block design with equal allocation to intervention or control arms [11]. Blinded study personnel performed screening assessments and approached eligible residents (or designated surrogates) for consent (ie, “prevalent participants”). After enrollment, the randomization status of the home was revealed. For homes randomized to intervention, study personnel trained nursing home staff about protocol procedures; there was no training in homes randomized to usual care. After prevalent participant enrollment was complete, subsequent screening continued to identify newly admitted or newly eligible residents at each home. These residents were consented and enrolled to optimize enrollment (ie, “incident participants”).
The intervention consisted of manual tooth/gum brushing plus 0.12% chlorhexidine oral rinse, administered twice per day, plus upright positioning during feeding. The intervention protocol was tailored to participants who could either perform self-care or required assistance (Supplementary Appendix). Control homes continued their usual oral care protocols, and we targeted surveys twice per year to determine if changes occurred during the study.
Data and Outcomes
Sociodemographic and clinical data were ascertained from the medical record, nursing staff, or direct resident assessment. The primary endpoint was development of first pneumonia. Pneumonia required the presence of (1) a compatible infiltrate on chest radiograph (CXR) (if previous CXR was available, the infiltrate had to be new or worsened) and (2) at least 2 of the following clinical features within 72 hours of the CXR-documented infiltrate: fever, pleuritic chest pain, respiratory rate >25 breaths/minute, worsening functional status (ie, decline in level of consciousness or activities of daily living), or new or increased cough, sputum production, shortness of breath, or chest examination findings.
The secondary endpoint was development of a first LRTI, defined as at least 3 pneumonia clinical criteria but with CXRs that were either not ordered or not compatible with pneumonia. CXRs were ordered at the discretion of primary providers. Two investigators adjudicated all outcomes, blinded to the randomization status of participants and the cumulative outcome incidence during the trial. A third blinded investigator resolved disagreements.
Chlorhexidine adherence within each home was determined by comparing expected with actual chlorhexidine volume expenditure. Oral brushing adherence was determined by comparing expected with actual residual toothpaste tube weight. The differences between expected and actual volume and weight were averaged over time periods to obtain an overall adherence measure. Upright feeding positioning adherence was evaluated qualitatively approximately once per month (Supplementary Appendix). Risk factor reduction was defined as improvement from baseline of oral hygiene (ie, ≥1 unit reduction in oral plaque score from baseline to final assessment) or swallowing (ie, absence of cough during swallowing at all observed meals at the final assessment).
Statistical Analyses
The target sample size was 828 participants to detect a 25% reduction in the cumulative 2.5-year first pneumonia rate with intervention relative to control assuming a type I error of 0.05 (2-sided), 80% power, an annual loss to follow-up rate of 20% (death, transfer out of the nursing home), equal allocation and an intracluster correlation (ICC) of 0.005 from a previous study [3]. Analyses of primary and secondary endpoints were by intent-to-treat. Analyses accounted for the cluster design; the unit of analysis was the participant. A P value of .05 (2-sided) was used for statistical significance. SAS 9.3 and R 2.15.2 software were used for analyses.
The treatment effect on the time to first pneumonia was analyzed by a Cox model employing a robust variance estimator [12] to account for participant clustering within nursing homes with adjustment for randomization strata and prespecified baseline covariates. Participants were censored who died or were transferred out of the nursing home. We tested the proportional hazards assumption using martingale residuals [13] and treatment by time interactions. The effect of intervention relative to control was estimated as a hazard ratio with 95% confidence intervals. Cumulative rates of pneumonia were calculated by the Kaplan–Meier method. Time to first LRTI and death were analyzed similarly to the primary outcome. In secondary analyses, a cluster competing risk model [14] was fit for the primary and secondary outcomes to account for the competing risk of death on estimates of treatment effects. Corresponding cumulative incidence rates for these outcomes were calculated by the method of Gray [15]. In exploratory analyses, we examined associations of intervention status with risk factor reduction (change in oral plaque score and swallowing difficulty), associations of adherence with risk factor reduction, and correlations of intervention nursing home adherence, measured as a continuous variable, with nursing home specific hazard functions and first pneumonia rates. One interim analysis was specified at 2.5–3 years for efficacy using a Haybittle–Peto stopping boundary of 0.001 (2-sided) with futility assessed by conditional power using 50% power under the current trend as a guideline for considering the study as futile [16, 17].
