Abstract
Social foraging provides animals with opportunities to gain knowledge about available food. Studies indicate that animals are influenced by social context during exploration and are able to learn socially. Carrion and hooded crows, which are opportunistic generalists with flexible social systems, have so far received little focus in this area. We combined observational and experimental approaches to investigate social interactions during foraging and social influences on crow behaviour within a free-ranging population at Vienna Zoo, which included 115 individually marked crows. We expected the crows to be tolerant of conspecifics during foraging due to high food abundance. We predicted that social context would enhance familiar object exploration, as well as a specific foraging strategy: predation by crows on other species. We found that crows were highly tolerant of one another, as reflected by their high rates of cofeeding – where they fed directly beside conspecific(s) – relative to affiliative or agonistic interactions. Evidence for social facilitation – when the observer’s behaviour is affected by the mere presence of a model – was found in both object exploration and predation behaviour. Specifically, crows touched the objects more frequently when others were present (whilst only approaching the objects when alone), and conspecifics were present more frequently during predation events involving the high-risk target species. Evidence for enhancement during object exploration – where the observer’s attention is drawn to a place or object by a model’s actions – was not confirmed in this context. Our results highlight the role played by the presence of conspecifics across different contexts: natural foraging behaviour, familiar object exploration and a specific foraging strategy. To our knowledge, this is one of the first corvid studies aimed at teasing apart specific social influence and learning mechanisms in the field. These crows therefore make promising candidates for studying social learning and its consequences under naturalistic conditions.
Keywords: social facilitation, tolerance, field, corvids, social learning
Introduction
Foraging in a social context allows animals to gain knowledge from conspecifics about where, what and how to eat (Galef & Giraldeau 2001). Whilst many studies have focused on the influence of social context in relation to novel foods and objects, the benefits of social foraging are also likely to apply to familiar items, such as aiding in the discovery of new familiar food sources (Galloway et al. 2005). Social facilitation – when the mere presence of another animal affects the observer’s behaviour (Hoppitt & Laland 2008) – has been found to play a role in familiar food consumption in a number of species. Humans (Herman et al. 2003) and rooks (Corvus frugilegus; Dally et al. 2008) will consume considerably more food, whilst others are present than alone. Capuchin monkeys (Cebus apella) start eating at the same time as the demonstrators and eat more of the familiar food than in control conditions (Galloway et al. 2005). Many of these non-human studies have been conducted in captive settings and findings could benefit from further testing in more naturalistic settings, removing issues like absence of feeding alternatives and fluidity of conspecifics present.
In this study, we therefore focused on a wild population of carrion and hooded crows (Corvus corone corone: C. c. cornix), which are worthy model species for investigating social foraging and social influences on behaviour for several reasons. Firstly, they are opportunistic generalists that utilise a wide variety of food sources (Goodwin 1976). They are well adapted to different habitats, including urban environments (Goodwin 1976), which can lead to large populations, particularly in areas where food availability is high. Like some other corvid species, such as common ravens (Corvus corax), crows have very flexible social structures based on fission–fusion dynamics (Goodwin 1976; Braun et al. 2012). Breeding pairs can be highly territorial, whilst non-breeders form communal roosts and foraging groups, which may also be joined by breeders outside the breeding season (Richner 1989; Baglione et al. 2002). This social system allows for a variety of different types and amounts of interactions to take place. For example, in common ravens, the quality of these relationships has been found to vary depending on value, compatibility and security (Fraser & Bugnyar 2010). In carrion crows, the quality and quantity of social interactions, such as the rate of tolerance or aggression within social groups, is still relatively unknown. This type of social living also provides opportunities for these birds to exploit conspecifics as a means of gaining information about available food. For instance, roosting together allows sharing of information, such as the location of food sources (Marzluff et al. 1996).
