Abstract
Background
Despite increased demand for contralateral prophylactic mastectomy (CPM), the survival benefit of this procedure remains uncertain.
Methods
We used the Surveillance, Epidemiology, and End Results database to identify 107 106 women with breast cancer who had undergone mastectomy for treatment between 1998 and 2003 and a subset of 8902 women who also underwent CPM during the same period. Associations between predictor variables and the likelihood of undergoing CPM were evaluated by use of χ2 analyses. Risk-stratified (estrogen receptor [ER] status, stage, and age) adjusted survival analyses were performed by using Cox regression. Statistical tests were two-sided.
Results
In a univariate analysis, CPM was associated with improved disease-specific survival (hazard ratio [HR] of death = 0.63, 95% confidence interval [CI] = 0.57 to 0.69; P < .001). Risk-stratified analysis showed that this association was because of a reduction in breast cancer–specific mortality in women aged 18–49 years with stages I–II ER-negative cancer (HR of death = 0.68, 95% CI = 0.53 to 0.88; P = .004). Five year–adjusted breast cancer survival for this group was improved with CPM vs without (88.5% vs 83.7%, difference = 4.8%). Although rates of contralateral breast cancer among young women with stages I–II disease undergoing CPM were independent of ER status, women with ER-positive tumors in the absence of prophylactic mastectomy also had a lower overall risk for contralateral breast cancer than women with ER-negative tumors (0.46% vs 0.90%, difference = 0.44%; P < .001).
Conclusions
CPM is associated with a small improvement in 5-year breast cancer–specific survival mainly in young women with early-stage ER-negative breast cancer. This effect is related to a higher baseline risk of contralateral breast cancer.
CONTEXT AND CAVEATS
Prior knowledge
To prevent subsequent breast cancer, some women with cancer in one breast will have the other breast surgically removed. Whether this treatment increases a woman's lifespan is unknown.
Study design
Population study of US women who had mastectomy (removal of the breast) during 1998–2003 for breast cancer and contralateral prophylactic mastectomy (removal of the other breast) during the same period. The associations of contralateral prophylactic mastectomy on breast cancer–specific survival were estimated, with further analyses by age, disease stage, and estrogen receptor status.
Contributions
Contralateral prophylactic mastectomy was associated with improved breast cancer–specific survival. This association was observed mainly among younger women (aged 18–49 years) with early-stage (I–II) estrogen receptor–negative breast cancer, whose 5-year breast cancer–specific survival rate increased by almost 5%.
Implications
Contralateral prophylactic mastectomy is associated with a small increase in 5-year breast cancer–specific survival, particularly among younger women with early-stage estrogen receptor–negative tumors.
Limitations
As an observational study, the results are subject to a variety of confounding factors, such as selection bias. The data used in the study were limited in terms of patient and tumor factors, such as BRCA mutation status, family history, and chemotherapy, which might affect the results.
From the Editors
The efficacy of prophylactic mastectomy in reducing the incidence of breast cancer is well established (1–4). Recent data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute show that women with unilateral breast cancer are increasingly seeking contralateral prophylactic mastectomy (CPM) (5). However, most women with unilateral breast cancer will not develop contralateral breast cancer during their lifetimes (2,6) and hence will derive no benefit from CPM (7). Furthermore, the potential benefit of CPM in reducing breast cancer mortality has not been adequately studied.
In a study of 148 BRCA1 and BRCA2 mutation carriers with unilateral breast cancer, patients who underwent CPM had improved overall survival but did not have improved breast cancer–specific survival (8). The improvement in overall survival came from a reduction in the number of ovarian cancers in the CPM cohort. After adjustment for bilateral prophylactic oophorectomy, no overall survival benefit was observed in patients who underwent CPM.
In contrast, another study showed that CPM was associated with a 43% relative (3.7% absolute) (hazard ratio [HR] for death = 0.57, 95% confidence interval [CI] = 0.45 to 0.72) reduction in the risk of death from breast cancer compared with a matched cohort of women who did not undergo CPM (6). However, the CPM cohort also had lower all-cause mortality (HR for death = 0.60, 95% CI = 0.50 to 0.72), raising the possibility that selection bias for an overall healthier cohort of patients was attributable to this association. Furthermore, analyses did not include adjustment for variables that may influence the decision to undergo CPM, such as age, stage of disease, and tumor histology (6). Because CPM is performed in breast cancer patients with heterogeneous characteristics, we hypothesized that the survival benefits of CPM are influenced by patient and tumor factors. To test this hypothesis, we examined the association between CPM and breast cancer–related survival among women with localized breast cancer within the SEER registry.
Methods
Data Source and Case Identification
Data from SEER, release 2008, were used for this study (10). Because this study used preexisting data with no personal identifiers, it was exempt from review by our institutional review board. SEER registry patients who were eligible for this cohort included women with adenocarcinoma of the breast diagnosed from January 1, 1998, through December 31, 2003, and treated with surgery. The first year when SEER coded data elements that would allow identification of patients with unilateral breast cancer who underwent a contralateral mastectomy as a component of the first course of treatment was 1998, and the last year of diagnosis that would allow patients to have at least 2 years of follow-up was 2003. All patients were reassigned a stage from the American Joint Committee on Cancer (AJCC), 5th edition (11), because SEER does not include data elements that would permit stage reassignment from the AJCC, 6th edition, for patients diagnosed during the study period. Only patients for whom the index breast cancer was the first cancer diagnosed were included.
