Abstract
Rates of colon cancer are much higher in African Americans (65:100,000) than in rural South Africans (<5:100,000). The higher rates are associated with higher animal protein and fat and lower fiber consumption, higher colonic secondary bile acids, lower colonic short chain fatty acid quantities and higher mucosal proliferative biomarkers of cancer risk in otherwise healthy middle aged volunteers. Here we investigate further the role of fat and fiber in this association. We performed two-week food exchanges in subjects from the same populations, where African Americans were fed a high-fiber, lowfat African-style diet, and rural Africans a high-fat low-fiber western-style diet under close supervision. In comparison to their usual diets, the food changes resulted in remarkable reciprocal changes in mucosal biomarkers of cancer risk and in aspects of the microbiota and metabolome known to affect cancer risk, best illustrated by increased saccharolytic fermentation and butyrogenesis and suppressed secondary bile acid synthesis in the African Americans.
INTRODUCTION
In the west, colon cancer is the second leading cause of cancer death. It afflicts approximately 150,000 Americans, 250,000 Europeans and 1 million people worldwide annually. Nearly one third will die1. Colonoscopy has permitted early detection and recent studies have shown this to be associated with a reduction in mortality rates, but overall impact has been small, particularly among African Americans who shoulder the greatest burden of the disease in the USA. Colon cancer is one of the westernized diseases, with an incidence in Americans of African descent of 65:100,000 compared to <5:100,000 in rural Africans. Migrant studies, such as those in Japanese Hawaiians, have demonstrated that it only takes one generation for the immigrant population to assume the colon cancer incidence of the host western population2. Whilst the change in diet is most likely responsible for this3, migration changes many other aspects of the environment. For example, cigarettes, chemicals, infections, antibiotics, might be equally responsible for the change in colon cancer risk. To focus on the importance of diet in African Americans and to explore the hypothesis that colon cancer risk is determined by the influence of the diet on the microbiota to produce anti- or pro-neoplastic metabolites, we have performed a series of investigations between African Americans and rural South Africans4,5. First, we observed that their diets were fundamentally different in preparation, cooking, and composition. Animal protein and fat intake was 2-3 times higher in Americans, whilst carbohydrate and fiber, chiefly in the form of resistant starch, were higher Africans. On colonoscopy, African Americans had more polyps and higher rates of mucosal proliferation, measured by Ki67 epithelial cell staining, confirming its potential use as a biomarker of cancer risk6. These differences were shown to be associated with profound differences in the microbiota (Americans dominated by genus Bacteroides, Africans by the genus Prevotella) and metabolic phenotype. Notable differences included higher levels of starch degraders, carbohydrate fermenters, and butyrate producers and their metabolites in Africans, and higher levels of potentially pathogenic proteobacteria (Escherichia, Acinetobacter) and bile acid deconjugators and their products in Americans5. As there is extensive experimental evidence that the products of fiber fermentation, in particular butyrate, are anti-inflammatory and anti-neoplastic7-9 and that the products of bacterial bile acid conjugation, secondary bile acids, are carcinogenic10, these findings suggested two potential mechanisms for diet-associated cancer risk: the protective effect of dietary fiber in increasing butyrogenesis, and the promotional effect of dietary fat on stimulating bile acid synthesis by the liver.
We know from the results of two recently published human studies that modifications of the fiber and fat content of the diets have a major impact on the colonic microbiota within a few days11,12. Here we provide critical information on the functional significance of these microbiota changes on the colonic mucosa by switching the fat and fiber proportions of the diet in our two high and low colon cancer risk study populations. Employing the study design outlined on Table 1, we showed the anticipated increase in saccharolytic fermentation and butyrogenesis and suppression of secondary bile acid synthesis produced by switching African Americans to a high fiber, low fat diet for 2 weeks was associated with a significant reduction in colonic mucosal inflammation and proliferation biomarkers of cancer risk. In stark contrast, the diet switch in rural Africans to a high fat low fiber diet resulted in reverse changes in all these parameters.
Table 1. Study design, time schedule and sampling.
ED 1 and ED 2 are the initial and final endoscopy days, HE is the home environment and DI is dietary intervention periods. The Xs corresponding to dietary evaluation indicate the frequency of dietary recordings; three days during the home environment when consuming their usual diets, and daily during the dietary intervention.
| Day | 0 | 7 | 14 | 15 | 22 | 29 |
|---|---|---|---|---|---|---|
|
HOME ENVIRONMENT (HE) STUDY |
DIETARY INTERVENTION (DI) |
|||||
| at home | in-house | |||||
| Period | ED 1 | HE 1 | HE 2 | DI 1 | DI 2 | ED 2 |
| Faecal Samples | X | X | X | X | X | X |
| Colonic evacuates | X | X | ||||
| Breath H2, CH4 | X | X | X | X | X | X |
| Bloods | X | X | ||||
| Mucosal biopsies | X | X | ||||
| Dietary observations | XXX | X X X X X X X X X X X X X X | ||||
Results
Changes in the colonic mucosa following diet switch
Measurements made in the home environment confirmed our preceding studies that the dietary intakes were quite different, with animal protein and fat intake being 2-3 times higher in Americans, and fiber intake, chiefly in the form of resistant starch, being higher in Africans4 (Supplementary Figure 1, Supplementary Tables 1-3). On colonoscopy, adenomatous polyps were found and removed in nine Americans and no Africans. Measurements of mucosal epithelial proliferation rates by Ki67 staining of biopsies from ascending, transverse and descending colon all showed significantly higher levels in African Americans than Africans (Figure 1, Supplementary Figure 2), again supporting its use as a biomarker of cancer risk. The dietary switch was well tolerated by both populations and body weights were all maintained with 2Kg. Remarkably, the switch decreased proliferative rates in African Americans to levels lower than those of Africans at baseline, whilst rates increased in Africans to levels greater than African Americans at baseline (Figure 1). In tandem, histological (Supplementary Tables 5a-d) and immunohistochemical markers of inflammation (Figure 1b, 1c) used, namely CD3+ intraepithelial lymphocytes and CD68+ lamina propria macrophages, increased on switching Africans to the high fat diet, and decreased in Americans given the high fiber diet, thus paralleling the measured changes in mucosal proliferation.
Figure 1. Colonic Mucosal Immunohistochemistry of Proliferative and Inflammatory Biomarkers.
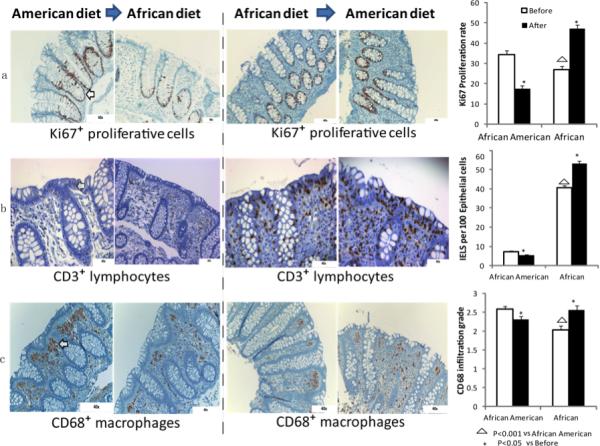
Immunohistochemical analysis of colonic mucosal biopsies taken at colonoscopy with their associated quantitative analysis on right panel. Panel ‘a’ provides an illustration of the paired changes in Ki67 staining of epithelial crypt cells in one African American and one rural African before and after dietary switch, panel ‘b’ shows in the same way paired changes in CD3+ staining, and panel ‘c’ the paired changes in CD68+ macrophages in the lamina propria. The bar graphs on the far right summarize the group mean ±SE results in 20 African Americans and 12 rural Africans. The two-tailed Mann-Whitney U-test was used for comparisons for non-paired samples and the Wilcoxon Rank Sum test for paired samples, with Bonferroni correction for multivariate comparisons. Triangles indicate significant (p<0.05) baseline differences, stars significant changes induced by diet switch
Changes In Colonic Microbial Metabolism Following Diet Switch
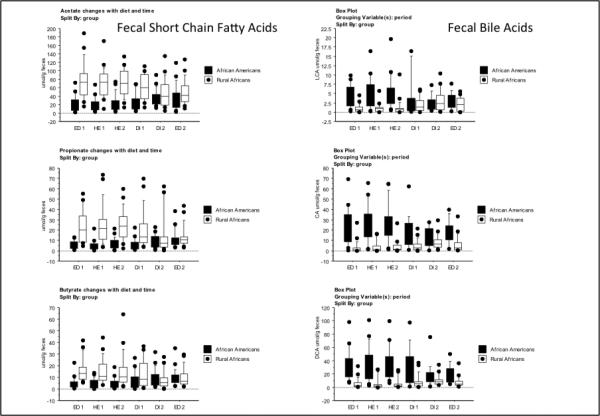
The reciprocal changes in mucosal proliferation and inflammation between Africans and Americans were associated with reciprocal changes in specific microbes and their metabolites that support our hypothesis that the mechanism whereby diet affects colon cancer risk is mediated by the microbiota. First, there was strong evidence that butyrogenesis was associated with lower mucosal proliferation, both in Africans consuming their usual high fiber diet and in African Americans switched to a high fiber African style diet (Figures 1 and 2, Supplementary Table 6). Because a wide variety of microbes have the capacity to produce butyrate, we targeted our analysis on the measurement of microbial genes encoding the final enzyme in butyrogenesis, namely butyryl-coenzyme-A-CoA transferase13. On their usual diet, Africans had significantly greater abundances of butyrate synthesizing genes, and higher fecal butyrate concentrations (Figure 2) and colonic evacuate quantities (Supplementary Table 7), whilst switching them to a western low fiber diet suppressed each of these parameters. Reciprocally, our measurements of butyrogenesis were lower in Americans consuming their usual low fiber diet and increased after dietary switch to a high fiber African style diet. Impressively, ‘Africanization’ of the diet increased total quantities of butyrate in colonic evacuates 2.5 times whilst ‘westernization’ reduced quantities by half. Recognizing that butyrogenesis is a final common pathway of accomplished by multiple microbial metabolic interactions fiber fermentation, which can only be, we examined the simultaneous changes in the group of microbes that remove the major metabolic end-product, hydrogen. If hydrogen is allowed to accumulate, it impairs the regeneration of oxidized pyridine nucleotides, which in turn inhibit the fermentation process and butyrogenesis14. The efficient removal of hydrogen by the hydrogenotrophic microbes, methanogens which bind hydrogen into non-toxic methane, sulfate-reducers which incorporate hydrogen into hydrogen sulfide, and acetogens which consume hydrogen to produce acetate and water, is therefore a prerequisite for enhanced butyrogenesis. Our observation that all these microbial groups were indeed in greater abundance, as were their metabolic products, both in Africans consuming their usual high fiber diet and when Americans were switched to the high fiber diet (Figure 2) well illustrates the importance of microbial partnership in the maintenance of colonic health.