The generalist diet and flexible social structure in these crows may lead to notable differences between different crow populations in where and how they find food, such as on beaches or rubbish dumps (Hori & Noda 2007; Baglione & Canestrari 2009). Crows will predate on other species including the eggs or young of small mammals and birds as well as occasionally fish, amphibians, reptiles and adult birds (von Glutz Blotzheim et al. 1993). In Scandinavia, there are reports of corvids stealing live fish directly from dangling fishing lines (von Glutz Blotzheim et al. 1993). Reliance on predation of other species as a foraging strategy may also differ between populations. It is not yet known whether these population differences are driven by ecological discrepancies leading to varied strategies or if social learning – learning from observing the behaviour of others (Zentall 2004) – might lead to differences in populations.
A few previous studies have focused on aspects of social learning in corvid species. Wild American crows (Corvus brachyrynchos) have been found to be able to learn to discriminate a ‘dangerous’ from a ‘neutral’ human face, firstly through individual learning, with this information then being transmitted socially (Cornell et al. 2011). In wild New Caledonian crows (Corvus moneduloides), juvenile birds appear to utilise a combination of individual learning and observing their parents, to develop Pandanus tool manufacturing skills (Holzhaider et al. 2010). A recent study in a cooperatively breeding carrion crow population did find evidence that fathers facilitated the access of their offspring to novel food (Chiarati et al. 2012). Further than that, however, there has been comparatively little focus, either in the laboratory or in field, on social influence and learning mechanisms on exploration and the relation to foraging behaviour in carrion crows which was the aim of this study.
The carrion and hooded crow population in the current study consisted of a large number of free-ranging birds that feed and/or live within Vienna Zoo, Austria – an area that presents the birds with a wide variety of easily accessible and reliable food sources. At the beginning of our study, we individually marked 115 crows. As this was the first study conducted with the crows at this site, we first aimed to explore the social interactions of the birds within the regular foraging context through observational methods. We expected the birds within the Zoo environment to rely heavily on the food provided for the zoo animals, which was indicated by the staff reports. We predicted that, although there were many birds present, the high food availability would promote social foraging and allow the birds to be fairly tolerant of one another during foraging.
Following on from this prediction, if the crows were indeed tolerant during foraging, this may indicate that the crows may also be tolerant in another context: exploration of non-food items. Specifically, we were interested in whether enhancement may play a role in familiar object exploration. Enhancement directs an observers’ attention towards a specific place (local enhancement) or object (stimulus enhancement) where the model acts (Giraldeau 1997). In terms of cognitive requirements, enhancement has been suggested to be a low-level social learning mechanism (Zentall 2004). However, it is still worthy of exploration as it can lead to benefits, such as increased foraging efficiency by providing animals with the opportunity to find and utilise food sources (Coussi-Korbel & Fragaszy 1995).
We selected familiar rather than novel objects for this experiment, to link the experiment findings with the observations of the natural foraging setting, which would have primarily consisted of familiar food sources. We looked at whether ‘observer’ birds were more likely to interact with the same object as the ‘model’ bird (first bird to act) in terms of the object’s position (local enhancement) or colour (stimulus enhancement). Studies with other closely related corvids have shown these birds are capable of making feature (including colour) discriminations (Corvus macrorhynchos, Bogale & Sugita 2014; Corvus corax, Range et al. 2008). Recent studies, such as Hoppitt et al. (2012) with wild meerkats, have indicated that animals are likely to be able to utilise a range of learning mechanisms. Although we expect that this may also be the case with the crows in general, we anticipated that in this particular context and population, in which high tolerance may allow birds to freely attend to the same location as conspecifics, we predicted evidence for local over stimulus enhancement.
Finally, we aimed to investigate how these two previous predictions may relate to a specific foraging strategy – predation on other species. Anecdotal reports from Vienna Zoo staff indicated that crows in this population predate on other species within the park, including the young of the zoo animals. We predicted that, as we expected the crows would be tolerant of one another and social context would facilitate object exploration, predation would be likely to take place in the presence of conspecifics. We were particularly interested in identifying the species targeted during predation events, to assess whether predation may occur more often in the presence of conspecifics if the target species presented a high risk to the crows.
