Abstract
Non-alcoholic fatty liver disease (NAFLD) is closely associated with metabolic syndrome. Prevalence of metabolic risk factors including diabetes mellitus, obesity, etc. is rapidly increasing in India putting this population at risk for NAFLD. Patients with NAFLD are at increased risk for liver-related morbidity and mortality and also cardiovascular disease risk and increased incidence of diabetes mellitus on long-term follow-up. Management of patients with NAFLD may require a multi-disciplinary approach involving not only the hepatologists but also the internists, cardiologists, and endocrinologists. This position paper which is a combined effort of the Indian National Association for Study of the Liver (INASL), Endocrine Society of India (ESI), Indian College of Cardiology (ICC) and the Indian Society of Gastroenterology (ISG) defines the spectrum of NAFLD and the association of NAFLD with insulin resistance and metabolic syndrome besides suggesting preferred approaches for the diagnosis and management of patients with NAFLD in the Indian context.
Keywords: NAFLD, nonalcoholic steatohepatitis, NASH, insulin resistance, cryptogenic cirrhosis
Abbreviations: ALT, Alanine Aminotransferase; APO C3, Apolipoprotein C3; ARFI, Acoustic Radiation Forced Impulse; AST, Aspartate Aminotransferase; ATPIII, Adult Treatment Panel III; BMI, Body mass index; CAD, Coronary artery disease; CC, Cryptogenic Cirrhosis; CIMT, Carotid Intima Media Thickness; CK 18, Cytokeratin 18; CT, Computed Tomography; DM, Diabetes Mellitus; EBP, Enhancer-Binding Protein; EMA, Anti-Endomysial antibodies; FFA, Free Fatty Acids; FMD, Flow-Mediated Vasodilatation; FPG, Fasting Plasma Glucose; GTT, Glucose Tolerance Test; HCC, Hepatocellular Carcinoma; HOMA-IR, Homeostasis Model Assessment for Insulin Resistance; HTG, Hepatic triglyceride; HTN, Hypertension; IFG, Impaired Fasting Glucose; IGT, Impaired Glucose Tolerance; IKK-β, Inhibitor of nuclear factor kappa-B kinase beta; IR, Insulin resistance; IRS-1, Insulin Receptor Substrate-1; ITT, Insulin Tolerance Test; LFTs, Liver Function Tests; MRE, Magnetic Resonance Elastography; MS, Metabolic syndrome; NAFLD, Non-alcoholic fatty liver disease; NASH, Non-Alcoholic Steatohepatitis; NF-κβ, Nuclear Factor Kappa Β; PCOS, Polycystic Ovarian Syndrome; PPG, Post-Prandial Glucose; PROCAM, Prospective Cardiovascular Munster study; SREBP, Sterol-Regulatory Element-Binding Protein; T2DM, Type 2 Diabetes Mellitus; TE, Transient elastography; TG, Triglycerides; TTG, Anti-tissue transglutaminase; VHCC, Virus-related HCC; VLDL, Very Low Density Lipoprotein; WHO, World Health Organization
Non-alcoholic fatty liver disease (NAFLD) is now considered a hepatic component of metabolic syndrome (MS) because of the close association between the two conditions. Prevalence of metabolic risk factors including diabetes mellitus, obesity, etc. is rapidly increasing which is consequently increasing the prevalence of NAFLD in India. Patients with NAFLD are at risk not only for the liver-related morbidity and mortality but also for the increased cardiovascular disease risk and increased incidence of diabetes mellitus on long-term follow-up. This asks for a call to involve not only the hepatologists but also the internists, cardiologists, and endocrinologists for the management of patients with NAFLD. This position paper which is a combined effort of the Indian National Association for the Study of Liver (INASL), Endocrine Society of India (ESI), Indian College of Cardiology (ICC) and Indian Society of Gastroenterology provides definitions of NAFLD, spectrum of NAFLD, pathogenesis of NAFLD, mainly discussing the association of NAFLD, insulin resistance (IR), and MS. Finally, this paper gives algorithms for the diagnosis and management of patients with NAFLD in the Indian context.