Figure 2. Targeted Analysis of Microbial Functional Genes of Specific Interest and Their Metabolites.
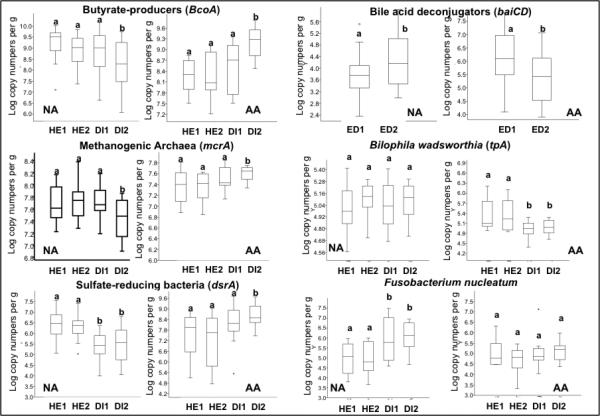
Box plots with whiskers and outliers summarizing the results for the targeted functional qPCR analysis of microbial genes of interest (upper panel), and measurements of their metabolic products in fecal samples (lower panel) in 20 African American (AA) and 20 rural Africans (NA) at sequential time points during the study. Group median and variability outside the upper and lower quartiles are shown. Measurements were taken before (Table 1: ED1, HE1, HE2), during (DI1, DI2) and after (ED2) 2 weeks of dietary change. With regard to the functional gene analysis, bcoA encodes for the enzyme butyryl-CoA:acetate CoA-transferase which is responsible for the last step in butyrate synthesis, mcrA for the enzyme responsible for methanogenesis, and baiCD for the 7-alpha-dehydroxylating enzyme responsible for secondary bile acid production. LCA represent lithocholic acid, CA cholic acid, DCA deoxycholic acid. As data was not normally distributed, the Kruskal-Wallis test was used to evaluate the significance of the group median differences across the different time points shown on the box plots. In the upper panel, comparison to baseline ED1, ‘a’ signifies no significant change, and ‘b’ a significant change. In the lower panel for the short chain fatty acids acetate, propionate and butyrate, and the secondary bile acids lithocholic acid (LCA) and deoxycholic acid (DCA) and primary bile acid cholic acid (CA), there were no significant differences between time points ED1, HE1 and HE2, while ED2 was consistently different (p<0.05, for details see Supplementary Table 6). This, together with Supplementary Figure 6, illustrates no significant effect from colonoscopy, and a profound effect from the dietary change.
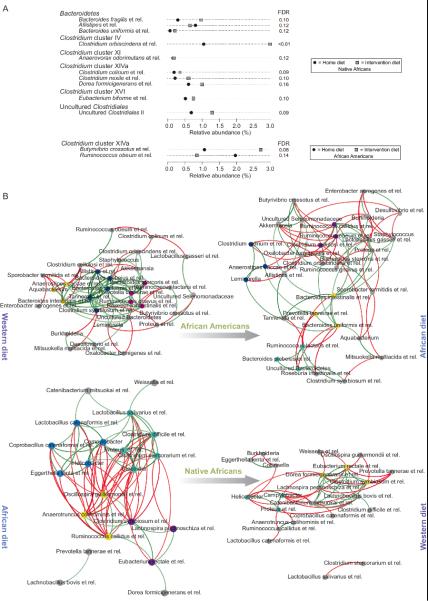
Flint's group has provided strong evidence that the most active butyrate producers, e.g. Roseburia spp. and Eubacterium rectale and Faecalibacterium prausnitzii are contained within Clostridium groups IV and XIVa15. Since Africans and African Americans have very distinct microbiota composition at baseline (Supplementary Table 8), we performed a global analysis of the microbiota by 16S rRNA gene phylogenetic microarray (Human Intestinal Tract Chip (HITChip)) to examine whether these groups were more prevalent with high fiber consumption16. Remarkably, these groups were no more apparent in Africans or Americans eating a high fiber diet. In fact, the compositional changes that were specifically associated to the diet switch were minor (ten genus-like groups for Africans and two for Americans; Figure 3a). These observations suggest either dissociation between structure and function or a change in microbial interactions within the community17. On the other hand, we observed stronger cooccurrence patterns between the genus-level taxa in Africans consuming their usual high fiber diet (Figure 3b), which included these potential butyrate producers, for example Eubacterium rectale et rel. and Clostridium symbiosum et rel., and bacteria associated with complex carbohydrate utilization, for example Oscillospira guillermondii et rel. Reduction in fiber consumption led to opposite associations (Figure 3b bottom right). Reciprocally, high fiber feeding in Americans was associated with a shift from correlations between Bacteroides and potential butyrate-producing groups (Roseburia intestinalis et rel. and Clostridium symbiosum et rel.) towards stronger co-occurrence patterns including Firmicutes that are typically associated with complex carbohydrate fermentation (Figure 3b top right).
Figure 3. The impact of diet switch on microbiota composition and co-occurrence networks.
Panel A provides an overview of significantly altered genus-like bacterial groups (FDR<0.2; linear model) following the 14-day diet change. Average fraction of total HITChip signal (proxy for relative abundance) over the samples for each comparison. Black and grey squares indicate the average abundances at home- and intervention diet, respectively. Panel B illustrates the microbiota genus-like group co-occurrence networks of Africans and African Americans under both dietary regimens for genus-like groups. Groups with correlation differences >1 before and after the diet and at least |r| >0.5 are shown. Positive correlations are indicated with green lines; negative correlations with red lines. Identically colored nodes indicate network modules with three or more co-occurring groups. Grey nodes represent modules of less than three co-occurring groups or those not represented by a module. The sample sizes are 20 African Americans and 17 rural (Native) Africans.
Global Changes in the Metabonome following Diet Switch
We have described metabonomics and the associated term, metabolomics, as the multiparametric metabolic responses of complex systems to perturbations through time18. Specifically metabonomics addresses such phenotypic changes at the level of small molecule metabolites, and maps these processes using appropriate analytical and statistical processes. Recognizing the extreme complexity of the microbiota and its ability to produce a wide array of other metabolites that could affect mucosal health, we performed compositional and pathway analysis19 on fecal water and urine samples by 1H NMR (Figures 4 and 5). Perhaps one of the most striking findings of this study was the dramatically higher quantities and diversity of metabolites in fecal water in Africans consuming their usual diet compared to African Americans (Figures 4a). Whilst the higher levels of products of saccharolytic fermentation were expected from our targeted analysis, the higher quantities of proteolytic fermentation products were not. The only significantly more abundant fecal metabolite identified in the Americans at baseline, and in Africans after westernization of their diets, was choline. This is highly significant as recent studies have shown that choline is extensively metabolized by the microbiota to trimethylamine20 which is absorbed and metabolized by the liver to trimethylamine-N-oxide (TMAO) which is strongly atherogenic21, providing yet another link between westernized diets, the microbiota, and westernized diseases. Evidence that this metabolic pathway was indeed stimulated in Africans given the western diet was the observed increase in urine TMA-O (Table 2). Reciprocal changes in urinary metabolites derived from microbial metabolism of green vegetables, e.g. N-acetyl-S-methyl-L-cysteine sulfoxide, were also observed following diet switch, suggesting that some of reduction in mucosal proliferation in Americans could also have been related to the effects of increased phytochemical consumption in the Africanized diets22. The urinary spectra obtained from African Americans following the change to a high fiber diet also revealed a marked increase in an unrecoginized metabolite (Figure 4a left upper panel), possibly a nicotinamide breakdown product, whose character and function have yet to be determined.