Exclusion criteria included age younger than 18 years or older than 90 years, lack of histological confirmation, lack of information on tumor size, presence of distant metastasis, lobular carcinoma in situ histology, bilateral involvement at presentation, and diagnosis not microscopically confirmed. Patients were also excluded if the cancer-reporting source was a nursing home, hospice, autopsy report, or death certificate because these patients would not have been likely to receive cancer-directed therapy. In addition, only patients whose breast cancer was treated with total mastectomy or modified radical mastectomy were included in the analysis. The stepwise case selection criteria are listed in Table 1.
Table 1.
Stepwise case ascertainment for analysis*
| Parameter | No. of subjects |
| Start: breast cancers diagnosed 1998 to 2003 | 311 643 |
| Exclude patients with nonductal or lobular histology | 293 036 |
| Exclude patients with LCIS | 286 585 |
| Exclude patients younger than 18 y or older than 90 y | 283 829 |
| Exclude patients lacking microscopic confirmation | 283 715 |
| Exclude patients with bilateral involvement | 283 383 |
| Exclude men | 281 575 |
| Exclude patients with a nursing home, convalescent home, hospice, autopsy, or death certificate source of diagnosis | 281 549 |
| Exclude patients treated with radical mastectomy, extended radical mastectomy, breast-conserving surgery, mastectomy NOS, surgery NOS, unknown surgery, no surgery at primary tumor site, or local tumor destruction NOS | 114 609 |
| Exclude patients with stage IV disease or unknown stage | 107 189 |
| Exclude patients with no survival time | 107 106 |
LCIS = lobular carcinoma in situ; NOS = not otherwise specified.
Prophylactic mastectomy patients were defined as those who underwent total (simple) mastectomy or modified radical mastectomy with removal of the uninvolved contralateral breast. Patients who had undergone the same index procedures without removal of the contralateral breast constituted the comparison group. Tumor grade was classified as well differentiated (grade 1) vs moderately differentiated (grade 2) vs poorly differentiated (grade 3) vs undifferentiated (grade 4). Lymph node status was classified as negative vs one to three positive lymph nodes vs four or more positive lymph nodes, to be consistent with clinical practice.
Contralateral breast cancer events were identified as metachronous primary breast cancers within the contralateral breast by following the index diagnosis among patients with the same SEER registry identification number. Breast cancer events occurring within 2 months of diagnosis of the index cancer or on the same side as the index cancer were considered synchronous cancers and not included in the determination of contralateral breast cancer risk.
Statistical Analysis
Baseline patient and tumor characteristics were compared by use of the χ2 test to identify associations between predictor variables and the likelihood of undergoing CPM. The SEER registry codes cause of death; therefore, we used SEER data through December 31, 2005, to evaluate breast cancer–specific survival. Patients were censored if death was from a cause other than breast cancer or if the patient was alive at last follow-up. Although patients with stage 0 breast cancer (ductal carcinoma in situ) were included in the selection criteria, the data from this group of women were used only for characterization of the CPM cohort; all survival analyses were limited to women with stages I–III breast cancer. Disease-specific survival outcomes were estimated by use of the Kaplan–Meier method, and univariate comparisons were performed by use of the log-rank test. Noncancer cause of death, with censoring for cancer-specific mortality, was also evaluated to compare the different risk strata and to evaluate the effect of incompletely adjusted selection bias on the performance of CPM and, consequently, survival outcomes. In addition, we evaluated relative survival proportions as an indirect method of evaluating cancer-specific survival to confirm our findings (12). Relative survival was calculated as the ratio of the observed survival to the expected survival for the US general population, individually matched for age, sex, and the year in which the patient was been diagnosed with breast cancer. Expected survival for the US population were obtained from Human Mortality Database (http:www.mortality.org) (13). Cox proportional hazards regression with censoring for non–breast cancer–related causes of death was performed to adjust for covariate effects. The final regression model to determine the effect of CPM on survival was constructed by use of stepwise forward selection with switching accounting for clinically relevant variables (CPM not performed vs CPM performed; 18–49 vs 50–59 vs 60–90 years; non-Hispanic white vs Hispanic white vs black vs Asian or Pacific Islander vs Other; stage 0 vs I vs IIA vs IIB vs IIIA vs IIIB; lymph node negative vs one to three positive vs four or more positive; tumor grade 1 vs 2 vs 3 vs 4; estrogen receptor [ER] positive vs ER negative; nonlobular vs lobular histology; first tumor diagnosis yes vs no) and the maximum likelihood function of the model. We evaluated the goodness of fit of the Cox model with Arjas plots after grouping into quintiles of risk (14). Risk-stratified regression analysis was performed to separately evaluate the effect of CPM within different age (18–49 vs 50–59 vs 60–90 years), stage (I/II vs III), and ER status (ER positive vs ER negative) groups within the final model. A multivariable analysis of relative survival was performed by use of the generalized linear models with a Poisson assumption for the observed number of deaths (15).
Statistical analyses were performed by use of Stata MP version 10.0 (Stata Corp, College Station, TX). SAS version 9.13 SP4 (SAS Institute, Cary, NC) was used for data retrieval and cleaning from SEER. A two-sided P value of less than .05 was defined to be statistically significant.
Results
Review of the SEER data revealed a total of 311 643 cases of breast cancer diagnosed during the 6-year period of the study (1998–2003). After selection of patients with unilateral breast cancer who underwent mastectomy for treatment of their index carcinoma and met inclusion criteria, 107 106 patients remained for analysis. Of these patients, 8902 (8.3%) also underwent CPM. Median follow-up was 47 months.