Need for the position paper on non-alcoholic fatty liver disease and metabolic syndrome from India
Increasing Obesity, Hypertension, and Diabetes Mellitus
Over the years, increasing obesity, hypertension, and diabetes mellitus has also led to higher incidence and prevalence of NAFLD in India. In India, the recent ICMR-India Diabetes (ICMR-INDIAB) study reported the prevalence of DM (both known and newly diagnosed) in four regions of the country: 10.4% in Tamil Nadu, 8.4% in Maharashtra, 5.3% in Jharkhand, and 13.6% in Chandigarh (Union Territory).1 The overall number of people with DM in India in 2011 based on this study was estimated to be 62.4 million, and this was confirmed by the Diabetes Atlas (5th edition) of the IDF, which gave a figure of 61.3 million people with DM in India in the age group of 20–79 years.1,2 Studies in India have shown an increasing trend in the prevalence of hypertension among urban adults: In Jaipur (1995), men 30% and women 33%; in Mumbai (1999), men 44% and women 45%; in Thiruvananthapuram (2000), men 31% and women 36%; in Jaipur (2002), men 36% and women 37%; in Chennai (2001), women 14%, were found to have hypertension.3 Among the rural populations, in Rajasthan (1994), HTN prevalence in men was 24% and that in women was 17%.4 The Chennai Urban Rural Epidemiology Study (CURES) reported a prevalence of 23.2% HTN among men and 17.1% among women in South India. Hypertension is responsible for 57% of all stroke mortality and 24% of all coronary artery disease (CAD) mortality in India.4 The prevalence of obesity differs mostly by location, gender, and socio-economicstratum.5 Higher prevalence is seen in urban areas, more so in the higher socio-economic stratum, and in women.5 Even though higher prevalence and rapidly increasing rate of obesity are seen in urban areas, rural-based Asian-Indians are also showing a growing trend of obesity.5 Increasing trend of overweight and obesity has been observed in children as well.
Insulin Resistance and Other Risk Factors in Indians
Studies have shown higher IR and higher hepatic triglycerides (HTGs) in Indians in comparison to other races suggesting that Indians are much prone to developing NAFLD. Insulin resistance is independently correlated with NAFLD regardless of adiposity. The prevalence of IR in Asian-Indians residing in India ranges from ∼7% to 55%.6–9 Since IR and the MS are widely prevalent in Asian-Indians, it is reasonable to presume that NAFLD would also be prevalent; however, data are scarce. Some recent data indicate that hepatic fat content of Asian-Indians is almost twice in amount for similar body mass index (BMI) when compared to white Caucasians.10 Insulin resistance has also been reported in children and adolescents. Such high prevalence of IR and the MS in Asian-Indians is likely to be due to multiple factors. It is important to note that all the associations of NAFLD, excess body fat, abdominal obesity (truncal subcutaneous fat and intra-abdominal fat), diabetes, hypertriglyceridemia, and IR are highly prevalent in urban Asian-Indians and may be important for pathogenesis of NAFLD. However, inter-relationships of NAFLD, IR, and the MS have been sparsely studied in Asian-Indians.11 By using proton magnetic resonance spectroscopy (MRS), Petersen et al measured hepatic triglyceride (HTG) content and plasma interleukin-6 (IL-6) concentrations in different ethnic groups.12 Interestingly, these authors reported that the HTG content and plasma IL-6 concentrations were nearly two-fold higher in Asian-Indians as compared to white Caucasians in USA.12 The increase in HTG content and IL-6 concentrations was associated with higher prevalence of IR in Asian-Indians (59%) as compared to white Caucasians (20%). Petersen et al also looked at the prevalence of various metabolic risk factors amongst different races and found that in spite of having lower BMI, Asian-Indians had the highest levels of fasting serum insulin and IR as measured by the homeostasis model assessment for insulin resistance (HOMA-IR) in comparison to other Eastern-Asians, Caucasians, Blacks, and Hispanics. Asian-Indians also had higher HTG content in comparison to Caucasians.12 This inter-ethnic difference in the prevalence of NAFLD and non-alcoholic steatohepatitis (NASH) is believed to be related not only to different lifestyles but also to a strong genetic predisposition. Of the various genes studied, polymorphisms of apolipoprotein C3 (APOC3) gene may be a phenomenon peculiar to Asian-Indians as demonstrated in a study.13 Since the results of APOC3 gene polymorphisms have not been replicated in other races with higher adiposity, it is possible that in pre-existing IR states such as obesity and MS, the effect of the APOC3 polymorphism may become redundant. Asian-Indians are less obese than their Western counterparts and this could be allowing a higher phenotypic expression of the APOC3 genetic polymorphism but this hypothesis requires further investigation.14 The recent concept of the association of gut microbiome with obesity and NAFLD may suggest a different bacterial flora in Indian patients, thus making them more prone to the development of NAFLD.
Even though majority of patients with NAFLD in India have overweight or obesity according to Asia–Pacific criteria, mean BMI in Indian patients with NAFLD is much lower than that in the West.15 Indian patients with NAFLD are further different from patients in the West because of lower prevalence of diabetes, hypertension, and full-blown MS in non-cirrhotic Indian patients with NAFLD presenting as raised transaminases.15–17 In contrast to West, data from India have also suggested that iron overload and HFE gene mutations do not play a major role in the pathogenesis of NAFLD. Even though NAFLD has emerged as an important cause of cryptogenic cirrhosis and hepatocellular carcinoma (HCC) in India, histological severity in Indians with NAFLD presenting to the Hepatologists with raised transaminases is milder in comparison to data from the West.16,17 Since Indian patients with NAFLD may have different pathogenesis and a different clinicopathological profile in comparison to the West, the available guidelines from the West for patients with NAFLD may not be applicable to patients with NAFLD in India.15,18 Hence, there is an urgent need to have separate guidelines and a combined position paper from different specialties involved in the care of patients with NAFLD.