Figure 4. The impact of diet switch on fecal and urinary metabolites and their correlation with faecal microbiota.
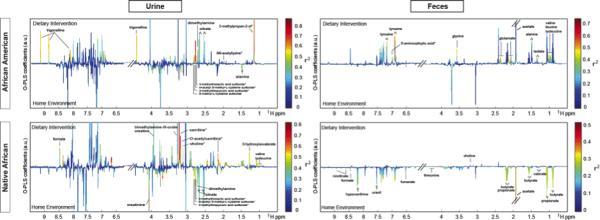
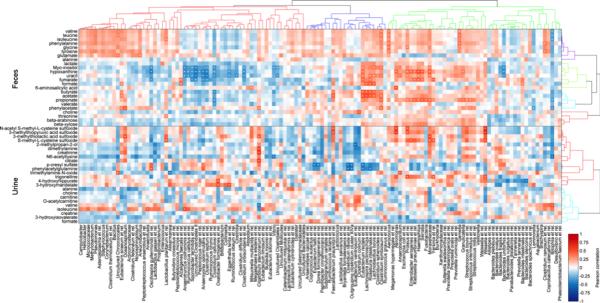
a) 1H NMR fecal and urinary spectra obtained from African Americans (AA) and native Africans (NA) during the home environment period (HE) and post dietary intervention (DI). Metabolites distinguishing the two groups are labelled either above the line (higher after dietary intervention) or below (higher in home environment) and the color represents the correlation coefficient (r2) of the metabolite with the groups. b) Two-way clustered heatmap of partial correlation between phylotypes assigned to genus-level taxa whose representation in the fecal microbiota (x axis) of African American (AA) and rural Africans (NA) and relative metabolite concentrations in urine and feces (y axis). P-values from correlation were adjusted for multiple testing using the Benjamini-Hochberg False Discovery Rate. Given a large number of tests performed, a cut-off of 0.3 was used to define the significance denoted by ‘+’. This level follows FDA guidelines for bioanalytical biomarkers and was chosen because of the relatively low number of samples it allows for less false negatives. Fecal metabolites (upper compartment) demonstrated a higher number of significant correlations with fecal microbiota compared with urinary metabolites (lower compartment). Eubacterium rectale, Lachnospira pectinoschiza, Roseburia intestinalis and Lactobacillus bovis correlate strongly with butyrate, acetate and formate. Adjustments were made for country (binary vector with 0=AA and 1=NA) and diet/stage (0=HE-2 and 1=DI-2) in calculating the partial correlations).
Figure 5. The impact of diet switch on faecal and urinary metabolic reaction networks.
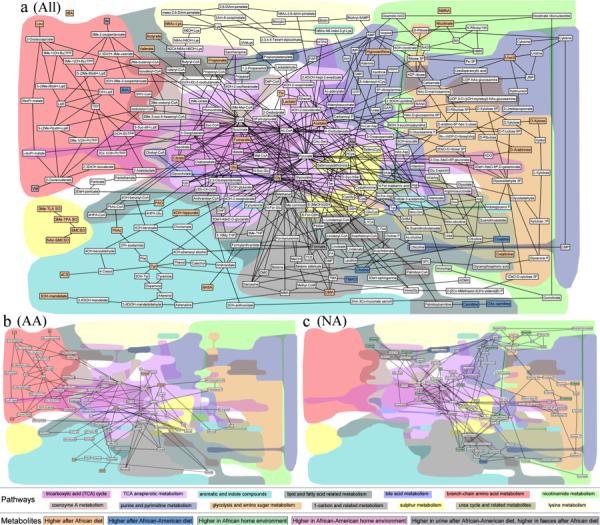
a-c) Metabolic reaction networks of metabolites found differentially expressed between different dietary comparisons, created using the MetaboNetworks software19 using information from KEGG on the reactions and enzymes within the biological system. The network shows links between metabolites if the reaction entry in KEGG indicates a main reactant pair and the reaction is either mediated by 1) an enzyme linked to human genes, 2) an enzyme linked to genes from identified bacterial groups using the HITChip or 3) it is part of a spontaneous process. The color of the metabolites indicate whether the metabolite is found in higher concentrations in the urine/feces of individuals consuming an African diet (orange), higher in individuals consuming an African-American diet (blue), higher in the African-American home environment (light red), higher in the African home environment (green) or whether the metabolites is not significantly associated with any comparison but is part of the metabolic network (white). Two metabolites (alanine and formate) are found with opposite associations in feces and urine (Table 2), these are therefore represented with a grey background color. A table with full names for each of the abbreviated metabolite names can be found in Supplemental Table 11. The background shading indicates different interconnected pathways. In the top part of the legend the color for each pathway, as represented in the figure, is shown. The bottom part of the legend, with black boxes around text box, indicates the association of each metabolite. a) Global network of changes (“All”) observed in fecal water and urine and in any comparison of the different groups. b) Network of fecal metabolites found differentially expressed in African-Americans before and after the dietary switch (“AA”). c) Network of fecal metabolites found differentially expressed in Africans before and after the dietary switch (“NA”).
Table 2.
Faecal and Urinary Metabolites
| a) Fecal Metabolites | Chemical shift Chemical shift (1H ppm) | Home environment African American (N=15) vs. African (N=19) | Dietary intervention African American (N=14) vs. African (N=20) | African American Home environment (N=15) vs. Dietary intervention (N=14) | African Home environment (N=19) vs. Dietary intervention (N=20) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q2Y=0.59; R2X=29.8% | Q2Y=0.74; R2X=26.8% | Q2Y=0.66; R2X=37.2% | Q2Y=0.37; R2X=30.7% | ||||||||||
| r | p | q | r | p | q | r | p | q | r | p | q | ||
| valine | 0.99 | 0.77a | <0.001 | 0.002 | 0.82c | <0.001 | 0.003 | ||||||
| leucine | 0.97 | 0.75a | <0.001 | 0.003 | 0.75c | 0.001 | 0.02 | ||||||
| isoleucine | 1.007 | 0.78a | <0.001 | 0.001 | 0.77c | <0.001 | 0.01 | ||||||
| glutamate | 2.36 | 0.82c | <0.001 | <0.001 | 0.84c | <0.001 | <0.001 | ||||||
| alanine | 1.48 | 0.7a | 0.003 | 0.007 | 0.79c | <0.001 | 0.009 | ||||||
| glycine | 3.57 | 0.72a | 0.002 | 0.005 | 0.82c | <0.001 | 0.002 | ||||||
| tyrosine | 6.91 | 0.74a | <0.001 | 0.003 | 0.81c | <0.001 | 0.001 | ||||||
| butyrate | 0.89 | 0.67a | 0.007 | 0.01 | 0.71a | <0.001 | 0.006 | ||||||
| propionate | 1.06 | 0.75a | <0.001 | 0.002 | 0.71a | <0.001 | 0.006 | ||||||
| valerate | 1.29 | 0.73a | 0.001 | 0.004 | 0.66a | 0.005 | 0.01 | ||||||
| acetate | 1.93 | 0.74a | <0.001 | 0.003 | 0.7c | 0.03 | 0.006 | 0.75a | <0.001 | 0.005 | |||
| lactate | 1.34 | 0.75a | <0.001 | 0.002 | 0.77c | <0.001 | 0.009 | ||||||
| formate | 8.45 | 0.69a | 0.002 | 0.009 | |||||||||
| choline | 3.2 | 0.78b | <0.001 | 0.001 | 0.66d | 0.006 | 0.01 | ||||||
| uracil | 5.81 | 0.78a | <0.001 | 0.001 | |||||||||
| hypoxanthine | 8.21 | 0.74a | <0.001 | 0.003 | 0.69a | 0.002 | 0.008 | ||||||
| 6-aminosalicylic acid* | 7.01 | 0.85c | <0.001 | <0.001 | 0.77c | <0.001 | 0.005 | ||||||
| fumarate | 6.53 | 0.69a | 0.002 | 0.008 | |||||||||
| phenylacetate | 3.54 | 0.81a | <0.001 | <0.001 | |||||||||
| β-xylose | 4.58 | 0.72a | <0.001 | 0.006 | |||||||||
| β-arabinose | 4.53 | 0.74a | <0.001 | 0.005 | |||||||||
| nicotinate | 8.62 | 0.71a | <0.001 | 0.005 | |||||||||
| threonine | 4.26 | 0.77a | <0.001 | 0.002 | |||||||||
| b) Urinary Metabolites | Chemical shift (1H ppm) | Home environment African American (N=8) vs. African (N=12) | Dietary intervention African American (N=7) vs. African (N=12) | African American Home environment (N=8) vs. Dietary intervention (N=7) | African Home environment (N=12) vs. Dietary intervention (N=12) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q2Y=0.41; R2X=26.6% | Q2Y=0.86; R2X=36.4% | Q2Y=0.51; R2X=20.8% | Q2Y=0.83; R2X=27.8% | ||||||||||
| r | p | q | r | p | q | r | p | q | r | p | q | ||
| N-acetyl S-methyl-L-cysteine sulfoxide* | 2.78 | 0.8a | 0.003 | 0.16 | 0.93c | <0.001 | <0.001 | 0.93c | <0.001 | 0.03 | 0.8a | <0.001 | 0.007 |
| 3-methylthiopyruvic acid sulfoxide* | 2.81 | 0.81a | 0.002 | 0.14 | 0.94c | <0.001 | <0.001 | 0.95c | <0.001 | 0.009 | 0.77a | <0.001 | 0.008 |
| 3-methylthiolactic acid sulfoxide* | 2.76 | 0.88c | <0.001 | <0.001 | 0.83c | 0.002 | 0.12 | 0.79a | 0.001 | 0.01 | |||
| S-methyl-L-cysteine sulfoxide | 2.84 | 0.84c | 0.003 | 0.13 | |||||||||
| p-cresyl sulfate | 2.35 | 0.7a | 0.03 | 0.28 | |||||||||
| phenylacetylglutamine | 2.27 | 0.71a | 0.02 | 0.26 | |||||||||
| 3-hydroxyisovalerate | 1.27 | 0.78b | 0.005 | 0.18 | 0.85d | <0.001 | 0.004 | ||||||
| 2-methylpropan-2-ol* | 1.14 | 0.91c | <0.001 | <0.001 | 0.89c | <0.001 | 0.07 | ||||||
| alanine | 1.49 | 0.8d | 0.003 | 0.02 | 0.75b | 0.03 | 0.33 | ||||||
| valine | 0.99 | 0.85d | <0.001 | 0.003 | |||||||||
| isoleucine | 0.93 | 0.88d | <0.001 | <0.001 | |||||||||
| carnitine | 3.23 | 0.88d | <0.001 | 0.001 | 0.91d | <0.001 | <0.001 | ||||||
| O-acetylcarnitine* | 2.15 | 0.91d | <0.001 | <0.001 | 0.91d | <0.001 | <0.001 | ||||||
| N6-acetyllysine* | 1.99 | 0.9c | <0.001 | 0.01 | |||||||||
| citrate | 2.53 | 0.85c | <0.001 | 0.003 | 0.71c | 0.04 | 0.39 | 0.71a | 0.006 | 0.04 | |||
| creatine | 3.94 | 0.73d | 0.004 | 0.03 | |||||||||
| creatinine | 4.065 | 0.82c | 0.001 | 0.01 | 0.77a | <0.001 | 0.007 | ||||||
| trigonelline | 4.44 | 0.9c | <0.001 | <0.001 | 0.86c | 0.002 | 0.12 | ||||||
| 4-hydroxyhippurate | 6.97 | 0.81c | 0.003 | 0.02 | |||||||||
| 3-hydroxymandelate | 6.92 | 0.87c | <0.001 | 0.002 | |||||||||
| dimethylamine | 2.73 | 0.88a | <0.001 | 0.07 | 0.79c | 0.01 | 0.25 | 0.78a | 0.001 | 0.01 | |||
| trimethylamine-N-oxide | 3.27 | 0.76d | 0.02 | 0.07 | |||||||||
| choline | 3.23 | 0.84d | <0.001 | 0.004 | |||||||||
| formate | 8.46 | 0.79d | 0.002 | 0.01 | |||||||||
