Abstract
B cell lymphoma/leukemia gene 6 (Bcl6) is well known as a master regulator of germinal center (GC) B cell differentiation. Now, three new publications demonstrate that Bcl6 also plays a second role in GC response, in that it is both required and sufficient to drive the differentiation of T follicular helper cells.
Germinal centers (GCs) are dynamic structures within secondary lymphoid tissues that are responsible for the generation of B cell memory and high-affinity antibodies (Manser, 2004). During a T cell-dependent antibody response, GCs form after naïve B cells are activated by cognate T cells, migrate to the boundary between the B and the T cell areas and initiate rapid focal expansion within a follicular dendritic cell network. Proliferating GC B cells diversify their immunoglobulin genes through class switch recombination and somatic hypermutation before eventually being selected to become memory or long-lived plasma cells. It is well accepted that, in addition to the initial T–B encounter, T cells also play a crucial role within the GC by facilitating the selection and maturation of mutated B cells. Yet, the exact nature of T cell help within this microenvironment remains an issue of debate. The identity of these T cells represents another conundrum. In the existing paradigm for T helper cell differentiation, various effector subsets are defined largely based on the lineage-specific cytokine secretion profiles, i.e. Th1, Th2, Th17 and iTreg characteristically produce IFN-γ, IL-4, IL-17 and TGF-β/IL-10, respectively. In comparison, GC T cells produce a spectrum of cytokines that trespass the boundary of Th1, Th2 and Th17. This posts a question as to whether GC T cells belong to a single, discrete effector cell lineage or are a collection of various T helper subsets that have somehow acquired the follicle-homing property.
The name follicular helper T cells (Tfh) was first proposed in 2000 to describe a population of CD4+ T cells expressing CXC chemokine receptor 5 (CXCR5) which enables them to migrate to the GCs enriched in CXCL13, the ligand for CXCR5 (Breitfeld et al., 2000; Schaerli et al., 2000). The initial suggestion that Tfh may represent a distinct effector cell lineage came with global gene expression analysis of Th1, Th2 and Tfh subsets (Chtanova et al., 2004). A large number of genes were shown to be preferentially expressed by Tfh over Th1 and Th2 cells including IL-10 and IL-21, two cytokines known to stimulate B cell proliferation. A strong association of Tfh with B cell lymphoma/leukemia gene 6 (Bcl6), a transcription factor required for GC B cell differentiation, was also highlighted, confirming an earlier report based on immunohistochemistry staining. Historically, major advances in the T helper cell research field were made by identifying the requirements for specific cytokines and ‘master regulator’ transcription factors during lineage specification. The importance of IL-21 in Tfh development was recognized recently. Using IL-21- or IL-21R-deficient mice, two groups demonstrated a critical role of IL-21 in Tfh development and GC formation (Nurieva et al., 2008; Vogelzang et al., 2008). Combined with previous data on the role of IL-21 in B cell proliferation and maturation, these studies support a model in which Tfh-derived IL-21 not only provides B cell help in conjunction with costimulatory molecules, but also promotes CXCR5 up-regulation and possibly supports the expansion of Tfh within the GC. Naturally, the next quest is to identify the transcription factor that can specify the Tfh cell fate.
Bcl6 is a potent transcription repressor frequently targeted by genetic alternations in GC-derived B cell lymphomas. In B cells, high-level Bcl6 expression is restricted to the GC stage and Bcl6-deficient mice cannot form GCs or produce high-affinity antibodies. Subsequent studies have shown that Bcl6 functions as a master regulator of GC B cell differentiation by controlling a vast gene expression network (Klein and Dalla-Favera, 2008). With respect to T cells, published studies have shown that Bcl6 inhibits Th2 differentiation but plays a positive role in the development of long-term CD4+ memory T cells and central memory CD8+ T cells. Although the mechanism of Bcl6 action remains obscure in many of these previous T cell studies, the candidacy of Bcl6 for the Tfh master regulator is fairly attractive. Supporting evidence includes the selective association of Bcl6 with Tfh compared with other T helper cell subsets, the striking GC phenotype in the germline Bcl6 knock-out mice and published work linking IL-21 exposure and Bcl6 up-regulation in naïve mouse and human B cells.