RESULTS
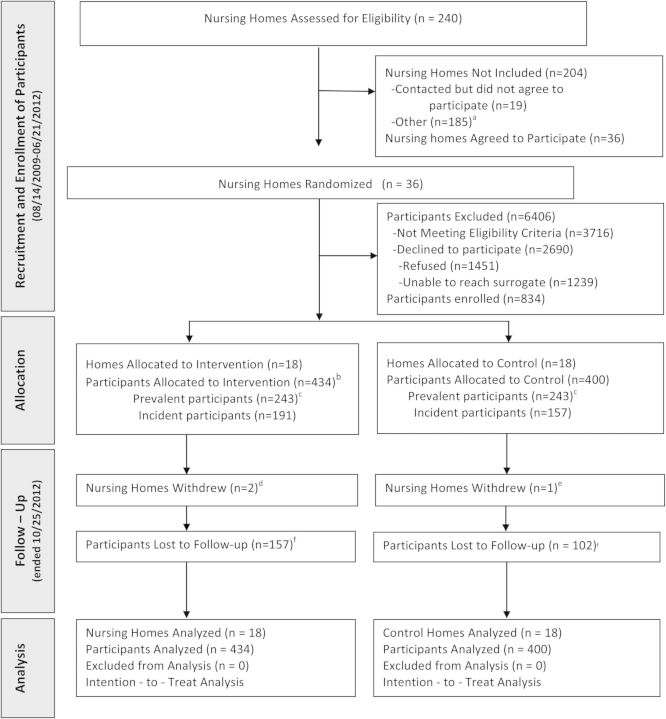
Among 36 nursing homes, 834 participants were enrolled: 434 to the intervention arm and 400 to the control arm (Figure 1). Three nursing homes withdrew during the study (2 intervention homes and 1 control home). The DSMB terminated the study for futility at the scheduled interim analysis because the conditional power under the observed treatment difference was nearly zero. At study termination, participants had a mean duration of follow-up until censoring or death of 1.13 years. Table 1 lists baseline participant characteristics. Results show no significant differences in age, sex, race or ethnicity, comorbidities, mental status, and functional status, between the 2 groups except for 1 measure of behavior (ie, resists care). Results show no statistically significant difference in the median star rating, the median number of beds per home, the proportion of nonprofit ownership, or the median number of nursing aide minutes per day spent with residents, in the intervention vs control homes (Supplementary Table 1). Prevalent and incident participants were also similar at baseline, except for ethnicity and 2 measures of mental status (Supplementary Table 2).
Figure 1.
Flow diagram of nursing homes and participants. aNursing home population mostly younger mental health residents, did not meet eligibility criteria, administrative reasons, or enrollment met; bTwo participants randomized to the intervention arm were identified after enrollment as having met a previously unidentified exclusion criterion: one participant had an existing pneumonia within 6 weeks and one participant was hospitalized (and had not resided in the nursing home for at least one month) prior to randomization. Both participants remained in the analyses; cPrevalent participants were those residents who were housed in the nursing home and recruited for the study at the time of initiation of the study at the home. Incident participants were recruited in subsequent waves of recruitment after the study was initiated at the home, approximately every three months; dWithdrew due to administrative leadership decision; eWithdrew due to foreclosure; fReasons for lost to follow-up included discharged from facility, facility withdrew from the study, and death.
Table 1.