Characteristics of patients who underwent CPM and those who did not were statistically significantly different (Table 2). Patients who elected CPM were more likely to be younger and to have earlier-stage disease (P < .001 for each). The percentage of women within each race and/or ethnicity who underwent CPM was greater among non-Hispanic white patients (9.5%) than among Hispanic white (5.4%), black (4.5%), or Asian or Pacific Islander (4.6%) patients (P < .001).
Table 2.
Baseline characteristics according to contralateral prophylactic mastectomy (CPM) status (N = 107 106) 1998–2003*
| CPM not performed (n = 98 204) |
CPM performed (n = 8902) |
||||
| Characteristic | No. | % | No. | % | χ2 statistic† |
| Age, y‡ | |||||
| 18–49 (referent) | 23 605 | 24 | 3731 | 42 | 2200 |
| 50–59 | 22 251 | 23 | 2629 | 30 | |
| 60–90 | 52 348 | 53 | 2542 | 29 | |
| Race and/or ethnicity§ | |||||
| Non-Hispanic white (referent) | 72 408 | 74 | 7584 | 85 | 575.97 |
| Hispanic white | 8211 | 8 | 469 | 5 | |
| Black | 8824 | 9 | 414 | 5 | |
| Asian or Pacific Islander | 8406 | 9 | 407 | 5 | |
| Other unspecified or unknown | 355 | 0 | 28 | 0 | |
| AJCC 5th cancer stage | |||||
| 0 (referent) | 12 833 | 13 | 1714 | 19 | 382.33 |
| I | 31 523 | 32 | 3058 | 34 | |
| IIA | 24 805 | 25 | 2081 | 23 | |
| IIB | 18 157 | 18 | 1260 | 14 | |
| IIIA | 5900 | 6 | 468 | 5 | |
| IIIB | 4986 | 5 | 321 | 4 | |
| Lymph node status | |||||
| Negative (referent) | 51 177 | 52 | 4802 | 54 | 160.70 |
| 1–3 Positive | 21 446 | 22 | 1863 | 21 | |
| ≥4 Positive | 15 198 | 15 | 1027 | 12 | |
| Unknown | 10 383 | 11 | 1210 | 14 | |
| Tumor grade | |||||
| I (referent) | 12 879 | 13 | 1289 | 14 | 122.93 |
| II | 36 032 | 37 | 3235 | 36 | |
| III | 34 601 | 35 | 2741 | 31 | |
| IV | 4031 | 4 | 474 | 5 | |
| Unknown | 10 661 | 11 | 1163 | 13 | |
| ER status | |||||
| Positive (referent) | 56 043 | 57 | 4959 | 56 | 43.18 |
| Negative | 17 689 | 18 | 1460 | 16 | |
| Unknown or not done | 24 472 | 25 | 2483 | 28 | |
| Histology type | |||||
| Nonlobular (referent) | 79 835 | 81 | 6570 | 74 | 293.78 |
| Lobular | 18 369 | 19 | 2332 | 26 | |
| First tumor diagnosis | |||||
| No (referent) | 25 789 | 26 | 2882 | 32 | 155.64 |
| Yes | 72 415 | 74 | 6020 | 68 | |
Stage according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 5th edition. ER = estrogen receptor.
All P values were less than .001. P values were calculated using the χ2 statistic. All statistical tests were two-sided.
Median (interquartile range) age of diagnosis for CPM performed was 52 (45–61) years and for CPM not performed was 61 (50–73) years.
Percent data shown represent data analysis down each column; corresponding percent values in the text represent analysis across each row.
In a univariate analysis, CPM was associated with improved disease-specific survival for women with stages I–III breast cancer (HR of death = 0.63, 95% CI = 0.57 to 0.69; P < .001) (Table 3); women with stage 0 breast cancer were excluded from survival analyses. Other variables that were associated with disease-specific survival were disease stage, lymph node status, tumor grade, ER status, race and/or ethnicity, histology, and age (P < .001 for each). In a Cox model, CPM remained associated with improved disease-specific survival (Table 3). Because we had determined that multiple covariates were important predictors for the performance of CPM (Table 2), we adjusted for these covariates—age, race and/or ethnicity, stage, lymph node status, tumor histology, grade, and ER status—in the survival analysis. In multivariable analysis, disease stage, lymph node status, tumor grade, ER status, race and/or ethnicity, histology, and age remained statistically significantly associated with survival outcome (P < .001 for each, Table 3).
Table 3.