Methods
Subjects
Subjects were free-ranging carrion crows (Corvus corone corone), hooded crows (C. c. cornix) and hybrids of these species that live and feed within Vienna Zoo, Austria (48°10′56″N, 16°18′09″E). A total of 115 crows were caught in ladder drop-in traps and ringed (combination of metal and coloured leg rings) with biometrics (bill, tarsus & wing measurements), species, age class (from oral cavity and eye colour: as per Bugnyar & Kotrschal 2002) and feather samples (for sexing analysis) taken. The animal experiment licence (number: BMWF-66.006/0005-II/10b/2010) was obtained by T.B. from the Austrian Ministry for Science and Research, and the project was authorised by Tiergarten Schönbrunn (Vienna Zoo).
Procedure
To investigate the social interactions of foraging crows, scan and focal sampling observations were collected during consecutive walks (‘rounds’) through the Zoo area on a given route. Each round lasted approx. 3 h and included three scan observations (hereafter ‘scans’) per location of all crows present, including unmarked crows, with 5-min breaks between scans, at eight Zoo locations. The number of crows present and food type involved – comprising of Zoo food: meat or non-meat (bread, vegetables, seed), leftovers by visitors (e.g. rubbish) and natural sources (grain, fruit, insects) – was recorded during these scans. A maximum of three rounds were run per day (morning, afternoon and evening – ensuring that rounds were balanced across these time periods) to control for light, weather and temperature (Bugnyar & Kotrschal 2002).
Neutral, affiliative and agonistic foraging-related social behaviours (Table 1) of marked crows only were recorded using 1-min focal observations. Focals were taken at any location where a marked bird was sighted with one focal follow per bird per location per round. Breeding status was determined through observation in a breeding-related context (e.g. nest building, feeding offspring). To record any predation events, ad-lib sampling was used of marked and unmarked crows at any observed location and target species. Observations ran from April to August 2010, to include both breeding (incubation phase, April–beginning of June) and post-breeding (fledging phase, mid-June–August) seasons.
Table 1.
Ethogram of social foraging-related behaviours recorded during scan and focal observations
| Category | Behaviour | Description |
|---|---|---|
| Agonistic | Cache raid | Recovering a food or other item from the cache of another bird, which may then be recached or consumed |
| Agonistic | Displace | Crow A- crow B (food), crow A approaches crow B, crow A obtains food, crow B leaves (without food). Crow A is alert (feathers upright), may involve harsh vocals and physical contact from Crow A to B |
| Agonistic | Steal | Crow A- crow B (food), crow A approaches crow B, crow A obtains food, crow A leaves (with food). Crow A is alert (feathers upright), may involve harsh vocals and physical contact from Crow A to B |
| Neutral | Co-feed | Feeding on same spot as another individual(s) within 0.5 m2 |
| Affiliative | Share | Feeding on same item as another individual(s) within 0.5 m2 |
| Affiliative | Beg | Crow A- crow B (food), crow A approaches crow B, crow A obtains food, crow A or B may leave (with/without food), crow B initiates, involves transfer of item between individuals |
| Affiliative | Bill feed | Crow B (adult) transfers food directly from bill to bill to crow A (adult/chick). May follow a beg |
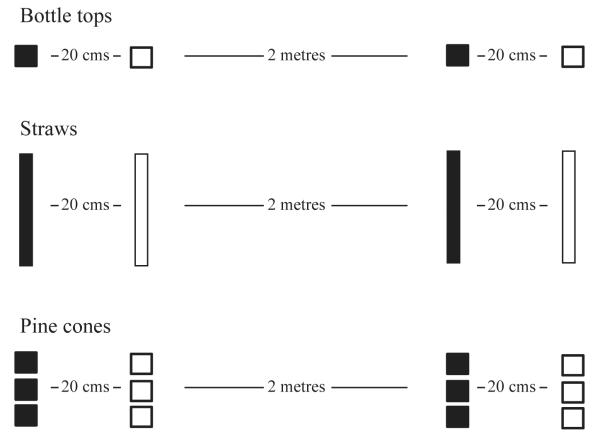
In addition to the observations, we presented the crows with a familiar object experimental set up at three Zoo locations in July to August 2010 (Fig. 1). At each session, four objects of the same size and shape were arranged in two object pairs, and each pair had objects of different colours. For example, one object pair was a red and a blue bottle top placed twenty centimetres apart on the ground with a second object pair (of the same type and colour) placed two metres from the first pair. Three types of objects were used, based on objects regularly found on the ground in the Zoo, comprising three configurations: bottle tops (red and blue), straws (yellow and green) and pinecones (red and purple). The different types of objects were presented in separate experimental sessions. The objects’ position and colours were counterbalanced within object pairs across the sessions.