Baseline Characteristics, Overall and by Treatment Status
| Characteristics | Total (n = 834) | Intervention (n = 434)a | Control (n = 400) | P Value |
|---|---|---|---|---|
| Age, y, mean ± SD | 86.3 ± 8.1 | 86.5 ± 8.0 | 86.1 ± 8.3 | .73 |
| Female sex | 636 (76.3) | 329 (75.8) | 307 (76.7) | .83 |
| Race/ethnicity | ||||
| Hispanic | 9 (1.1) | 5 (1.2) | 4 (1.0) | .88 |
| White | 780 (93.5) | 413 (95.2) | 367 (91.7) | .52 |
| Coexisting conditions | ||||
| Dementia | 659 (79.0) | 341 (78.6) | 318 (79.5) | .81 |
| COPD | 144 (17.3) | 75 (17.3) | 69 (17.3) | .99 |
| Stroke | 192 (23.0) | 100 (23.0) | 92 (23.0) | .99 |
| Congestive heart failure | 184 (22.1) | 98 (22.6) | 86 (21.5) | .74 |
| Cancer | 161 (19.3) | 81 (18.7) | 80 (20.0) | .65 |
| Diabetes | 244 (29.3) | 118 (27.2) | 126 (31.5) | .20 |
| Liver disease | 13 (1.6) | 7 (1.6) | 6 (1.5) | .89 |
| Kidney disease | 174 (20.9) | 96 (22.1) | 78 (19.5) | .49 |
| Depression | 511 (61.3) | 275 (63.4) | 236 (59.0) | .37 |
| No. of comorbid conditions, mean ± SD | 3.7 ± 1.4 | 3.7 ± 1.4 | 3.7 ± 1.4 | .90 |
| Vaccination statusb | ||||
| Influenza vaccination | 699 (91.4) | 373 (91.2) | 326 (91.6) | .95 |
| Pneumococcal vaccination | 604 (88.3) | 326 (86.7) | 278 (90.3) | .34 |
| Functional and behavioral status | ||||
| Swallowing difficulty | 165 (19.8) | 79 (18.2) | 86 (21.5) | .40 |
| Presence of teeth | 539 (64.6) | 280 (64.5) | 259 (64.7) | .94 |
| No. of ADL disabilitiesc | ||||
| 1–3 | 184 (22.1) | 83 (19.1) | 101 (25.2) | .50 |
| 4–7 | 197 (23.6) | 101 (23.3) | 96 (24.0) | … |
| Resists care | 94 (11.3) | 34 (7.8) | 60 (15.0) | <.001 |
| Restraint use in the past 2 wk | 39 (4.7) | 21 (4.8) | 18 (4.5) | .89 |
| Cognitive and mental status | ||||
| Confusiond | 676 (84.7) | 344 (82.9) | 332 (86.7) | .44 |
| Delirium severityd | ||||
| Low | 193 (23.1) | 93 (21.4) | 100 (25.0) | .68 |
| High | 140 (16.8) | 70 (16.1) | 70 (17.5) | … |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ADL, activities of daily living; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
a P values for categorical variable by treatment group comparisons were estimated using Rao–Scott χ2 statistics, which account for stratification and the clustering of study participants in nursing homes. P values for continuous variable by treatment group comparisons were estimated with variance estimators using the Taylor series method to account for stratification and the clustering of study participants in nursing homes.
b There were 70 missing values for the influenza vaccination variable and 151 missing values for the pneumococcal vaccination variable.
c ADL disabilities was defined as total dependence in the number of 7 ADLs (ie, bed mobility, transfer, dressing, eating, toilet use, personal hygiene, and bathing). There were 453 participants without total dependence in any of the 7 ADLs.
d There were 36 missing values for the confusion variable. Delirium was defined by presence of 6 indicators of delirium (ie, easily distracted, periods of altered perception or awareness of surroundings, episodes of disorganized speech, periods of restlessness, periods of lethargy, and mental function varies over the course of the day). Low delirium severity was defined as 1–2 indicators present, and high severity was defined as 3–6 indicators present. Five hundred one participants had no indicators present at baseline.
Table 2 presents counts, unadjusted person-year rates, and adjusted Cox regression model data for first pneumonia, first LRTI, and death. The number of participants with a first pneumonia was 119 (27.4%) with intervention vs 94 (23.5%) with control. Results show no significant differences for either outcome between intervention and control arms. Figure 2A and 2B present the cumulative incidence of first pneumonia and first LRTI, respectively. Results show no significant difference for either outcome. Relative risk findings were similar using a competing risk model (Supplementary Table 3 and Supplementary Figure 1A and 1B).