Univariate and multivariable analyses for factors associated with survival (N = 107 106)*
| Characteristic | No. (%) | Unadjusted HR† (95% CI) | Adjusted HR*,† (95% CI) |
| CPM | |||
| CPM not performed | 98 204 (91.69) | 1 (referent) | 1 (referent) |
| CPM performed | 8902 (8.31) | 0.63 (0.57 to 0.69) | 0.84 (0.76 to 0.92) |
| Age, y | |||
| 18–49 | 27 336 (25.52) | 1 (referent) | 1 (referent) |
| 50–59 | 24 880 (23.23) | 0.85 (0.80 to 0.90) | 1.00 (0.94 to 1.05)‡ |
| 60–90 | 54 890 (51.25) | 0.92 (0.88 to 0.96§ | 1.35 (1.28 to 1.41) |
| Race and/or ethnicity | |||
| Non-Hispanic white | 79 992 (74.68) | 1 (referent) | 1 (referent) |
| Hispanic white | 8680 (8.10) | 1.35 (1.26 to 1.45) | 1.01 (0.94 to 1.09)‖ |
| Black | 9238 (8.63) | 2.03 (1.92 to 2.15) | 1.44 (1.36 to 1.53) |
| Asian or Pacific Islander | 8813 (8.23) | 0.82 (0.76 to 0.89) | 0.87 (0.78 to 0.92) |
| Other unspecified or unknown | 383 (0.36) | 0.32 (0.17 to 0.59) | 0.37 (0.20 to 0.70)§ |
| AJCC 5th cancer stage | |||
| 0 | 14 547 (13.58) | 1 (referent) | 1 (referent) |
| I | 34 581 (32.29) | 3.33 (2.78 to 3.99) | 8.97 (7.39 to 10.89) |
| IIA | 26 886 (25.10) | 8.80 (7.38 to 10.50) | 19.06 (15.75 to 23.07) |
| IIB | 19 417 (18.13) | 19.20 (16.12 to 22.85) | 32.22 (26.55 to 39.09) |
| IIIA | 6368 (5.95) | 33.14 (27.75 to 39.59) | 45.94 (37.71 to 55.96) |
| IIIB | 5307 (4.95) | 59.86 (50.19 to 71.40) | 74.49 (61.47 to 90.28) |
| Lymph node status | |||
| Negative | 55 979 (52.27) | 1 (referent) | 1 (referent) |
| 1–3 positive lymph nodes | 23 309 (21.76) | 2.90 (2.74 to 3.07) | 1.19 (1.11 to 1.27) |
| ≥4 positive lymph nodes | 16 225 (15.15) | 7.86 (7.46 to 8.28) | 2.40 (2.24 to 2.57) |
| Unknown | 11 593 (10.82) | 1.88 (1.73 to 2.03) | 2.31 (2.12 to 2.52) |
| Tumor grade | |||
| 1 | 14 168 (13.23) | 1 (referent) | 1 (referent) |
| 2 | 39 267 (36.66) | 2.37 (2.13 to 2.65) | 1.59 (1.43 to 1.78) |
| 3 | 37 342 (34.86) | 6.56 (5.90 to 7.29) | 2.61 (2.34 to 2.91) |
| 4 | 4505 (4.21) | 3.30 (2.86 to 3.81) | 2.86 (2.47 to 3.31) |
| Unknown | 11 824 (11.04) | 2.09 (1.84 to 2.38) | 1.66 (1.46 to 1.89) |
| ER status | |||
| Positive | 61 002 (56.95) | 1 (referent) | 1 (referent) |
| Negative | 19 149 (17.88) | 3.38 (3.23 to 3.53) | 2.36 (2.25 to 2.48) |
| Unknown or not done | 26 955 (25.17) | 0.90 (0.85 to 0.95) | 1.49 (1.40 to 1.58) |
| Histology type | |||
| Nonlobular | 86 405 (80.67) | 1 (referent) | 1 (referent) |
| Lobular | 20 701 (19.33) | 0.73 (0.69 to 0.78) | 0.86 (0.81 to 0.92) |
| First tumor diagnosis | |||
| No | 28 671 (26.77) | 1 (referent) | 1 (referent) |
| Yes | 78 435 (73.23) | 1.56 (1.48 to 1.64) | 0.59 (0.56 to 0.63) |
Adjusted for all other variables shown in the table. Stage according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 5th edition. All P values were calculated using Cox regression with the Wald test for significance. All P values were two-sided. CI = confidence interval; CPM = contralateral prophylactic mastectomy; ER = estrogen receptor.
All P values were less than .001.
P = .97.
P < .01
P = .61.
To consider the possibility that the improved breast cancer–specific survival that was associated with CPM reflected a selection bias for an overall healthier patient cohort (16), we evaluated cancer-specific and non–breast cancer causes of death among age-stratified cohorts (Figure 1). On adjusted analysis, we found that the cancer-related survival associated with CPM declined with age, with women younger than 50 years having a modest risk reduction (HR of death = 0.84, 95% CI = 0.72 to 0.97; P = .02) and with those older than 60 years having no risk reduction from CPM (HR of death = 0.88, 95% CI = 0.75 to 1.03; P = .13). Conversely, among young women, there was no association between CPM and noncancer causes of death (HR of death = 0.62, 95% CI = 0.36 to 1.06; P = .09), whereas a strong association was observed for women older than 60 years (HR of death= 0.63, 95% CI = 0.53 to 0.74; P < .001). For women between the ages of 50 and 59 years, CPM was associated with both an improved cancer-specific and noncancer survival (HR of death = 0.79, 95% CI = 0.66 to 0.95, P = .01; and HR of death = 0.53, 95% CI = 0.32 to 0.87, P = .01, respectively). These data strongly suggest that with increasing age, a bias exists for selecting a healthier cohort of women for CPM.
Figure 1.