Fig. 1.
Experimental set-up. At each session, four of the same types of familiar objects were presented in two differently coloured object pairs arranged two metres from each other. The observer (second bird to act) may interact with either object within the same object pair (local enhancement) or the same coloured object within the other object pair (stimulus enhancement) as the model (first bird to act).
Object pairs were presented 4× per location without food (step 1, 36 sessions) and 4× per location with a small piece of bread underneath each object (step 2, 36 sessions). Each object pairing was shown in a randomised order for both steps, with a total of 72 sessions. Sessions were videotaped lasting 20 min each starting when the objects were placed in position, and the experimenter had moved out of the area. The experimenter remained approximately 20 m from the objects, sitting in a visitor area, in order to be able to identify any marked individuals in the area as the leg rings were not always clear on the videotape. A ‘food only’ condition was included on a separate day (28 sessions) to maintain interest by the birds in the experiment locations.
Actions towards the displayed items were recorded as ‘approach’ (approach within 0.5 m without touching the object) or ‘touch’ (approach with touching the object). The position and colour of objects attended to (approach/touch) by each crow, as well as the number of crows present in the area (sighted within 20 m2 of the experiment objects), were also recorded. For example, the ‘model’ bird (first bird to attend to the items) attends (approach or touch) to the red bottle top in the object pair on the left side. If the ‘observer’ bird (second bird to attend to the items) then attends to either coloured bottle top on the left side, we would consider this to be local enhancement. Alternatively, if the observer bird attends to the red bottle top on either side (and not the blue bottle top), we would consider this to be stimulus enhancement.
Data Analysis
Nonparametric tests (SPSS 19) were run, as most variables had a non-normal distribution. In cases with multiple related conditions, Friedman’s ANOVAs were run, which were followed by Wilcoxon matched-pair signed-rank tests if significant. Observation data were analysed using ‘rounds’ as replicates (n = 100). Ad-lib observation and experimental data were analysed using binomial or Fisher’s exact tests. All tests were exact and two-tailed (Mundry & Fischer 1998). Bonferroni corrections were applied in cases with multiple comparisons. Interobserver reliability between the two observers (R. Miller and M. Schiestl) was high (Cohen’s kappa κ = 0.87, p < 0.01).
Results
Social Interactions During Foraging
Over 2222 scans, data on a total of 4858 observations of crows (including unmarked crows) were recorded, with a mean of 2.2 crows recorded per scan and 48.6 crows per observation round. Between 0 and 22 crows (including unmarked crows) were recorded per location per round – indicating that up to 22 different individuals could be present at a location at any one time. Over 100 scan observation rounds, using the mean frequency of observations for each food type per round, the crows were found to frequently exploit food provided for the Zoo animals: eating non-meat items from these diets more than meat (Wilcoxon signed ranks: z-score = −2.52, p = 0.01) or human leftovers (z-score = −3.36, p = 0.001).