| N-methyl-2-pyridone-5-carboxamide* | 6.66 | 0.93d | <0.001 | <0.001 | |||||||||
Table 2 a) and b) show significantly changed fecal and urinary metabolites in each pairwise comparison (e.g. African American vs. African during home environment, African American at home environment vs. dietary intervention). Representative chemical shift of each metabolite is provided. The r values are Pearson's correlation coefficient from the correlation of the metabolite concentrations with classification (e.g. African Americans vs. Native Africans). P and q values represent the significance of the metabolite changes and false discovery rate-adjusted p values76, respectively.
higher in home environment – Africans
higher levels of metabolites in home environment – African Americans
higher in dietary switch – African Americans
higher in dietary switch – Africans.
Tentative assignment.
The dietary intervention caused, in tandem with the microbiota changes, substantial modifications in the metabolic phenotypes of both groups (Table 2, Figure 4a), with Africans demonstrating less metabolic variation and Americans becoming more diverse. This is probably best appreciated from the metabolic pathway analysis (Figure 5b and 5c) of stool and urine samples from both groups, with color coding identifying the significant baseline differences, based on integration of the enzymes of bacterial groups identified by HITChip microarray16, host metabolic enzymes, and fecal and urinary metabolites by 1H NMR associated with reactions from the KEGG database. The lower two panels illustrate the significant shifts in metabolic pathways identified in fecal samples after dietary switch, showing mirror image changes reflecting the above, most notably involving reductions in short chain fatty acids and increases in glycosylated proteins and choline metabolism in Africans, and the unexpected increases in amino acid metabolism in Americans. Consistent with the results of our targeted analyses, the effects on saccharolytic fermentation were reciprocal, with increases in acetate and lactate in Americans and reductions in Africans. Unlike the results obtained from targeted gas chromatography shown of Figure 2, the increases in butyrate and propionate measured by global NMR did not achieve statistical significance probably because the method is more qualitative than quantitative. Again, contrary to expectation faecal concentrations of colonic branched-chain amino acids, which are likely to be derived from proteolytic fermentation, decreased in Africans given the high protein western diet for unclear reasons.
Global Microbe-Metabolite Associations
To investigate this, we performed a partial correlation analysis between genus level microbes and urine and fecal metabolites (Figure 4b), adjusted for nationality and diet. Although we cannot biologically explain all associations (for example, propionate correlates with enterobacteriaceae and Streptococcus which are not known to produce propionate) one striking feature was that the metabolite butyrate was exclusively associated with microbial groups that are known to contain butyrate producers, well exemplified by Roseburia intestinalis et rel, Eubacterium rectale et rel, and Clostridium symbiosum et rel which are shown in the co-occurrence networks on Figure 3B to be enhanced when high fibre diets were consumed by both population groups.
High Fat, Low Fiber Diets and Toxic Microbial Metabolites
It is unlikely that butyrogenesis can alone explain diet-associated cancer risk. Our preceding studies had shown that the higher fat diet of African Americans was associated a greater abundance of fecal microbial genes that encode for one of the key enzymes responsible for deconjugation of bile acids (the baiCD gene encodes for the 7-alpha-dehydroxylating enzyme23), as well as their metabolic products, secondary bile acids5. Secondary bile acids have been shown to be carcinogenic in experimental models and multiple human studies have shown that their fecal levels are associated with cancer risk10,24,25. Furthermore, there is substantial experimental evidence that the carcinogenic potential of these metabolites is potentiated by colonic butyrate deficiency26-29. Once again, we show that there were reciprocal changes in both baiCD gene abundance and the secondary bile acids, lithocholic and deoxcholic acid, after diet switch, with levels increasing in Africans given the high fat western style diet and reductions in Americans fed the low fat traditional African diet (Figure 2, lower panel). Whilst the use of these functional genes may not cover all the enzymatic pathways of interest, they do provide legitimate tracers for them30. Remarkably, Africanization reduced colonic evacuate secondary bile acids by 70%, and westernization increased them by 400% (Supplementary Table 7).
Dietary fat may influence colon cancer risk through other pathways as illustrated by a recent study in an Il10−/− mouse model which showed that a high-fat diet cultivated an increase in the abundance of Biophila wadsworthia, a member of the Desulfovibrionaceae family which generates hydrogen sulfide via taurine respiration, leading to acute inflammation31. Exogenous hydrogen sulfide is a potent genotoxin in vitro32. Finally, although no strong evidence has yet been found to indict a specific microbe in colonic carcinogenesis, a recent study found that Fusobacterium nucleatum was enriched in human colon cancer tissue, and suggested that the microbe might promote colorectal neoplasia progression through recruitment of proinflammatory tumor-infiltrating immune cells33. Alternatively, members of our group have hypothesized that fusobacteria may promote genotoxity by their ability to convert cysteine to hydrogen sulfide34. Figure 2 (upper panel) shows that the presence and activity of both of these microbes may have played a role in the changes in mucosal biomarkers: the high fiber, low fat dietary intervention in African Americans was associated with in a significant decrease of B. wadsworthia, whilst the low fiber high fat intervention in Africans was associated with an increase of F. nucleatum.
Discussion
We have shown in individuals from high risk and from low cancer risk populations that changes in the food content of fiber and fat had remarkable effects on their colonic microbiota and metabonome within 2 weeks, and, critically, that these changes were associated with significant changes in mucosal inflammation and proliferation. Whilst we cannot claim from our results that these changes in mucosa will result in changes in the development of cancer, there is good experimental evidence that increased epithelial proliferation predicts neoplastic change because it increases the risk of development of DNA mutations due to the higher rate of exposure of sensitive proliferating cells to luminal carcinogens. The evidence for the use of proliferation biomarkers in humans is based on the known increase in the percentage of epithelial cells engaged in DNA synthesis (measured as “proliferation index”) in all premalignant conditions of the gastrointestinal tract, as well as the observed expansion of the proliferative zone, causing a shift of the proliferating cells from the base to the surface of the crypt35-37. There is also substantial evidence that chronic inflammation increases cancer risk38 and it has been observed in human studies that colon cancer is increased 5-fold in patients with chronic ulcerative colitis38 and reduced 50% by anti-inflammatory drugs39. In keeping with this, it was noteworthy that our two biomarkers on mucosal inflammation, CD3+ intraepithelial lymphocytes and CD68+ lamina propria macrophages responded in the same direction as our Ki67 proliferative biomarker. CD3+ intraepithelial lymphocytes provide a measure of T cell activation and therefore the pivotal balance between immune activation and quiescence in response to luminal antigens, such as bacteria40,41, while CD68+ lamina propria macrophages play an active role in the persistence of chronic inflammation42 and neoplastic progression43,44. Interestingly, the microbial metabolite, butyrate, was shown recently to reduce the responsiveness of lamina propria macrophages to commensal bacteria45, suggesting that the dietary change in Americans may have resulted in a decrease in both macrophage numbers and their activity.