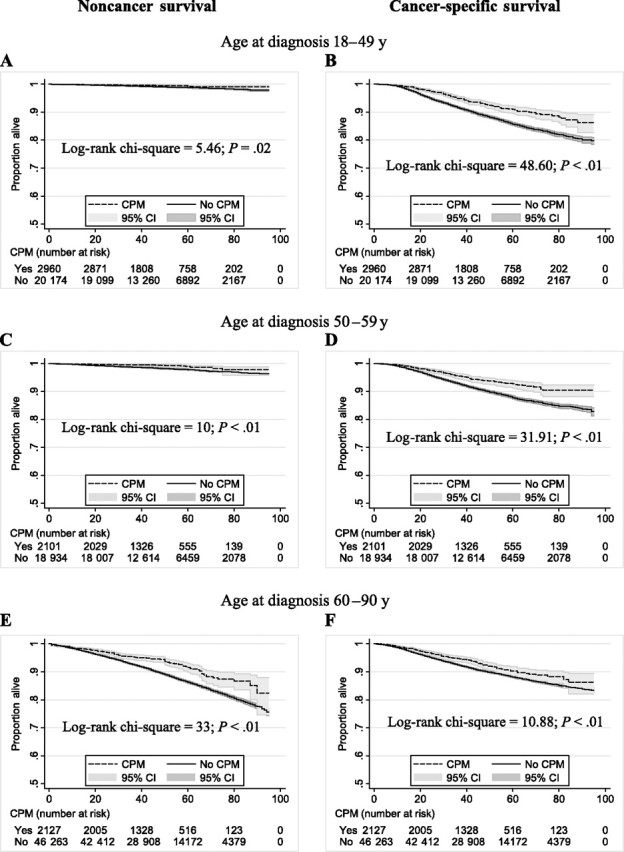
Noncancer and cancer-specific survival for contralateral prophylactic mastectomy (CPM) vs no CPM by age at diagnosis. A) Noncancer survival associated with CPM among women aged 18–49 years at time of breast cancer diagnosis (hazard ratio [HR] for death = 0.62, 95% confidence interval [CI] = 0.36 to 1.06; P = .09). B) Cancer-specific survival associated with CPM among women aged 18–49 years at time of breast cancer diagnosis (HR for death = 0.84, 95% CI = 0.72 to 0.97; P = .02). C) Noncancer survival associated with CPM among women aged 50–59 years at time of breast cancer diagnosis (HR for death = 0.53, 95% CI = 0.32 to 0.87; P = .01). D) Cancer-specific survival associated with CPM among women aged 50–59 years at time of breast cancer diagnosis (HR for death = 0.79, 95% CI = 0.66 to 0.95; P = .01). E) Noncancer survival associated with CPM among women aged 60–90 years at time of breast cancer diagnosis (HR for death = 0.63, 95% CI = 0.53 to 0.74; P < .001). F) Cancer-specific survival associated with CPM among women aged 60–90 years at time of breast cancer diagnosis (HR for death = 0.88, 95% CI = 0.75 to 1.03; P = .13). The scale for x-axis is time after diagnosis in months. Adjusted for age, race, tumor stage, number of positive lymph nodes, tumor grade, tumor histology, and first tumor indicator in Cox regression model. All P values are two-sided and were calculated using the log-rank test.
From our analysis of noncancer mortality, it was clear that simple adjusted analysis was insufficient to explain the findings, and we further tested our hypothesis that the survival benefits associated with CPM are influenced by patient and tumor factors by performing risk-stratified multivariable regression analyses. Given the importance of age on clinical decision making, disease stage on survival, and the importance of ER status as an indication for hormonal therapy, we stratified patients on the basis of these three risk factors.
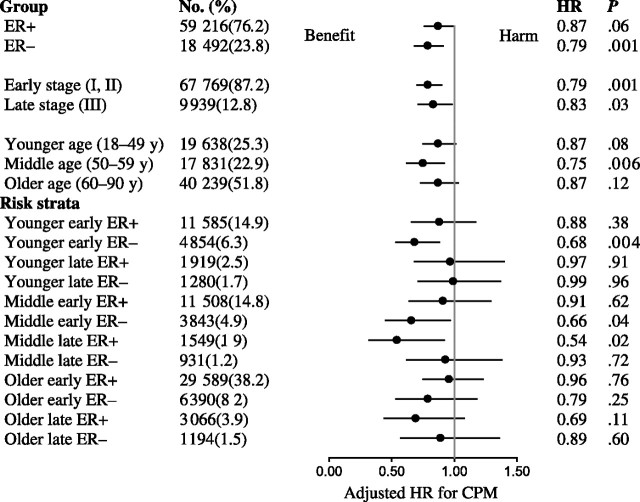
We found that in patients diagnosed before age 50 years, those with stage I or II ER-negative breast cancer had a reduction in the risk of disease-specific mortality associated with CPM (HR for death = 0.68, 95% CI = 0.53 to 0.88; P = .004) (Figure 2). A similar association was not seen among young women with early-stage ER-positive breast cancer (HR for death = 0.88, 95% CI = 0.66 to 1.17; P = .38). These reductions translated into an increase in 5 year–adjusted breast cancer–specific survival of 4.8% (88.5% vs 83.7%) in favor of CPM among the young, early-stage, ER-negative group compared with 0.5% (96.4% vs 95.9%) among their ER-positive counterparts (Figure 3).
Figure 2.
Forest plot of risk-stratified hazard rates for disease-specific mortality associated with contralateral prophylactic mastectomy (CPM). Hazard ratios (HRs; solid circles) and 95% confidence intervals (shown by the whiskers on both sides of the solid circles) were derived by multivariable Cox regression analysis, adjusted for number of positive lymph nodes (0 vs 1–3 vs ≥4), tumor grade (1 vs 2 vs 3 vs 4), ethnicity and/or race (non-Hispanic white vs Hispanic white vs Black vs Asian or Pacific Islander vs other), tumor histology (nonlobular vs lobular), and first tumor indicator (yes vs no). All P values were calculated using Cox regression with the Wald test for significance. All P values were two-sided. ER = estrogen receptor.
Figure 3.