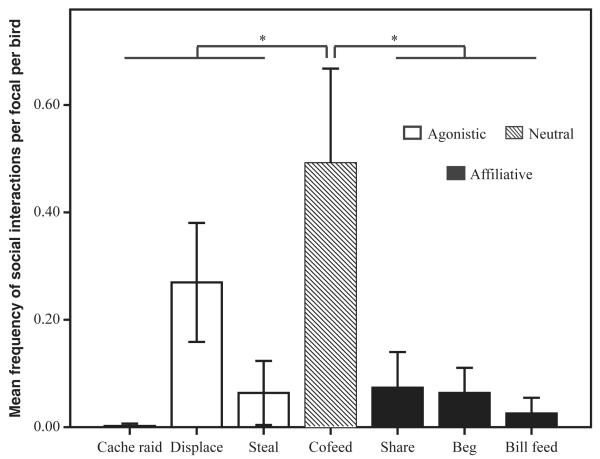
The 115 marked crows included both sexes (male 63.5%; female 36.5%), all age classes (juvenile 5.5%; subadult 85%; adult 9.5%) and breeding statuses (breeder 8.3%; non-breeder 91.7%), with a high proportion of resighting following marking (79%). The focal data of 40 marked crows (minimum five focals per bird), using the mean frequency of social interactions per focal per bird, indicated that foraging crows were found to be highly tolerant, as cofeeding (neutral behaviour) occurred significantly more frequently than affiliative (Wilcoxon signed ranks: z-score = −2.6, p = 0.009) or agonistic behaviours (Wilcoxon signed ranks: z-score = −2.4, p = 0.015; Fig. 2). Interestingly, affiliative and agonistic behaviours occurred at similar rates, with no significant differences between occurrences of these behaviours in general (Wilcoxon signed ranks: z-score = −1.2, p = 0.24), or specifically between the most prominent variables, displace and beg behaviours (Wilcoxon signed ranks: z-score = −1.93, p = 0.054).
Fig. 2.
Frequency of foraging-related social interactions for 40 marked crows (minimum of 5 focal observations per bird). Cofeeding (neutral) was significantly more frequent than affiliative or agonistic behaviours. Significant differences (p < 0.05) are indicated by*.
Social Influences on Object Exploration
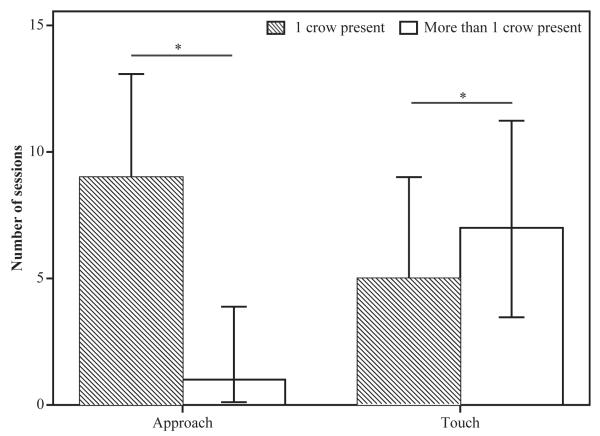
We explored whether social context influenced action towards the items. Of 72 sessions, during the 10 sessions where crows approached the items, only 1 crow was present in nine cases (90%). Of the 12 sessions where the items were touched, more than one crow was present in seven sessions (58.3%). There was a significant effect of the presence of conspecifics on action towards the displayed items (Fisher’s exact test: p = 0.03, Fig. 3). Crows were more likely to approach-only when alone and to actually touch the displayed items when conspecifics were present. This experiment therefore shows a clear effect of social facilitation on familiar object exploration behaviour.
Fig. 3.
Number of sessions by action: approach or touch the displayed items and number of crows present. Crows were significantly more likely to approach (no touching) when alone and to touch the items when conspecifics were present. Significant differences (p < 0.05) are indicated by*.