Our study revealed two unanticipated colonic mucosal findings in rural Africans consuming their usual baseline diets. First, despite the significantly lower epithelial proliferation and population colon cancer rates, mucosal inflammation detected endoscopically, histologically, and by immunohistochemistry, was more common and pronounced compared to high risk African Americans (Figure 1). Secondly, despite the lower protein intake and lower plasma amino acid levels (Supplementary Table 9), proteolytic fermentation was also increased, releasing metabolites that are known to be inflammatory and pro-carcinogenic46. The explanation for these findings is unclear, but may be linked to the high prevalence of chronic parasitic infestations in rural Africans causing inflammation and exudation of mucinous proteins. Of note, schistosoma were incidentally identified in two subjects, and a tapeworm segment in another (Supplementary Figure 3). Intestinal protozoa have been hypothesized to stimulate immunosurveillance against colon cancer47. Clearly further focused investigations of these paradoxical findings are needed, but possible explanations involve the complex associations between inflammation, immunosurveillance, and carcinogenesis, and the ability of butyrogenesis to arrest the progression of inflammation to neoplasia. In murine models, lymphocytes can suppress carcinogenesis as part of the process of immunosurveillance, and in patients with tumors, specific intra-tumor lymphocyte markers for Th1 polarization and cytotoxic and memory T cells have been shown to suppress growth and metastasis, a process termed immunoediting48,49. Furthermore, Thus, T cell infiltration may suppress or enhance tumorigenesis, depending upon the type, density and location of the immune cells. There is substantial experimental evidence, recently reviewed by us7, that the major products of saccharolytic fermentation, acetate, propionate, and butyrate, have potent anti-neoplastic properties 50, but butyrate is unique in its maintenance of colonic mucosal health and defense. First, it is the preferred energy source for colonocytes51. Second, it has a remarkably wide variety of antineoplastic properties, best exemplified by its function as a histone deacetylase inhibitor52, its capacity to down-regulate the key canonical Wnt-signaling pathway linked to colonic carcinogenesis 53, and its ability to reduce the burden of carcinogens, such as bile acids28,29,54 and red meat products26. Further studies are needed to explore the intriguing possibility that increased saccharolytic fermentation and butyrogenesis may counter the recognized proliferative properties of T helper type 17 T cells (TH17)55
Although we have focused on the potential for a high fiber and high fat to modify metabolic pathways that have been shown experimentally to impact mucosal biomarkers of cancer risk, it must be stressed that a change in one aspect of an otherwise isocaloric diet will inevitably result in a change in another. Consequently, the associated change in animal protein or digestible carbohydrate might also be responsible for the observed mucosal changes. Be that as it may, our results provide fairly substantial evidence for an powerful influence of butyrate on mucosal health, and raise hope that an increase in fiber consumption, together with a moderation in fat intake, may reduce the disproportionately high incidence of colon cancer in African Americans, and indeed in most westernized communities. Clearly, these suggestions need now to be confirmed by longitudinal studies. With regard to fiber, total quantities may be critical, as fiber supplementation at ≈35 g/d has generally failed to reduce polyp recurrence in clinical trials56 and recent experimental studies demonstrate that a threshold effect of butyrate availability determines its inhibitory effect on proliferation52. Furthermore, human studies by Burkitt concluded that total fiber intake needs to exceed 50 g/d (as in our study) to prevent colon cancer57. Our results also raise serious concern that the progressive westernization of African communities may reduce butyrogenesis, allowing their high basal state of chronic inflammation to progress through proliferation to neoplasia38, culminating in the emergence of colon cancer as a major health issue58.
Methods
Study Design
First, the study was submitted to the University of Pittsburgh and University of KwaZulu-Natal Institutional Review Boards for approval. We employed a unique design (Table 1, Supplementary Note 1), where 20 healthy middle aged African Americans and 20 rural Africans (Supplemental Table 10: Demographics) from the same communities previously studied 5 were studied first for 2 weeks in their own home environment, eating their usual food (HE study), and then again in-house whilst they were fed the intervention diet for 2 weeks (DI study). Consequently, each subject served as his/her own control, which is important given the known wide individual variation in colonic microbiota composition. We chose intervention diets that were at the same time palatable and contained reverse quantities of fiber and fat, such that African Americans would be given ‘African style’ foods increasing their average fiber intake from 14g/d to 55g/d and reducing their fat from 35% to 16% of total calories, whilst Africans were given a ‘western style’ diet reducing their fibre from 66g/d to 12g/d and increasing their fat from 16% to 52% (Supplementary Tables 1-3). Cogniscient of the problems of compliance to acute dietary change and the accuracy of dietary recall to estimate actual intakes within the community, we elected to perform all the dietary intervention studies in-house, where meals could be prepared and given under close supervision. With African Americans, participants were housed the University of Pittsburgh Clinical Translational Research Center (CTRC) and with rural Africans, we employed in a rural lodging facility, close to their homes, with full kitchen facilities. Body weights were maintained within 2 kg by adjusting food quantities while keeping overall macronutrient composition the same. The sampling schedule is given on Table 1, showing that fresh fecal samples were taken at 3 intervals during the HE study and again 3 times after the diet switch. Colonoscopy was performed to identify latent disease, polyps, or cancer, to obtain 3h colonic evacuates for analysis and biopsies for biomarkers of cancer risk4 at the beginning of the HE study and end of the DI study. Full details of the menus, cooking methods, and total dietary compositions are given under Supplementary Tables 4a-c, 2, and 3; Supplementary Note 2).
Subjects and Recruitment
Age and sex matched healthy volunteers, from the age range 50-65 years, were randomly selected from the African American population in the Pittsburgh region of Pennsylvania and from the rural native South Africans from the rural Kwazulu region. We collaborated with Dr Stephen Thomas, Director of Minority Studies at the University of Pittsburgh School of Public Health to recruit healthy African American volunteers from the Pittsburgh region, and also advertised the study with the approval of our IRB in public areas. In South Africa, volunteers were be recruited through advertisements placed in public community centers, e.g. post offices, town halls, civic centers, and through the iZulu Community Health Center. Appropriate compensation (as advised by Minority studies and the iZulu Community and ratified by the University of Pittsburgh and KwaZulu-Natal) for time and testing was paid to volunteers for participation.
Screening
Informed signed consent was taken from each participant. All African volunteers could understand English, but a nurse-translator participated in the consent process to ensure proper understanding of the details of the research procedures. Screening was performed in Pittsburgh at the CTRC and in Africa at Ngwelezana Hospital out-patient clinic, Empangeni, KwaZulu-Natal, South Africa. A detailed medical history was first taken. With rural Africans, a local bilingual nurse acted as interpreter. A 20ml blood sample was taken for full blood count, ESR, electrolytes and urea, albumin, alkaline phosphatase, AST and bilirubin. If the results were normal and if they satisfied the eligibility criteria, they were invited to participate in the study.
Subject Eligibility
Details are given under Supplementary Note 1. Inclusion Criteria: Healthy volunteers, from GI standpoint between 40-65 years (age at which colon cancer screening/colonoscopy is recommended in this population) and BMI between 18-35 Kg/m2 (Supplementary Discussion). Exclusion criteria: Participants pre-colonoscopy were ineligible if they had a history of familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, inflammatory bowel disease, or invasive cancer within 5 years before enrollment (h/o adenomatous polyps acceptable). Also ineligible were individuals with known renal, hepatic, or bleeding disorders; previous GI surgery resulting in disturbed gut function due to of loss of bowel or altered anatomy; or any form of chronic GI disease resulting in disturbed gut function, diarrhea, and malabsorption. Individuals with antibiotic use within the past 12 weeks (Supplementary Discussion), current steroids use, or diabetes. Exclusion criteria post-colonoscopy were detection of previously unrecognized ulceration (with depth and >0.5cm), stricture, severe inflammation, and polyps >1cm diameter or cancer.