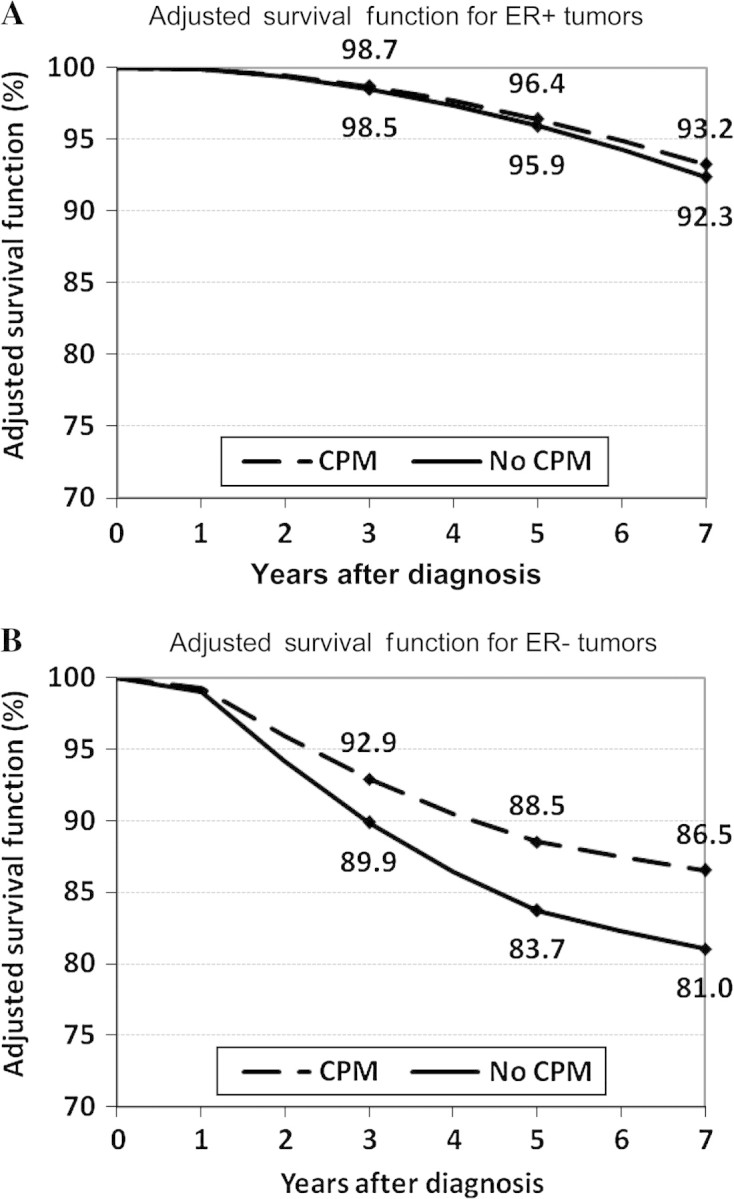
Adjusted breast cancer–specific survival function associated with contralateral prophylactic mastectomy (CPM) for young women with early-stage breast cancer. A) Women with estrogen receptor (ER)–positive tumors (hazard ratio [HR] for death = 0.88, 95% confidence interval [CI] = 0.66 to 1.17; P = .38). B) Women with ER-negative tumors (HR for death = 0.68, 95% CI = 0.53 to 0.88; P .004). Hazard ratios and 95% confidence intervals were derived by multivariable Cox regression analysis, adjusted for number of positive lymph nodes (0 vs 1–3 vs ≥4), tumor grade (1 vs 2 vs 3 vs 4), ethnicity and/or race (non-Hispanic white vs Hispanic white vs Black vs Asian or Pacific Islander vs other), tumor histology (nonlobular vs lobular), and first tumor indicator (yes vs no). Percentages of women alive within the CPM and non-CPM groups at 3, 5, and 7 years are indicated on the curves. All P values are two-sided and were calculated using Cox regression with the Wald test for statistical significance.
In older patients, we found no reduction in breast cancer–related death associated with CPM in any of the subgroups of women older than 60 years (Figure 2). Among women between the ages of 50 and 59 years, CPM was associated with improved breast cancer–specific survival for women who had early-stage ER-negative disease (HR for death = 0.66, 95% CI = 0.45 to 0.97; P = .04) and those with later-stage ER-positive disease (HR for death = 0.54, 95% CI = 0.32 to 0.92; P = .02). These findings likely reflect the mixed effects of a true association with CPM and unexplained model variance that are caused by differences in the health status among women in this group. No association was observed between CPM and survival among patients with ductal carcinoma in situ, and thus, this subgroup was excluded from further analysis (data not shown). Similarly, no association was observed between CPM and survival in patients with pure lobular histology in the index breast (data not shown). This result is consistent with more contemporary data that suggest no differences in contralateral breast cancer rates between women with invasive ductal and invasive lobular tumor histology (17).
Given the potential limitations of SEER in coding for cause of death, we further examined the robustness of our findings by performing a risk-stratified analysis of the association of CPM with relative survival (Table 4) to compare the survival of the breast cancer cohorts (stages I–III only) identified through the SEER database against the general population free of cancer. Again, we found that for young women with early-stage ER-negative breast tumors, CPM was associated with an improvement in relative survival (HR for death = 0.69, 95% CI = 0.53 to 0.89; P .005). In addition, for all subgroups, the directionality of the hazard ratios for breast cancer–specific and relative survival and the relative magnitude of the associations were similar to those observed for breast cancer–specific survival. These findings suggest that the limitations associated with SEER cause of death coding did not influence the associations that we observed between CPM and disease-specific survival.
Table 4.