Of the 22 sessions where at least one bird (‘model’) attended to the items (approach/touch), a second bird (‘observer’) attended to the items in 8 sessions. After the model bird attended to the object, the observer bird was numerically more likely to attend to the same position (local enhancement) rather than the same colour (stimulus enhancement) as the previous bird’s item (7 of 8 cases; 88%). However, the comparison between the number of cases in which the birds attended to the object’s position vs. colour failed to reach significance (Binomial test: N = 7:1, p = 0.07). Between 1 and 11 crows were present (within 20 m of the items) per session, with an average of 3.4 birds present per session. When the model bird attended to the object, the other crows were generally 1–3 m (9 of 17 cases; 53%) or more than 3 m (5 of 17 cases; 29%) away from the model.
Predation by Crows on Other Species
We observed a total of 24 predation events. There was more than 1 crow present in 11 events (46%), which was not significant (Binomial test: N = 11:13, p = 0.84). However, we also wanted to explore any relation between target species and number of crows present. The target species were peccary (Pecari tajacu) piglets (15 events: 63%), parma wallaby (Macropus parma) joeys (2 events: 8%), wild mallard (Anas platrhynchos) ducklings (6 events: 25%) and a wild European mole (Talpa europaea) (1 event: 4%). There were three other species reported by Zoo staff to be targeted by crows, including greater flamingo (Phoenicopterus roseus); however, there was no predation event recorded with these species despite the presence of eggs/young as potential targets and their inclusion in observation areas.
Of these target species, the peccary adults pose the most significant threat to the crows, as they are capable of killing a crow (M. Schiestl, pers. obs.). In most peccary related events (10 of 15 events: 67%), there was more than 1 crow present, whilst there was more than 1 crow present in only one (1 of 9 events: 11%) of the other (non-dangerous) species events. The follow-up test of number of crows present and target species was significant (Fisher’s exact test: p = 0.01). We therefore found that more crows were present during the predation events on peccaries – the species posing the highest threat to the crows – than on other target species.
Discussion
A large number (4858) of observations of crows were collected during scan observations, with up to 22 crows potentially observed in any one location per round. This finding reflects the presence of a relatively large population of crows utilising the Zoo. As predicted, it does appear that the crows rely heavily on food provided for the Zoo animals – a source that appears to attract many crows to the site. This food consisted of both meat and non-meat items, which were available to the crows on a regular and reliable basis, as several enclosures are open-access. As expected, potentially due to the wide variety of food types available throughout the day, the crows were highly tolerant of conspecifics whilst foraging, which was reflected in the high level of cofeeding behaviour recorded relative to affiliative and agonistic social interactions.
The finding that these crows are highly tolerant during foraging differs from a recent study with free-ranging common ravens at the Cumberland Wild Park in Upper Austria, which showed a high proportion of agonistic behaviours in the presence of food (Braun et al. 2012). However, the study sites’ habitats differ (urban vs. rural), which presents potentially significant differences in availability, predictability and type of food available (Braun et al. 2012). Further, raven and crow feeding ecology differs insofar as ravens rely more on meat, in the form of carcasses, than crows in their diet, which is a rather clumped and therefore monopolisable food source. This is also reflected in the crows’ feeding behaviour in our study. The birds often relied on non-meat food provided for the Zoo animals on a regular basis, which is high-quantity, low-quality food that is fairly easy to share. This may explain the different findings to the raven study, but it may also be the case that other carrion crow populations differ in tolerance and aggression levels – this would need to be examined through comparative field studies.
The level of tolerance in our population may allow for a facilitating influence of social context on other aspects of behaviour, such as object exploration. In our familiar object exploration experiment, we found evidence for social facilitation, as when conspecifics were present, crows were more likely to touch the displayed items, whilst only approaching the items without touching when alone. This finding is similar to those of previous corvid studies, where the presence of specific others (kin) has been found to facilitate novel food and object exploration in carrion crows and common ravens (Stówe et al. 2006; Schwab et al. 2008; Chiarati et al. 2012). We did not find clear evidence for enhancement, insofar as the ‘observer’ birds were not significantly more likely to attend (approach or touch) to the same object as the ‘model’ bird in terms of the object’s position or colour. This lack of significance was potentially due to the small sample size, as a relatively low number of birds interacted with the items, despite the potentially large sample size available and inclusion of food in some trials.