Fecal, Colonic, and Mucosal Sampling and Colonoscopy
To synchronize the measurements of the microbiota, the metabolome, and the colonic mucosa, the sampling was tied to the preparation for and conduct of a colonoscopy, as previously4 described, at baseline whilst on their usual diet (ED1, Table 1), and then again at the conclusion of the dietary change (ED2, Table 1). In this, fresh fecal samples are collected prior to colonic evacuation and immediately frozen at −80°C, and total colonic contents are collected for 3 h during evacuation with a simple PEG solution. In this, two liters of polyethylene glycol (60g/l, molecular weight 3350) solution was consumed rapidly as possible over ½ h. We avoided using the commercial preparation ‘Golytely’, as it contains sodium sulfate, which is known to disturb the microbiota composition. Our experience had shown that the quality of bowel preparation with this technique was similar to the more conventional overnight bowel washout. The mucosa health status was assessed by visualization and biopsy. Polyps, when encountered, were removed per standard practice, and biopsies were taken from normal mucosa from the proximal (cecum/ascending colon) mid (transverse) and distal (sigmoid) colon at 25cm from the anal verge. Mucosal samples for immunohistochemistry were collected in formalized saline, and for gene expression in RNAlater (Qiagen, Germantown, MD) before being stored frozen at −80°C.
Measurement of Mucosal Biomarkers
Histology
Colonic mucosal biopsies were obtained by colonoscopy before and after dietary switch from 3 different sites (ascending, transverse and descending) and stored in 10% buffered formalin. Later, the biopsy samples were embedded in paraffin and 5-mm sections were cut and stained with either routine hematoxylin and eosin (H&E) or immunohistochemical stains (see below). The histologic findings on the H&E stained sections were evaluated by one blinded experienced gastroenterological histopathologist (AK). Measurements focused on the numbers of inflammatory cells and eosinophils in the lamina propria and the numbers of intraepithelial lymphocytes. Scoring of the H&E stained section was done as shown on Supplementary Table 5a. Any pathologic finding, such as presence of parasitic organisms, was recorded.
Immunohistochemistry
Slides for CD3 and Ki67 staining were deparaffinized at 60° for 2 hours. To inhibit endogenous peroxidase, the slides were pre-treated using 3% hydrogen peroxide/methanol at room temperature (RT) for 10 min, followed by antigen retrieval with 0.2% pepsin solution (# P7012, Sigma, 3050 Spruce St., St. Louis, MO, USA) at 37° for 10 min. Serum Free Protein Block (# X0909, Dako, 6392 Via Real, Carpinteria, CA 93013, USA) was used at RT for 10 min. The slides were drained and incubated with Primary antibodies CD3 and Ki-67 (# A0452, 1:100, rabbit polyclonal, Dako; # MIB-1, 1:100, mouse monoclonal, Dako) respectively at RT for 1 hr. Secondary detection was applied using Immpress universal antibody Polymer detection kit (# MP-7500, Vector Labs, 30 Ingold Road, Burlingame, CA 94010, USA) at RT for 30 min. The slides were stained with DAB substrate kit (# SK-4100, Vector Labs) for 10 min and counter stained using Shandon Hematoxylin (# 6765015, Thermo Scientific, 81 Wyman St, Waltham, MA 02451, USA).
Slide staining for CD68 was done on a Ventana Benchmark Ultra slide stainer. Deparaffinized slides were pretreated using the ultra CC1 (# 950-224, Ventana, 1910 Innovation Park Dr., Tucson, AZ 85755, USA) for 24 min for antigen retrieval. Slides were incubated with the Primary antibody CD68 (# M087601, 1:100, mouse monoclonal, Clone PG-M1, Dako,) for 32min RT. The Optivew DAB kit (# 760-700, Ventana) was used for secondary detection.
Quantification of immunohistochemical staining
Counting of the proportions of positive staining cells using light microscopy at x400 magnification was performed by a single investigator (KM), under blinded conditions. To assess inter-observer variability, 40 slides were randomly selected and recounted by a second senior pathologist (AK), showing a concurrence of 88% for Ki67+ and 80% for CD68+ densities.
Ki67
The proportion of Ki67 positive staining cells were counted in well-oriented crypts (average/slide 8, range 4-14). Ki67 proliferation rate was defined as number of Ki67+ cells divided by the total number of crypt cells and expressed as percentage. The differences were found to be the same in the total crypt and in the upper crypt, so only the total crypt proportions are reported here.
CD3
Only CD3+ staining intraepithelial lymphocytes (IEL) were counted in a representative area of at least 300 epithelial cells. The density of IELs was expressed as an index of number of CD3-positive lymphocytes per 100 epithelial cells.
CD68
The number of CD68 positive cells (macrophages) within the lamina propria were counted and graded on a scale from 1-3: Grade 1 (None/rare), Grade 2 (scattered superficial collections) and Grade 3 (strong, diffuse or band-like infiltrate in the superficial lamina propria) as shown in Figure 1c.
Targeted Analysis of Fecal and Colonic Microbes and Metabolites of Special Interest
Details of the materials and methods used for the collection, preparation and analysis of fecal samples for targeted analysis of microbes of special interest (real-time quantitative PCR) and their metabolites (Agilent Technologies 6890N Network GC System with a flame-ionization detector for short chain fatty acids and Shimadzu HPLC-MS for quantification using electrospray ionization in negative ion mode by monitoring the (M-H)− ion for bile acids) in African Americans and rural Africans have been previously published5. Justification for the use of the BcoA functional gene for butyrate production is that Flint's group in Aberdeen have demonstrated that whilst there are a number of different metabolic pathways that use different enzymes that culminate in butyrate synthesis, butyryl-CoA:acetate CoA-transferase, the product of the BcoA gene, is responsible for the last step in butyrate synthesis in the vast majority of intestinal butyrate producers13. Similarly, there are other microbial enzymes that participate in bile acid deconjugation, but Wells et al demonstrated that there was a good correlation between human fecal bacterial dehydroxylating activity measured by fecal dilution assay and their PCR assay for the baiCD gene which encodes the key enzyme responsible for the bile acid 7alpha-dehydroxylation pathway23
Plasma Amino Acids
fasting blood concentrations were measured in extracted plasma by reverse-phase C-18 precolumn derivatization HPLC (AccQ•Tag™ Ultra derivatization, Waters, Milford, MA) as previously5.
Statistical analysis
Statistical analysis of the group differences in continuous variables was conducted using SPSS 16.0 (SPSS Inc). The significance of group differences for normally distributed data was assessed with unpaired and paired Student's t tests. The nonparametric data were analyzed with a Mann-Whitney U test or Kruskal-Wallis one-way analysis of variance by ranks for unpaired data, and Wilcoxon signed-rank test tests for paired data. The significance of the association was evaluated with Spearman's rank correlation test. A level of p < 0.05 was accepted as statistically significant. Data are presented as means ±SEs. Complex microbiota and metabonome data were analyzed by several multivariate ordinations detailed below (principal component analyses, nonmetric multidimensional scaling), Kruskal-Wallis independent tests, and multivariate ANOVA with Bonferroni correction.
Global Analysis of the Microbiota Composition and Diversity
We chose the Human Intestinal Tract (HIT)Chip phylogenetic microarray for the global profiling of microbiota composition. It has been demonstrated that the HITChip analysis of fecal samples provides highly concordant results concerning the microbiota composition when compared to 16S rRNA gene or metagenome sequencing59-62 since it allows deep profiling of phylotypes at high resolution, down to <0.1% relative abundance; corresponding to a duplicated set of 100,000 pyrosequencing reads per sample with very high reproducibility (>98%)59 and at considerably lower cost.
HITChip Analysis
DNA was isolated from fecal or colonic samples and subsequently used for phylogenetic profiling of the intestinal microbiota using the HITChip phylogenetic microarray16. Standardized quality control was maintained through our library of a duplicated set of 3,631 probes targeting the 16S rRNA gene sequences of over 1,000 intestinal bacterial phylotypes. Briefly, the full-length 16S rRNA genes were amplified, and PCR products were transcribed in vitro into RNA, labeled with Cy3 and Cy5 and fragmented. Hybridizations were performed in duplicate and data was extracted from microarray-scanned images using Agilent Feature Extraction software, version 10.7.3.1 (http://www.agilent.com). Array normalization was performed as previously described using a set of custom R scripts (http://r-project.org) and stored in a custom MySQL database (http://www.mysql.com). Duplicate hybridizations with a Pearson correlation >98% were considered for further analysis.
Microbiota profiles were generated by pre-processing the probe-level measurements with min-max normalization and the frozen-RPA probe summarization62,63 into three phylogenetic levels: level 1, defined as order-like 16S rRNA gene sequence groups; level 2, defined as genus-like 16S rRNA gene sequence groups (sequence similarity >90%); and level 3, phylotype-like 16S rRNA gene sequence groups (sequence similarity >98%). In the present work we primarily focus on the genus-level (level 2) variation. Significance of the differences between the time points (Fig. 3A) were estimated based on a (paired) linear model for microarrays (limma) with the threshold of FDR<0.2 estimated based on Benjamini-Hochberg procedure and a minimum fold-change of 25% (0.1 at the Log10 scale)64.