Table 2.
Frequency, Person-year Rates, and Cox Model Results for Outcomes and Death
| Outcomes | Total (N = 834) |
Intervention (n = 434) |
Control (n = 400) |
Adjusted Cox Modela |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | Rateb (95% CI) | No. (%) | Rate (95% CI) | No. (%) | Rate (95% CI) | Hazard Ratio | (95% CI) | P Value | |
| First Pneumoniac | 213 (25.5) | 0.27 (.22–.33) | 119 (27.4) | 0.28 (.22–.37) | 94 (23.5) | 0.26 (.19–.36) | 1.12d,e | (.84–1.50) | .44 |
| First LRTI | 225 (27.0) | 0.28 (.23–.34) | 125 (28.8) | 0.29 (.23–.37) | 100 (25.0) | 0.27 (.21–.36) | 1.07 | (.79–1.46) | .65 |
| Death | 210 (25.2) | 0.22 (.19–.26) | 122 (28.1) | 0.24 (.20–.28) | 88 (22.0) | 0.20 (.16–.27) | 1.16 | (.88–1.53) | .29 |
Abbreviations: CI, confidence interval; ICC, intracluster correlation; LRTI, lower respiratory tract infection.
a These models are adjusted for prespecified variables for stratum, age, race, sex, comorbid conditions, chronic obstructive pulmonary disease, swallowing difficulty, and the presence of teeth.
b Rates per person-year of surveillance were generated by a Poisson regression model using a robust variance estimator to account for the clustering of study participants in nursing homes.
c The observed ICC for the first pneumonia outcome was 0.035; it was estimated using data obtained from the early termination of the study.
d The proportional hazards assumption is not met in this model, so the hazard ratio in this case represents an average measure of risk over time rather than a measure of instantaneous risk.
e An indicator variable for prevalent (vs incident) participant was added to the multivariable Cox model and was not significant (P = .680). An interaction term, crossing the prevalent participant with treatment group variables, was added to the model and was not statistically significant (P = .849).
Figure 2.
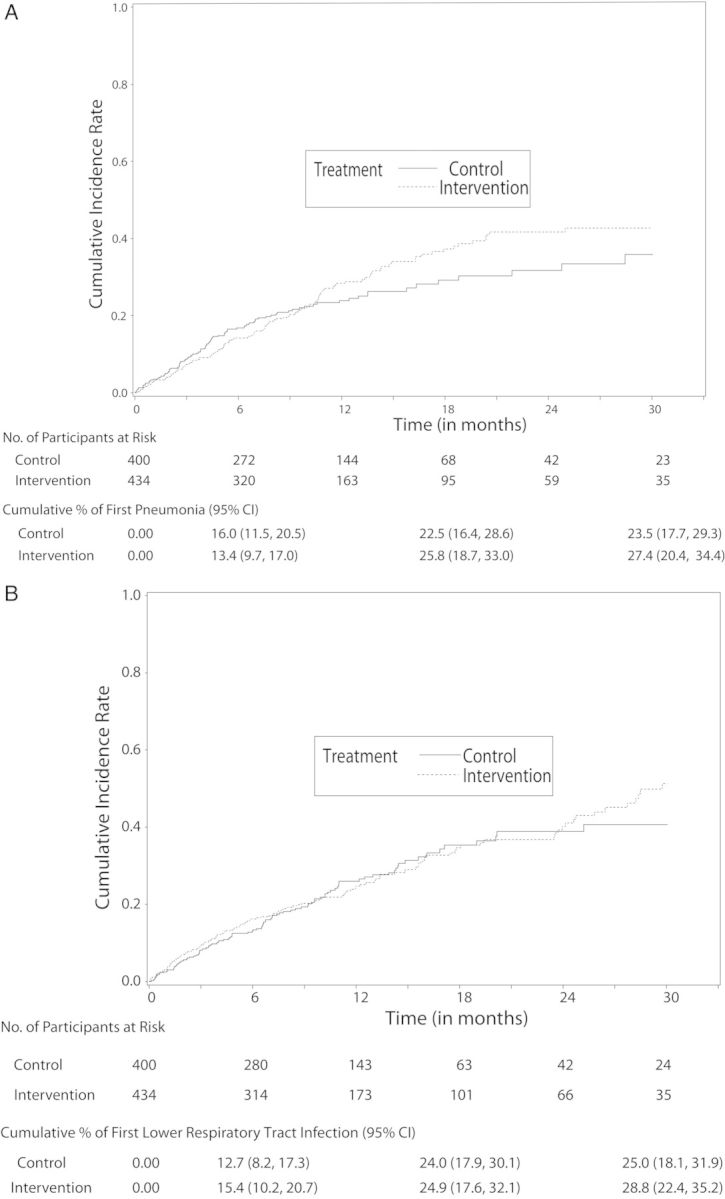
A, Cumulative incidence of first pneumonia by treatment (1 – Kaplan–Meier estimates). The number of participants at risk at 30 months represents participants who were censored after they completed the 30 months of follow-up as specified in the study protocol. B, Cumulative incidence of first lower respiratory tract infection by treatment (1 – Kaplan–Meier estimates). The number of participants at risk at 30 months represents participants who were censored after they completed the 30 months of follow-up as specified in the study protocol. The cumulative percentages in (A and B) are calculated by dividing the number of outcome events in each treatment arm at each time point by the total number of participants enrolled in the respective treatment arm (ie, 400 in the control arm and 434 in the intervention arm), and then by multiplying by 100; 95% confidence intervals (CIs) for the percentages account for the clustering of participants within nursing homes.