With the recent publication of three elegant studies, the status of Bcl6 as a master regulator of Tfh fate is firmly established (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). Using gain-of-function and loss-of-function approaches, these researchers reached the same conclusion that Bcl6 is both required and sufficient to drive Tfh differentiation. Specifically, Bcl6-deficient naïve CD4+ T manifested severe defects in Tfh development both in cell culture system (Nurieva et al., 2009) and in vivo (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). On the contrary, Bcl6 over-expression caused up-regulation of Tfh markers including CXCR4/5, ICOS, PD-1 and IL-21R, and markedly enhanced Tfh and GC development in vivo. The most impressive demonstration was provided by Johnston et al. who showed that, in a TCR transgenic context, 80–90% of Bcl6-transduced CD4+ T cells adopted a Tfh phenotype. Using fetal liver chimeras or adoptive cell transfer approaches, all three groups concluded that the requirement for Bcl6 in Tfh differentiation was T cell autonomous. Since each group approached the Bcl6-Tfh link from a different angle and uncovered unique insights, the three studies also nicely complemented each other. Johnston et al. were intrigued by the reciprocal expression pattern between Bcl6 and Blimp-1, a transcription factor well known to antagonize Bcl6's function in late GC B cells. Thus, after defining the role of Bcl6 in Tfh differentiation, they turned to Blimp-1 and showed that Blimp-1 works as an antagonist of Bcl6 in the Tfh context as well. In another interesting experiment, they transferred T cells with different Bcl6 status into recipient mice that either lacked B cells or expressed a transgenic B cell receptor of the wrong specificity. This experiment confirmed that in vivo Tfh differentiation requires cognate T–B interaction. Following up their previous work on IL-21, Nurieva et al. explored the mechanism of Bcl6 up-regulation by studying the relationship between IL-6, IL-21 and Bcl6 in a cell cultured-based Tfh differentiation system. They observed that IL-6-primed activated CD4+ T cells increased the expression of IL-21, IL-21R and, most importantly, Bcl6. They also showed that much of the IL-6 and IL-21 effects in this system, e.g. up-regulation of IL-21R, IL-6R, CXCR5 and Bcl6, can be reproduced by forced expression of exogenous Bcl6. These experiments addressed the critical issue of cytokine–Bcl6 link and nicely demonstrated that the general principles driving Tfh differentiation also conform to the existing paradigm of effector T cell specification. Still, much mechanistic details remain to be elucidated regarding the precise contributions of IL-6, IL-21 and Bcl6 in establishing a stable Tfh transcription program. For instance, Bcl6 alone did not up-regulate IL-21 (Johnston et al., 2009), neither could it elevate ICOS or CD40L expression (Yu et al., 2009).