Microbial Co-Occurrence Network Analysis
We constructed the co-occurrence networks between the 130 genus-like bacterial groups based on their logarithmic abundances (HITChip log10 signal) within each treatment group (African Americans and Native Africans; before and after the dietary intervention). For a robust correlation analysis we applied the SparCC algorithm using 20 iterations, and with 50 bootstrap data sets for significance testing, followed by q-value65 correction of the pseudo p-values from the bootstrap analysis. We focused on the significant (|r|>0.5; q<0.01) correlations between the genus-like groups where a qualitative change (change of sign) in the correlation was observed following the dietary intervention. To ensure robust analysis, we included only the correlations that changed drastically in the intervention, with a difference of >1 between the correlation values before and after intervention (so that the correlations would change for instance from −0.5 to 0.5 or higher). This provided a list of genus-like bacteria that had significant changes in their mutual correlation networks following the dietary intervention in either rural African or African American group. To simplify interpretation, we clustered the associated genus-like groups into coherent network modules with complete-linkage hierarchical clustering based on the SparCC correlations. We defined a module as a connected sub-network where the correlations between all genus-like groups within the module are r>0.5. Networks were visualized using the network visualization and exploration platform Gephi66. The resulting modules are highlighted in Figure 3B.
Global Analysis of the Metabolome: Sample preparation for NMR spectroscopic analysis
All urinary samples were thawed at room temperature and vortexed for 10 sec. A total of 400 μl of urinary sample was thoroughly mixed with 250 μl of 0.2 M sodium phosphate buffer containing 20% D2O, pH=7.4, 0.01% 3-(trimethylsilyl)-[2,2,3,3-2H4]propionic acid sodium salt (TSP) and 3 mM sodium azide (NaN3). The mixture was subsequently centrifuged at 10,000 x g for 10 min and 600 μl of supernatant was transferred into an NMR tube with an outer diameter of 5 mm. Approximately 200 mg of wet fecal sample was mixed with 600 μl of H2O (HPLC grade) in a 1.5 ml Eppendorf tube. A cycle of 30-sec vortexing, 5-min sonicating at 4 °C and 30-sec vortexing was then carried out on the mixture, followed by centrifuging at 10,000 x g for 10 min. A total of 400 μl of supernatant was added into a 1.5 ml Eppendorf containing 250 μl of aforementioned sodium phosphate buffer, vortexed and spun at 10,000 × g for 10 min. An amount of 600 μl of supernatant was put into an NMR tube.
1H NMR spectroscopy
1H NMR spectra of urinary and fecal water extract samples were acquired using a Bruker 600 MHz spectrometer (Bruker, Rheinstetten, Germany) at the operating 1H frequency of 600.13 MHz at a temperature of 300 K. An NMR pulse sequence (recycle delay −90°-t1-90°-tm-90°-acquisition) and standard parameters were used to obtain standard one-dimensional 1H NMR spectral data as described in Beckonert et al 67.
Multivariate statistical data analysis
1H NMR spectra of urine and fecal extracts were automatically phased, referenced to TSP at 1H δ0.00 and baseline-corrected using an in-house developed MATLAB script (Dr. Tim Ebbels, Imperial College London). The processed NMR spectra (1H δ 0 to 10) were imported to MATLAB (R2012a, MathWorks) and digitized into 20 k data points with a resolution of 0.0005 ppm using an in-house developed script. The water peak region in urine (4.7-5.14 ppm) and fecal water spectra (4.7-5.18 ppm) were removed to minimize the effect of the disordered baseline caused by water suppression. In addition, regions (1H 0-0.3) in urine spectra, and regions (1H 0-0.25) in fecal water spectra containing only noise were therefore removed, together with the urea signal in urine (1H 5.48-6.24). Due to the heavy peak shifting in urinary spectra, a recursive segment-wise peak alignment method was applied in order to improve metabolic biomarker recovery68. Probabilistic quotient normalization was subsequently performed on the resulting datasets in order to account for dilution of complex biological mixtures69. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) was carried out with a unit variance scaling method in SIMCA (P+13.0) and MATLAB software. P values of metabolites were calculated using ANOVA and false discovery rate (FDR) was also calculated for each metabolite marker70. For the two-way clustered heatmap (Figure 4b) between fecal microbiota and metabolite concentrations in urine and feces, the partial correlation was calculated for each phylotype and metabolite. The correlation was adjusted for country and dietary intervention to account for baseline differences. A pFDR cut-off of 0.3 was used to define the significance (denoted by ‘+’) to account for the different numbers of samples in each data set. For each data set (microbiota, fecal metabolites, urinary metabolites) the data was clustered using hierarchical cluster analyses with complete linkage. The optimal number of clusters was chosen as the splitting that maximized the modularity using the correlation as weight of each edge in the network.
Metabolic Reaction Network Generation
Using the freely available MetaboNetworks software19 reactions that occur spontaneously or by means of enzymes linked to human and/or bacterial genomes were identified in the Kyoto Encyclopaedia of Genes and Genomes (KEGG). All complete genome sequences listed in KEGG that could be associated to the bacterial groups represented on the HITChip were included in creating the database, resulting in a total of 817 bacterial genomes included in the database. Next, the software was used to construct a shortest-path metabolic reaction network between all (fecal and urinary) metabolites significantly different in any of the different dietary comparisons. From this ‘global network’, 2 sub-graphs were generated for each of the two dietary switches using significantly associated fecal metabolites. In order to highlight the differential expression of the metabolites associated with specific pathways, the background shading was added to the graphs to indicate the different interconnecting pathways (Figure 5a-c). The abbreviations and full names of these metabolites can be found in Supplementary Table 11.
Supplementary Material
Acknowledgements
We thank Chadd and Kate Bain and the iZulu Lodge and Projects team in KwaZulu-Natal for help with the recruitment and housing of the African volunteers and Dr Iain Thirsk and his surgical endoscopy staff at Ngwelezana Hospital, Empangeni, KwaZulu-Natal, for providing the endoscopic facilities for the study. We acknowledge the help of Dr Robert Branch and the staff of the University of Pittsburgh Clinical Translation and Research Center for help with the dietary switch studies on African Americans. We thank Ms. Kayellen Umeakunne for help with the design of the intervention diets. Primary funding for the study was provided by a grant from the National Institutes of Health R01 CA135379 (O'Keefe), and CTRC support from UL1 RR024153 and UL1TR000005. The research was also supported by the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. We would also like to acknowledge the Academy of Medical Sciences who funded part of the metabolic profiling work. Additional funding for the microbiota studies was provided by the Spinoza Award of the Netherlands Organization (de Vos) for Scientific Research, the ERC Advanced Grant 250172 (Microbes Inside) of the European Research Council and the Academy of Finland (Grant 141140 to WMdV; Grant 256950 to LL). The University of Pittsburgh Genomics and Proteomics Core Laboratory (GPCL) produced the mucosal gene expression microarray data.
Footnotes
Author contributions:
SJDOK designed and conducted the human studies, supervised the analysis and evaluation the results, prepared, edited and revised the manuscript. EGZ: Assisted with study design, managed the global microbiota analysis and interpretation, and assisted with preparation of the manuscript. HRG: Assisted with study design and execution of the human studies, managed the targeted microbiota analysis and interpretation, assisted with preparation of the manuscript. JK was responsible for writing the text relating to the 1H NMR analysis and for interpreting the spectral data. JN provided strategic oversight for the metabonomic analytical strategy and was responsible for data interpretation. JL performed the 1H NMR analysis and the multivariate analysis. JO was responsible for targeted analysis of metabolites, data management, sample distribution and manuscript preparation. LL and ST: conducted the microbiota data analysis and interpretation, and assisted with preparation of the manuscript. PP: Sample coordination and microbiota phylogenetic profiling. WMDV: Managed, interpreted and supported microbiota analysis. KM and HK performed the mucosal immunohistochemistry. EW (USA), ER (USA) and FB (S Africa) performed the dietary analysis, meal design and production. KAN and VN helped organize the South African studies and endoscopies. KV was responsible for the screening, recruitment and management of African Americans dietary switch studies. FC performed the targeted microbiota analysis. LM supervised the recruitment of African subjects and helped with the dietary exchange studies. AK supervised the histological assessments of biopsy samples. ACB coordinated the targeted microbiota analyses. JD supervised the targeted metabolite analysis and helped with m/s preparation. JP carried out the metabolic reaction network analysis and interpretation.
Accession codes
Microbiome and metabolome data have been deposited in the XXXXXX and YYYYY databases with accession codes AAAA and BBBB, respectively.