Model identity, such as kin, is likely to influence social learning (Laland 2004; Hoppitt et al. 2012). However, despite the relatively high number of birds individually marked (115 birds) in our study, there were still many unmarked birds present within the Zoo. The crows present during the experiment may therefore not have included close affiliates, kin or the operation of other factors that may influence the likelihood of social learning, which may have contributed to a non-significant result in relation to enhancement. Nevertheless, we have shown that presence of others does facilitate familiar object exploration. Increasing the number of identifiable birds would allow us to tease out other social influence such as model identity and learning mechanisms within a free-ranging crow population in the future.
Our findings may also relate to the ‘risky shift’ phenomenon (Wallach et al. 1964) that reflects increased risk taking in the social context. Birds did touch the items in our study at higher rates when conspecifics were present but only approached items without touching, when alone. Certainly, the perception of risk likely accounted for our result on the predation behaviour of the crows. Here, social context did have a facilitating influence on predation on other species, but only when the target species was potentially dangerous to the predating crow. The presence of conspecifics may lead to increased risk taking, as it reduces the threat level to the single crow as the target’s parent(s) is required to keep track of and defend its young against several potential predators at once. An alternative explanation is that there were simply more crows present at the peccary site in general. However, this explanation seems unlikely as there were also other locations with potential predation targets (e.g. flamingo young) where crow numbers were comparably high, but predation events remained very low.
In conclusion, we show here that free-ranging carrion and hooded crows are tolerant of each other at abundant food sources. There was evidence for social facilitation in relation to familiar object exploration, and predation when the target species is potentially dangerous to the predating crow. Future studies with these crows incorporating a range of social learning-related experiments and increasing the number of identifiable birds would be beneficial for the continued study of social learning and its consequences in the natural environment.
Acknowledgements
We thank the Vienna University Cognitive Biology team, Dr Markus Böckle and Tiergarten Schönbrunn (Vienna Zoo) for project support. Thank you to the Editor and two reviewers for their invaluable comments on the manuscript. Thanks are also due to the Austrian National Bank, FWF Austrian Science Fund (Y366B17), WWTF Vienna Science and Technology Fund (CS11-008) and the Royal Zoological Society of Scotland for project funding.
Footnotes
We declare that we have no conflict of interests.
Literature Cited
- Baglione V, Canestrari D. Kleptoparasitsm and temporal segregation of sympatric corvids foraging in a refuse dump. AUK. 2009;126:566–578. [Google Scholar]
- Baglione V, Marcos JM, Canestrari D. Cooperatively breeding groups of carrion crow in northern Spain. AUK. 2002;119:790–799. [Google Scholar]
- Bogale BA, Sugita S. Shape discrimination and concept formation in the jungle crow. Anim. Cog. 2014;17:105–111. doi: 10.1007/s10071-013-0642-y. [DOI] [PubMed] [Google Scholar]
- Braun A, Walsdorff T, Fraser ON, Bugnyar T. Socialized sub-groups in a temporary stable Raven flock? J. Ornith. 2012;153:97–104. doi: 10.1007/s10336-011-0810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T, Kotrschal K. Scrounging tactics in free-ranging ravens. Ethology. 2002;108:993–1009. [Google Scholar]
- Chiarati E, Canestrari D, Vera R, Baglione V. Subordinates benefit from exploratory dominants: response to novel food in cooperatively breeding carrion crows. Anim. Behav. 2012;83:103–109. [Google Scholar]