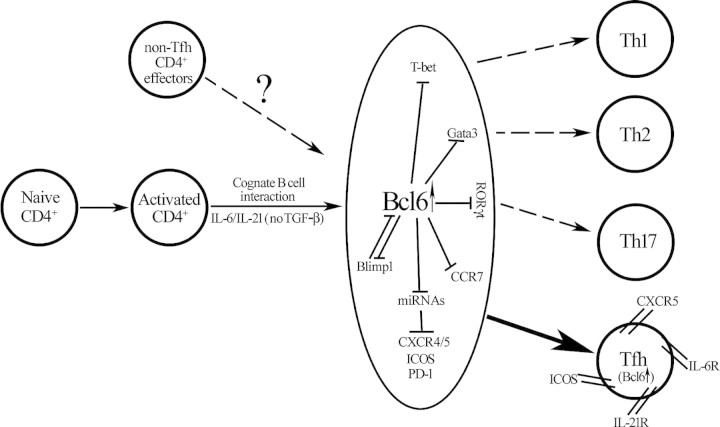
Although certain transcription factors can both promote and suppress transcription, no evidence exists of today that Bcl6 can directly transactivate target genes. Then, how does a transcription repressor positively promote the Tfh cell fate? Johnston et al. went an extra mile in this regard and proposed that Bcl6 achieves this via two complementary strategies. First, results from their chromatin immunoprecipitation and gene expression analyses suggested that Bcl6 may inhibit non-Tfh cell fates by inhibiting lineage-inappropriate master regulators such as T-bet, GATA-3 and possibly RORγc (Figure 1). Nurieva et al. suggested that, under a Tfh polarizing condition, Bcl6 can inhibit RORγc function but not expression. Johnston et al. also proposed a very interesting ‘suppression of the suppressor’ strategy to account for Bcl6's ability to promote Tfh cell fate. This approach depends upon the ability of Bcl6 to inhibit a set of microRNAs (miRNAs) capable of targeting Tfh marker genes. The authors conducted expression profiling and over-expression experiments to support this hypothesis. For instance, Bcl6 over-expression down-regulated miR-101/103 and the miR-17-92 cluster which can inhibit ICOS and CXCR5, respectively. This hypothesis has inherited appeal since miRNAs are known to fine-tune as opposed to shutting off gene expression, and Tfh is distinguished from other CD4+ effector subsets by quantitative differences in the expression levels of the Tfh signature genes. Interestingly, human GC B cells (Bcl6 high and CXCR5+) have been shown to highly express the miR-17-92 cluster compared with other B cell subsets. At the moment, it is unclear if this apparent discrepancy reflects a human versus mouse difference or cell-type specific activity of Bcl6.
Figure 1.
A model showing the Bcl6-regulated gene network that specifies the Tfh cell fate while inhibiting other T helper cell lineages. At the commitment stage, naïve CD4+ T cells are first activated by antigen-presenting cells (not shown) before falling under the influence of IL-6 and/or IL-21. Presumably, cognate T–B interaction is also required at this step, although the relationship between cognate B cell interaction and IL-6/IL-21 signaling is unclear. Committed Tfh cells start to secrete IL-21 and up-regulate IL-21R, thus completing an autocrine loop that triggers the up-regulation of Bcl6. As the level of Bcl6 arises, the lineage potential for Th1, Th2 and Th17 is suppressed due to Bcl6-mediated down-regulation or functional antagonism of T-bet, GATA3 and RORγc, respectively. Bcl6 also inhibits Blimp-1, a transcription factor that can antagonize Bcl6 activity during T helper cell differentiation. At the meantime, Bcl6 reprograms the traffic pattern of the developing Tfh cells by down-regulating CCR7 and up-regulating CXCR5. CXCR5 and other Tfh signature markers (CXCR4, ICOS and PD-1) are elevated by Bcl6 in an indirect manner due to Bcl6's ability to suppress the expression of miRNAs that normally target their 3′ UTR. A possibility for Bcl6 to redirect non-Tfh effectors to Tfh fate was also hinted in the studies.
In summary, these three studies have convincingly demonstrated a specific requirement for Bcl6 in Tfh differentiation and expanded our appreciation for Bcl6 as the ‘master regulator’ of GC response. As with all breakthrough discoveries, a new series of questions are also raised and await future investigations. Since a considerable amount of insights on Bcl6 function has already been gathered from years of investigations on the B cell side, this newly recognized role of Bcl6 in Tfh cells provides an exciting opportunity to further understand the Bcl6 biology from both a cell-type-specific perspective and in the dynamic GC microenvironment setting.
References
- Breitfeld D., Ohl L., Kremmer E., Ellwart J., Sallusto F., Lipp M., Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T., Tangye S.G., Newton R., Frank N., Hodge M.R., Rolph M.S., Mackay C.R. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., Ditoro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Manser T. Textbook germinal centers? J. Immunol. 2004;172:3369–3375. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P., Willimann K., Lang A.B., Lipp M., Loetscher P., Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang A., McGuire H.M., Yu D., Sprent J., Mackay C.R., King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]