Stratified multivariable analysis of relative survival (N = 77 708)*
| Variable | Estrogen receptor–positive status |
Estrogen receptor–negative status |
||||
| No. | HR† (95% CI) | P | No. | HR (95% CI) | P | |
| Younger (18–49 y), stage (I, II) | 11 585 | 0.79 (0.58 to 1.08) | .15 | 4854 | 0.69 (0.53 to 0.89) | .005 |
| Younger (18–49 y), stage (III) | 1919 | 0.88 (0.62 to 1.26) | .51 | 1280 | 1.03 (0.74 to 1.43) | .83 |
| Middle (50–59 y), stage (I, II) | 11 508 | 0.65 (0.39 to 1.09) | .11 | 3843 | 0.64 (0.42 to 0.96) | .03 |
| Middle (50–59 y), stage (III) | 1549 | 0.47 (0.26 to 0.86) | .01 | 931 | 1.06 (0.73 to 1.56) | .73 |
| Older (60–90 y), stage (I, II) | 29 589 | 0.61 (0.35 to 1.08) | .10 | 6390 | 0.75 (0.45 to 1.23) | .26 |
| Older (60–90 y), stage (III) | 3066 | 0.73 (0.45 to 1.18) | .21 | 1194 | 0.73 (0.45 to 1.17) | .20 |
Estrogen receptor unknown and stage 0 were not included in this analysis. All P values were calculated using general linear model. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
Hazard ratios for survival among patients who did or did not undergo contralateral prophylactic mastectomy, adjusted for number of positive lymph nodes, tumor grade, race, tumor histology, and first tumor indicator.
Last, we examined the rates of contralateral breast cancer among young women with early-stage ER-negative disease. In this group, the cumulative incidence of contralateral breast cancer was 0.90% in women who did not undergo CPM and 0.16% in women who did undergo CPM (P = .05) (Table 5). Among young women with early-stage ER-positive cancer, the rates of contralateral breast cancer were similarly low for those who had undergone CPM (0.13% vs 0.16%, P = .90), and rates were also lower in the absence of CPM (0.46% vs 0.90%, P < .001). This lower baseline risk of contralateral breast cancer may account for the lack of benefit associated with CPM in young women with early-stage ER-positive disease.
Table 5.
Rates of contralateral breast cancer in women younger than 50 years and with early-stage (I, II) disease*
| % with estrogen receptor–positive status | % with estrogen receptor–negative status | P † | |
| No CPM | 0.46 | 0.90 | <.001 |
| CPM | 0.13 | 0.16 | .90 |
| P | .07 | .05 |
CPM = contralateral prophylactic mastectomy.
Two-sided P value for two-sample proportion t test.
Discussion
Prophylactic mastectomy has consistently been shown to reduce the risk of breast cancer. However, despite a greater than 90% relative reduction in the odds of developing breast cancer, it has been estimated that most women undergoing prophylactic mastectomy will derive no survival benefit (7). Using data from the SEER registry, we have demonstrated an association between CPM and improved survival among a subgroup of women with breast cancer. This association was most clearly observed among women younger than 50 years with early-stage ER-negative tumors.
Our results are consistent with several corollary reports in the literature. First, without any prevention strategies, women with breast cancer are estimated to have a constant annual risk for a subsequent primary breast cancer of 0.5% to 1% (18–20). Our observation of a reduced breast cancer–specific mortality associated with CPM in younger women (18–49 years) may thus be due in part to the larger absolute lifetime risk of metachronous contralateral breast cancer combined with low probability of competing causes of death. In intermediate-age patients (50–59 years), any potential benefit associated with CPM will be more greatly influenced by their remaining time at risk during their life expectancy horizon.
Second, our findings are consistent with the established role of antiestrogen therapy in reducing the risk of contralateral breast cancer. Tamoxifen therapy is effective in reducing the risk of contralateral breast cancer by approximately 50% (21). For postmenopausal women, aromatase inhibitors increase this benefit even further (22). Although our analysis could not directly incorporate use of antiestrogens, our finding that women with ER-positive disease had a 50% reduction in rate of subsequent contralateral breast cancer compared with women with ER-negative disease is consistent with the known clinical benefits of tamoxifen therapy.
Finally, our observation that disease stage at presentation affects the survival benefit that is associated with CPM supports the importance of considering the competing risk of death from the index carcinoma. Our data suggest that the risk of death from a second contralateral breast cancer is outweighed by the risk of death from the initial breast cancer in women who are diagnosed with locally advanced (stage III) disease.
Important in our study is that stratification by the three risk categories—age younger than 50 years, ER-negative disease, and stage I or II disease—identified the primary group in which a survival benefit associated with CPM was observed. Thus, a high, absolute lifetime risk of contralateral breast cancer, lack of availability of chemoprevention options, and a low risk of death from the index tumor combined create the optimal conditions under which to consider CPM. It is of interest that we saw a statistically significant association between CPM and breast cancer survival despite relatively short follow-up. With longer durations of follow-up, we anticipate that the degree of benefit associated with CPM in ER-negative breast cancer patients will increase. Whether longer follow-up would demonstrate a benefit associated with CPM among women with ER-positive tumors is difficult to speculate and will likely be linked to age at presentation and duration of antiestrogen therapy. Trends toward longer durations of treatment, particularly in perimenopausal patients, may offer continued substantial protection against a second contralateral breast cancer event, thus offsetting any benefit associated with CPM over time.
Our finding that CPM was associated with an improvement in noncancer survival in women older than 50 years also highlights the complex interplay between health status and the performance of CPM. Therefore, whether our findings of CPM-associated cancer-specific survival benefit among young women with early-stage ER-negative breast cancers can be extended to any of the women older than 50 years is uncertain.