In the intervention arm, the overall mean adherence was 87.9% (standard deviation [SD], 20.5%) to chlorhexidine and 75.0% (SD, 23.4%) to toothpaste. Overall adherence to upright feeding position when observed was 100%. There were no protocol-related serious adverse events, and there were 64 protocol-related nonserious adverse events, all of which were anticipated. The most common protocol-related nonserious adverse events were oral cavity disturbances and dental staining.
In exploratory analyses, risk factor reduction was assessed in a subset of the cohort (n = 197 for plaque score; n = 23 for swallowing difficulty). Results show no significant association between intervention status or level of adherence with reduction from the first to last measurement in either swallowing or oral plaque score (data not shown). In addition, results show no correlation between level of adherence, as measured by toothpaste and chlorhexidine expenditure, and first pneumonia outcomes (Supplementary Table 4A and 4B). We ascertained usual daily oral care protocol information from 16 of the 18 control homes in which the information was available. All 16 reported oral brushing twice per day and 5 reported oral antibacterial rinsing twice per day as part of their usual oral care; 2 reported flossing twice per day as part of their usual oral care. Among 12 control homes that provided follow-up information during the study, none showed changes to their oral brushing and rinsing protocols.
DISCUSSION
In this cluster-randomized controlled trial, the multicomponent intervention protocol did not significantly reduce the cumulative incidence of a first radiographically confirmed pneumonia compared with usual care. Similarly, there was no significant difference in the incidence of a first LRTI between intervention and control arms.
Nursing home–acquired pneumonia occurs at an average rate of 1 episode per 1000 days of care, which is 10-fold greater than the rate in elderly community dwellers [18–20]. It has been reported to constitute a major proportion of patients with healthcare-associated pneumonia [21, 22]. Although Streptococcus pneumoniae and influenza vaccination remain important for nursing home residents [23, 24], the persistent clinical burden of pneumonia suggests the need for additional prevention strategies. Interventions to improve oral hygiene and swallowing decrease the risk of pneumonia in selected healthcare-associated populations, but there is a need for large high-quality randomized trials [4–9].