None of the authors declared competing financial interests statements.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 Dec 15;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Le Marchand LKL. Cancer among Japanese migrants to Hawaii: Gene-environment interactions. Rev Epidemiol Santé Publique. 1992;40:425–430. [PubMed] [Google Scholar]
- 3.Norat T, Aune D, Chan D, Romaguera D. Fruits and Vegetables: Updating the Epidemiologic Evidence for the WCRF/AICR Lifestyle Recommendations for Cancer Prevention. Cancer treatment and research. 2014;159:35–50. doi: 10.1007/978-3-642-38007-5_3. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe SJ, Chung D, Mahmoud N, et al. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007 Jan;137(1 Suppl):175S–182S. doi: 10.1093/jn/137.1.175S. [DOI] [PubMed] [Google Scholar]
- 5.Ou J, Carbonero F, Zoetendal EG, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013 Jul;98(1):111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponz de Leon M, Roncucci L, Di Donato P, Tassi L, Smerieri O, Amorico MG, Malagoli G, et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res. 1988 Jul 15;48(14):4121–4126. [PubMed] [Google Scholar]
- 7.Greer JB, O'Keefe SJ. Microbial induction of immunity, inflammation, and cancer. Front Physiol. 2011;1:168. doi: 10.3389/fphys.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vipperla K, O'Keefe SJ. The Microbiota and Its Metabolites in Colonic Mucosal Health and Cancer Risk. Nutrition in Clinical Practice. 2012 Oct 1;27(5):624–635. doi: 10.1177/0884533612452012. 2012. [DOI] [PubMed] [Google Scholar]
- 9.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013 Aug 2;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein C, Holubec H, Bhattacharyya A, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Archives of Toxicology. 2011;85(8):863–871. doi: 10.1007/s00204-011-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu GD, Chen J, Hoffmann C, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 2011 Oct 7;334(6052):105–108. doi: 10.1126/science.1208344. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environmental microbiology. 2010 Feb;12(2):304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibson GR, Macfarlane GT, Cummings JH. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993 Apr;34(4):437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcenilla A, Pryde SE, Martin JC, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000 Apr;66(4):1654–1661. doi: 10.1128/aem.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajilic-Stojanovic M, Heilig HG, Molenaar D, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol. 2009 Jul;11(7):1736–1751. doi: 10.1111/j.1462-2920.2009.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Structure, function and diversity of the healthy human microbiome. Nature. 2012 Jun 14;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature. 2008 Oct 23;455(7216):1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 19.Posma JM, Robinette SL, Holmes E, Nicholson JK. MetaboNetworks, an interactive Matlabbased toolbox for creating, customizing and exploring sub-networks from KEGG. Bioinformatics. 2014 Mar 15;30(6):893–895. doi: 10.1093/bioinformatics/btt612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu RH. Dietary bioactive compounds and their health implications. Journal of food science. 2013 Jun;78(Suppl 1):A18–25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 23.Wells JE, Hylemon PB. Identification and Characterization of a Bile Acid 7α-Dehydroxylation Operon in Clostridium sp. Strain TO-931, a Highly Active 7α-Dehydroxylating Strain Isolated from Human Feces. Applied and Environmental Microbiology. 2000 Mar 1;66(3):1107–1113. doi: 10.1128/aem.66.3.1107-1113.2000. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kok TM, van Faassen A, Glinghammar B, et al. Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Digestive diseases and sciences. 1999 Nov;44(11):2218–2225. doi: 10.1023/a:1026644418142. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutation Research/Reviews in Mutation Research. 2005;589(1):47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Winter J, Nyskohus L, Young GP, et al. Inhibition by resistant starch of red meat-induced promutagenic adducts in mouse colon. Cancer prevention research (Philadelphia, Pa.) 2011 Nov;4(11):1920–1928. doi: 10.1158/1940-6207.CAPR-11-0176. [DOI] [PubMed] [Google Scholar]
- 27.Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biology & Therapy. 2006;5(3):267–272. doi: 10.4161/cbt.5.3.2382. [DOI] [PubMed] [Google Scholar]
- 28.van Munster IP, Tangerman A, Nagengast FM. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci. 1994;39(4):834–842. doi: 10.1007/BF02087431. [DOI] [PubMed] [Google Scholar]
- 29.Hylla S, Gostner A, Dusel G, et al. Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention. Am J Clin Nutr. 1998;67(1):136–142. doi: 10.1093/ajcn/67.1.136. [DOI] [PubMed] [Google Scholar]
- 30.Wells JE, Williams KB, Whitehead TR, Heuman DM, Hylemon PB. Development and application of a polymerase chain reaction assay for the detection and enumeration of bile acid 7α-dehydroxylating bacteria in human feces. Clinica Chimica Acta. 2003;331(1-2):127–134. doi: 10.1016/s0009-8981(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 31.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/−mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR. Evidence That Hydrogen Sulfide Is a Genotoxic Agent. Molecular Cancer Research. 2006 Jan;4(1):9–14. doi: 10.1158/1541-7786.MCR-05-0126. 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome research. 2012 Feb;22(2):292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Frontiers in physiology. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terpstra OT, van Blankenstein M, Dees J, Eilers GA. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- 36.Lipkin M, Blattner WA, Gardner EJ, et al. Classification and risk assessment of individuals with familial polyposis, Gardner's syndrome, and familial non-polyposis colon cancer from [3H]thymidine labeling patterns in colonic epithelial cells. Cancer Res. 1984 Sep;44(9):4201–4207. [PubMed] [Google Scholar]
- 37.Yu CC, Filipe MI. Update on proliferation-associated antibodies applicable to formalin-fixed paraffin-embedded tissue and their clinical applications. The Histochemical journal. 1993 Dec;25(12):843–853. [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2009;181:223–229. doi: 10.1007/978-3-540-69297-3_21. [DOI] [PubMed] [Google Scholar]
- 40.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunological reviews. 2007 Feb;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunisawa J, Takahashi I, Kiyono H. Intraepithelial lymphocytes: their shared and divergent immunological behaviors in the small and large intestine. Immunological reviews. 2007 Feb;215:136–153. doi: 10.1111/j.1600-065X.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 42.Caprioli F, Bose F, Rossi RL, et al. Reduction of CD68+ macrophages and decreased IL-17 expression in intestinal mucosa of patients with inflammatory bowel disease strongly correlate with endoscopic response and mucosal healing following infliximab therapy. Inflamm Bowel Dis. 2013 Mar-Apr;19(4):729–739. doi: 10.1097/MIB.0b013e318280292b. [DOI] [PubMed] [Google Scholar]
- 43.Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. 1999 Oct;81(3):496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka S, Tatsuguchi A, Futagami S, et al. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut. 2006 Jan;55(1):54–61. doi: 10.1136/gut.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014 Feb 11;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Molecular Nutrition & Food Research. 2012;56(1):184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 47.Juckett DA, Aylsworth CF, Quensen JM. Intestinal protozoa are hypothesized to stimulate immunosurveillance against colon cancer. Medical hypotheses. 2008;71(1):104–110. doi: 10.1016/j.mehy.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 48.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006 Sep 29;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 49.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002 Nov;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 50.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. nhibition of histonedeacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. The Journal of Nutritional Biochemistry. 2008;19(9):587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- 52.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Molecular cell. 2012 Nov 30;48(4):612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bordonaro M, Lazarova DL, Sartorelli AC. Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle. 2008;7(9):1178–1183. doi: 10.4161/cc.7.9.5818. [DOI] [PubMed] [Google Scholar]
- 54.Rosignoli P, Fabiani R, De Bartolomeo A, Fuccelli R, Pelli MA, Morozzi G. Genotoxic effect of bile acids on human normal and tumour colon cells and protection by dietary antioxidants and butyrate. Eur J Nutr. 2008 Sep;47(6):301–309. doi: 10.1007/s00394-008-0725-8. [DOI] [PubMed] [Google Scholar]
- 55.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature medicine. 2009 Sep;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asano TKMR. Dietary fibre for the prevention of colorectal adenomas and carcinomas (Review). The Cochrane Library. 2008;(4) [Google Scholar]
- 57.Burkitt D. Diseases of the alimentary tract and western diets. Pathol Microbiol. 1971;39(3):177–186. doi: 10.1159/000162646. [DOI] [PubMed] [Google Scholar]
- 58.Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012 Sep 15;118(18):4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 59.Claesson MJ, O'Sullivan O, Wang Q, et al. omparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4(8):e6669. doi: 10.1371/journal.pone.0006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013 Aug 29;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 61.Van den Bogert B, De Vos WM, Zoetendal EG, Kleerebezem M. Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples. Appl Environ Microbiol. 2010;77(6):2071–2080. doi: 10.1128/AEM.02477-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lahti L, Elo LL, Aittokallio T, Kaski S. Probabilistic analysis of probe reliability in differential gene expression studies with short oligonucleotide arrays. IEEE/ACM transactions on computational biology and bioinformatics / IEEE, ACM. 2011 Jan-Mar;8(1):217–225. doi: 10.1109/TCBB.2009.38. [DOI] [PubMed] [Google Scholar]
- 63.Lahti L, Torrente A, Elo LL, Brazma A, Rung J. A fully scalable online pre-processing algorithm for short oligonucleotide microarray atlases. Nucleic Acids Res. 2013 May 1;41(10):e110. doi: 10.1093/nar/gkt229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth G. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry WH R, editors. Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer, New York; New York: 2005. pp. 397–420. [Google Scholar]
- 65.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bastian M HS, Jacomy M. International AAAI Conference on Weblogs and Social Media. California; San Jose: 2009. Gephi: An Open Source Software for Exploring and Manipulating Networks. 2009. [Google Scholar]
- 67.Beckonert O, Keun HC, Ebbels TMD, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2(11):2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 68.Veselkov KA, Lindon JC, Ebbels TMD, et al. Recursive Segment-Wise Peak Alignment of Biological (1)H NMR Spectra for Improved Metabolic Biomarker Recovery. Anal Chem. 2009 Jan 1;81(1):56–66. doi: 10.1021/ac8011544. [DOI] [PubMed] [Google Scholar]
- 69.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem. 2006 Jul 1;78(13):4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 70.Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B. 2002;64:479–498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.