This study has several limitations. Although our study shows that CPM is associated with improved disease-specific survival, SEER collects only limited information on patient and tumor factors. Therefore, we were not able to incorporate more precise risk measurements for development of contralateral breast cancer, such as the presence of BRCA1 or BRCA2 mutations or a high-risk family history. In addition, data regarding chemotherapy are not available from SEER and thus could not be evaluated in our model. However, on the basis of guidelines from the National Comprehensive Cancer Network during the study period, the majority of young women with ER-negative stage I or II breast cancer would be expected to have received chemotherapy (23,24) (www.nccn.org version 2, update 2008). Third, we were not able to incorporate into our analyses the method of detection of the index breast cancer (eg, by mammogram or clinical examination) as a separate variable as this information is not available within the SEER dataset. Because women with mammogram-detected breast cancers have been shown to have better survival outcomes even after adjusting for stage (25), it is possible that related bias in our cohort may have influenced the results. Last, with the median actual follow-up duration for the study cohort of 47 months, and the potential for patient migration out of SEER regions after their index cancer diagnosis, our study may have underestimated the true contralateral breast cancer rate in the cohort. This, in addition to other unexplained variance in the model (such as treatment disparities), may account for the observation that the adjusted absolute survival benefit associated with CPM among young women with ER-negative breast cancer exceeded the absolute reduction in contralateral breast cancer risk. However, the survival analysis is less affected by such factors and with longer follow-up, and the contralateral breast cancer rates along with differences in survival between the risk groups are expected to be greater.
There are limitations to simple covariate adjustment for examining survival outcomes after CPM, including selection bias for the procedure (26). We attempted to address these limitations by performing risk stratification to identify the subcohorts who did and did not show associated benefit from CPM and by examining the association of CPM and survival from non–breast cancer causes of death as an indicator of the overall health of the patients. Furthermore, to ensure that potential errors in cause of death coding within SEER did not bias the results, we also performed relative survival analysis and confirmed the validity of our observations.
Finally, our analyses characterize and highlight the associations between the use of CPM and breast cancer survival among population-based cohorts. Our observation that the association between CPM and survival is most relevant among young women with early-stage ER-negative breast cancer is consistent with the survival benefit of CPM being inversely related to the risk of death from the index cancer and directly related to the cumulative lifetime risk of death from a contralateral breast cancer event. The analyses have been performed to account for as many potential influences on outcomes as possible within the available data, and the results are internally consistent across a number of different approaches to the data, which suggest robustness of our findings. However, despite these efforts, a causal relationship between survival and CPM cannot be proved, that is only possible in a randomized controlled trial, unlikely to be completed in the foreseeable future.
Funding
American Society of Clinical Oncology Career Development Award (G.J.C.).
Footnotes
The sponsors had no role in the study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication.
References
- 1.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340(2):77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell SK, Schaid DJ, Myers JL, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol. 2001;19(19):3938–3943. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- 3.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22(6):1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 4.Meijers-Heijboer H, van Geel B, van Putten WL, et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345(3):159–164. doi: 10.1056/NEJM200107193450301. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 6.Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23(19):4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 7.Hamm RM, Lawler F, Scheid D. Prophylactic mastectomy in women with a high risk of breast cancer. N Engl J Med. 1999;340(23):1837–1838. author reply 9. [PubMed] [Google Scholar]
- 8.van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer. 2005;93(3):287–292. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guern AS, Vinh-Hung V. Statistical distribution of involved axillary lymph nodes in breast cancer. Bull Cancer. 2008;95(4):449–455. doi: 10.1684/bdc.2008.0620. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Surveillance, Epidemiology, and End Research (SEER 17) Program. Public-Use Data (1973-2005) [DVD-ROM] Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; 2008. DCCPS, Surveillance Research Program, Cancer Statistics Branch. www.seer.cancer.gov. Accessed May 19, 2008. [Google Scholar]
- 11.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual. 5th ed. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 12.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 13.Human Mortality Database. Berkeley, CA: University of California; Rostock; Germany: Max Planck Institute for Demographic Research. http://www.mortality.orgorhttp://www.humanmortality.de. Accessed October 2, 2008. [Google Scholar]
- 14.Arjas E. A graphical method for assessing goodness-of-fit in Cox's proportional hazards model. J Am Stat Assoc. 1988;83(401):204–212. [Google Scholar]
- 15.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23(1):51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vo TN, Meric-Bernstam F, Yi M, et al. Outcomes of breast-conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg. 2006;192(4):552–555. doi: 10.1016/j.amjsurg.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Robbins GF, Berg JW. Bilateral primary breast cancer: a prospective clinicopathological study. Cancer. 1964;17(12):1501–1527. doi: 10.1002/1097-0142(196412)17:12<1501::aid-cncr2820171202>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Adami HO, Bergstrom R, Hansen J. Age at first primary as a determinant of the incidence of bilateral breast cancer. Cumulative and relative risks in a population-based case-control study. Cancer. 1985;55(3):643–647. doi: 10.1002/1097-0142(19850201)55:3<643::aid-cncr2820550328>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56(4):1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 22.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 23.Carlson RW, Anderson BO, Bensinger W, et al. NCCN Practice Guidelines for Breast Cancer. Oncology (Williston Park) 2000;14(11A):33–49. [PubMed] [Google Scholar]
- 24.Update: NCCN practice guidelines for the treatment of breast cancer. National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13(5A):41–66. [PubMed] [Google Scholar]
- 25.Shen Y, Yang Y, Inoue LY, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97(16):1195–1203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 26.Miller CC, Reardon MJ, Safi HJ. Risk Stratification: A Practical Guide for Clinicians. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]