Our clinical trial tested a multicomponent protocol, targeted to both oral hygiene and swallowing, for its effectiveness in reducing pneumonia in a large nursing home cohort in the United States at high risk for pneumonia. The methodological strategy used a cluster design in which nursing homes, instead of individuals, were randomized to either adopt the multicomponent intervention protocol or continue their usual care. Advantages of the cluster design included avoidance of potential control-arm contamination when randomizing at the level of participant or nursing unit, efficiency in staff training, and improved ability of adopting the intervention facility-wide. The main disadvantage to the cluster design was the risk of imbalance of home and resident characteristics between intervention and control arms [25]. To reduce potential baseline imbalances, homes were stratified prior to randomization by the number of minutes per day that nursing aides spent with residents. Although baseline characteristics of the participants were well balanced between the intervention and control homes (Table 1), the intervention protocol did not significantly reduce the rate of first pneumonia, prompting study termination for futility.
The lack of effectiveness of the intervention protocol in pneumonia reduction among nursing home residents was similar to the recent meta-analysis of oral chlorhexidine use and ventilator-associated pneumonia prevention among non–cardiac surgery patients [9]. Ineffectiveness of the intervention protocol for pneumonia reduction among our nursing home cohort has several potential explanations. First, staff adherence to the intervention protocol may have been inadequate to prevent pneumonia. Although overall measured rates of adherence to oral brushing (75.0%), topical oral chlorhexidine (87.9%) and upright feeding positioning (100%) appeared high, it is possible that our quantitative measures of toothpaste and chlorhexidine expenditure by staff overrepresented actual participant administration. Second, control home usual oral care may have been modified after study initiation to more closely resemble the intervention arm. This is unlikely, as information available from control homes in which >1 assessment of their oral care policy was obtainable, there were no changes in usual oral brushing and rinsing protocols over the course of the study. Third, although we stratified homes to reduce imbalances in the cluster design, there may have been unidentified confounders, such as imbalances that were more common among the intervention homes that increased pneumonia outcomes (eg, respiratory viral outbreaks, lower vaccination rates among staff, low community childhood pneumococcal vaccination impact on herd immunity [26], decisions to perform CXR or hospitalize residents). Finally, it is plausible that the oral microbiota of participants changed during the intervention protocol and had a heterogeneous impact on pathogen acquisition over time. Recent studies have demonstrated that the microbiota of nursing home residents are distinct from young and older adult community dwellers [27]. Therefore, the oral bacterial community of nursing home residents may represent a vulnerable ecological niche in which daily oral brushing and topical chlorhexidine may initially reduce existing pathogen colonization, but over time alter the entire oral bacterial community enough to facilitate new bacterial pathogen acquisition (eg, Staphylococcus aureus, Enterobacteriaceae, Pseudomonas) and counteract any initial benefit.
Our study had several advantages over previous reports. First, the multicomponent protocol had both biological plausibility to reduce pneumonia and demonstrated feasibility to administer in a nursing home setting [10]. Second, our cohort was large and assembled among community nursing homes that enhanced real-world effectiveness assessment of the intervention and generalizability of the results. Third, we used a rigorous primary outcome of pneumonia that required radiographic documentation, with an intent-to-treat analysis.
Nonetheless, our study had limitations. First, our cohort was limited to a single state and a subset of residents at high risk for pneumonia, not all nursing home residents. Second, to maximize enrollment, our cohort included participants (ie, “incident participants”) who were screened and enrolled after the initial enrollment (ie, “prevalent participants”) and home randomization. Although this raised the risk of potential bias, baseline characteristics were similar between prevalent and incident participants (Supplementary Appendix). Third, our longitudinal assessment of oral plaque scores, in a subset of the cohort, was done by dental study staff that participated in training of intervention home staff, so their assessments were unblinded. Despite potential for bias favoring improvement in plaque scores among intervention participants, results show no significant differences in plaque scores between intervention and control arms.