- Cornell HN, Marzluff JM, Pecorano S. Social learning spreads knowledge about dangerous humans among American crows. Proc. Biol. Sci. 2011;279:499–508. doi: 10.1098/rspb.2011.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussi-Korbel S, Fragaszy SM. On the relation between social dynamics and social learning. Anim. Behav. 1995;50:1441–1453. [Google Scholar]
- Dally JM, Clayton NS, Emery NJ. Social influences on foraging by rooks. Behaviour. 2008;145:1101–1124. [Google Scholar]
- Fraser ON, Bugnyar T. The quality of social relationships in ravens. Anim. Behav. 2010;79:927–933. doi: 10.1016/j.anbehav.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Giraldeau LA. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Galloway AT, Addessi E, Fragaszy DM, Visalberghi E. Social facilitation of eating familiar food in tufted capuchins: does it involve behavioural coordination? Int. J. Primatol. 2005;26:181–189. [Google Scholar]
- Giraldeau LA. The ecology of information use. In: Krebs JR, Davis NB, editors. Behavioural Ecology. Blackwell Science; Oxford: 1997. pp. 42–68. [Google Scholar]
- von Glutz Blotzheim UN, Bauer KM, Haffer J. Corvidae – Rabenvögel. In: von Blotzheim G, editor. Hanbuch der Vögel Mitteleuropas. part 4. Vol. 13/III. Aula-Verlag; Wiesbaden: 1993. p. 2018. [Google Scholar]
- Goodwin D. Crows of the World. Cornell Univ. Press; Ithaca, NY: 1976. [Google Scholar]
- Herman CP, Roth D, Polivy J. Effects of the presence of others on food intake: a normative interpretation. Psychol. Bull. 2003;129:873–886. doi: 10.1037/0033-2909.129.6.873. [DOI] [PubMed] [Google Scholar]
- Holzhaider JC, Hunt GR, Gray RD. Social learning in New Caledonian crows. Learn. Behav. 2010;38:206–219. doi: 10.3758/LB.38.3.206. [DOI] [PubMed] [Google Scholar]
- Hoppitt WJE, Laland KN. Social processes influencing learning in animals: a review of the evidence. Adv. Study Anim. Behav. 2008;38:105–165. [Google Scholar]
- Hoppitt WJE, Samson J, Laland KN, Thornton A. Identification of learning mechanisms in a wild meerkat population. PLoS ONE. 2012;7:e42044. doi: 10.1371/journal.pone.0042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M, Noda T. Avian predation on wild and cultured sea urchin in a rocky shore habitat. Fish. Sci. 2007;73:303–313. [Google Scholar]
- Laland KN. Social learning strategies. Learn. Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Marzluff JM, Heinrich B, Marzluff CS. Raven roosts are mobile information centres. Anim. Behav. 1996;51:89–103. [Google Scholar]
- Mundry R, Fischer J. Use of statistical programs for nonparametric tests of small samples often leads to incorrect p values: examples from Animal Behaviour. Anim. Behav. 1998;56:256–259. doi: 10.1006/anbe.1998.0756. [DOI] [PubMed] [Google Scholar]
- Range F, Bugnyar T, Kotrschal K. The performance of ravens on simple discriminate tasks: a preliminary study. Acta Ethol. 2008;11:34–41. doi: 10.1007/s10211-008-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner H. Habitat-specific growth and fitness in carrion crows. J. Anim. Ecol. 1989;58:427–440. [Google Scholar]
- Schwab C, Bugnyar T, Schloegl C, Kotrschal K. Enhanced social learning between siblings in common ravens. Anim. Behav. 2008;75:501–508. doi: 10.1016/j.anbehav.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöwe M, Bugnyar T, Loretto MC, Schloegl C, Range F, Kotrschal K. Novel object exploration in ravens: effects of social relationships. Behav. Proc. 2006;73:68–75. doi: 10.1016/j.beproc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Wallach MA, Kogan N, Bem DJ. Diffusion of responsibility and level of risk taking in groups. J. Abnorm. Psychol. 1964;68:263–274. doi: 10.1037/h0042190. [DOI] [PubMed] [Google Scholar]
- Zentall TR. Action imitation in birds. Learn. Behav. 2004;32:15–23. doi: 10.3758/bf03196003. [DOI] [PubMed] [Google Scholar]