We believe that oral brushing, upright feeding positioning, and oral topical chlorhexidine, when clinically indicated, remain important strategies for maintaining oral health and subjective comfort among elderly nursing home residents; in some healthcare settings, they have been shown to reduce the incidence of LRTIs [4–9, 28]. However, our study results do not support their utility as a pneumonia prevention strategy in this high-risk population of elderly nursing home residents. Other innovative strategies require further investigation to reduce this important public health burden.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We appreciate the participation of all 36 nursing homes in this study.
Author contributions. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the National Institutes of Health, the National Institute on Aging (NIA) (K23AG028691, R01AG030575, K07AG030093, and P30AG021342). This study was conducted in part at the Yale Claude D. Pepper Older Americans Independence Center.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Health statistics and information systems. Available at: http://www.who.int/healthinfo/global_burden_disease. Accessed 2 December 2014.
- 2.High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:149–71. doi: 10.1086/595683. [DOI] [PubMed] [Google Scholar]
- 3.Quagliarello V, Ginter S, Han L, Van Ness P, Allore H, Tinetti M. Modifiable risk factors for nursing home-acquired pneumonia. Clin Infect Dis. 2005;40:1–6. doi: 10.1086/426023. [DOI] [PubMed] [Google Scholar]
- 4.Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Amer Geriatr Soc. 2003;51:1018–22. doi: 10.1046/j.1365-2389.2003.51318.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Amer Geriatr Soc. 2002;50:430–3. doi: 10.1046/j.1532-5415.2002.50106.x. [DOI] [PubMed] [Google Scholar]
- 6.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296:2460–6. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 7.Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Noque S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–8. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 8.Alhazzani W, Smith O, Muscedere J, Medd J, Cook D. Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta-analysis of randomized trials evaluating ventilator-associated pneumonia. Crit Care Med. 2013;41:646–55. doi: 10.1097/CCM.0b013e3182742d45. [DOI] [PubMed] [Google Scholar]
- 9.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174:751–61. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 10.Quagliarello VJ, Juthani-Mehta M, Ginter S, Towle V, Allore H, Tinetti M. Pilot testing of intervention protocols to prevent pneumonia among nursing home residents. J Amer Geriatr Soc. 2009;57:1226–31. doi: 10.1111/j.1532-5415.2009.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival analysis: state of the art. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 13.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative martingale residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 14.Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13:371–83. doi: 10.1093/biostatistics/kxr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 16.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan GKK, Wittes J. The B-value: A tool for monitoring data. Biometrics. 1988;44:579–85. [PubMed] [Google Scholar]
- 18.Muder R. Pneumonia in residents of long term care facilities: epidemiology, etiology, and prevention. Amer J Med. 1998;105:319–30. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 19.Marrie TJ. Community acquired pneumonia. Clin Infect Dis. 1994;18:501–15. doi: 10.1093/clinids/18.4.501. [DOI] [PubMed] [Google Scholar]
- 20.Muder R. Management of nursing home acquired pneumonia: unresolved issues and priorities for future investigation. J Am Geriatr Soc. 2000;48:95–6. doi: 10.1111/j.1532-5415.2000.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 21.Shindo Y, Sato S, Maruyama E, et al. Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest. 2009;135:633–40. doi: 10.1378/chest.08-1357. [DOI] [PubMed] [Google Scholar]
- 22.Attridge RT, Frei CR. Health care-associated pneumonia: an evidence-based review. Amer J Med. 2011;124:689–97. doi: 10.1016/j.amjmed.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomized and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monto AS, Hornbuckle K, Ohmit SE. Influenza vaccine effectiveness among elderly nursing home residents: a cohort study. Am J Epidemiol. 2001;154:155–60. doi: 10.1093/aje/154.2.155. [DOI] [PubMed] [Google Scholar]
- 25.Van Ness PH, Peduzzi PN, Quagliarello VJ. Efficacy and effectiveness as aspects of cluster randomized trials with nursing home residents: methodological insights from a pneumonia prevention trial. Cont Clin Trials. 2012;33:1124–31. doi: 10.1016/j.cct.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin M, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 28.Terpenning M. Geriatric oral health and pneumonia risk. Clin Infect Dis. 2005;54:138–43. doi: 10.1086/430603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.