Non-alcoholic Fatty Liver Disease in India vis-à-vis Asian Data
The data on the prevalence of fatty liver in Asian population are limited. Chitturi et al highlighted the potential burden of NAFLD in the Asian-Pacific area, with estimated 1.8 million Asians with NASH and at least 400,000 Australians with the disease, thus eclipsing the disease burden of hepatitis B and C.19 In China, the prevalence of NAFLD measured by ultrasonography was 14.7%.20,21 In Japan, the prevalence of NAFLD was reported to be 3.3% and 3.8% in non-obese and21.6% and 18.8% in obese males and females, respectively.22 In a hospital-based study in Taiwan, the prevalence of NAFLD was 36.9%, being higher in men than in women.23 Among 13,768 Korean adults, 25% were diagnosed with NAFLD on abdominal ultrasound.24 Overall, studies from India do suggest a high prevalence of NAFLD in general population and in high risk groups. In the general population, prevalence of NAFLD varies from 9% to 35% in India (Table 1). The lower prevalence is from a community-based epidemiological study from the rural part of West Bengal, whereas prevalence of NAFLD is much higher in other studies from India carried out in urban populations.28 In Mumbai, the prevalence is reported to be 16.6%,26 while it is reported as 32% in Chennai.27 In a hospital-based study from Cuttack, the prevalence was similarly high at 24.5%.25 The prevalence of NAFLD is even higher in high-risk groups such as diabetics and the obese. In one of the studies 35 out of 40 (88%) nonalcoholic patients with type 2 diabetes mellitus had evidence of fatty liver on ultrasound.29 In another study from Mumbai, 49 out of 100 patients with type 2 diabetes mellitus had evidence of fatty liver on ultrasound.30 Thirty-two of these patients underwent liver biopsy. Mild, moderate, and severe disease was present in 21/32 (65.5%), 4/32 (12.5%), and 3/32 (9.35%) patients, respectively, and fibrosis was present in 7/32 (21.8%) patients with one having grade 4 and three having grade 3 fibrosis.30 The prevalence data from India are given in Table 1. Extrapolation of Indian data suggests that India may have at least 25 million patients with NAFLD who may be at risk for significant liver disease (considering 5% of 1000 million Indians to be diabetic and prevalence of NAFLD to be in at least 50% of these diabetics).31
Table 1.
Prevalence Data of Non-alcoholic Fatty Liver Disease in the General Population of India.
| Author and year | No./type of patients | Diagnostic method for hepatic steatosis | Comments |
|---|---|---|---|
| Singh et al 200425 | 159 healthy subjects | Liver ultrasonography | Fatty liver in 39 (24.5%, M: 26.9%, F: 13.8%) |
| Amarapurkar et al 200726 | 1168 healthy subjects | Liver ultrasonography | NAFLD is 16.6%; age > 40, central obesity Male, BMI > 25↑ AST/ALT |
| Mohan V et al—Urban South India 200927 | 541 subjects | Liver ultrasonography | 32% (173/541 subjects) (M: 35.1%, F: 29.1%) |
| NAFLD, NAFLD with elevated alanine ami- | |||
| Das et al—Rural West Bengal 201028 | n = 1911 | Liver ultrasonography and blood sampling | notransferase, and cryptogenic cirrhosis was 8.7%, 2.3%, and 0.2%, respectively; M:F = 1.1:1 ↑ income, BMI FBS > 100 central obesity |
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CT: computed tomography; FBS: fasting blood sugar.
Definitions and spectrum
Definition of Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease has been defined as the accumulation of fat in the liver in the absence of recent or ongoing intake of significant amount of alcohol. The significant amount of alcohol has been defined variably but a cut-off of intake up to 20 g/day seems reasonable both for males and females (approximately 30 ml of whisky = 100 ml of wine = 240 ml of beer = 10 g of alcohol). In addition, the definition of primary NAFLD also includes the exclusion of other secondary causes of hepatic steatosis including but not limited to various medications and hepatitis C virus, surgical procedures, total parenteral nutrition, and various inborn errors of metabolism. The fat deposition in the liver is best defined histologically on liver biopsy as the macrovesicular steatosis occupying at least 5% of the hepatocytes. In spite of the limitations (most of the imaging modalities including ultrasound, computed tomography (CT), and magnetic resonance imaging are best at picking up hepatic steatosis when it is occupying more than 30% of hepatocytes) for practical purposes, hepatic steatosis is usually defined on imaging with MRS being the best modality. Since MRS is not readily available, for practical purposes, most commonly used imaging modality to define NAFLD is ultrasound abdomen. The differentiation of simple steatosis from NASH is possible only on histology with a limited role of imaging in differentiating the two.
Spectrum of Non-Alcoholic Fatty Liver Disease
Non-cirrhotic Non-alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease is a broad term consisting of patients with simple steatosis, NASH, NASH-related cirrhosis, and NASH-related HCC. Patients with simple steatosis also called as having non-alcoholic fatty liver have the presence of fat in the liver with or without the presence of lobular inflammation on histology, whereas NASH is defined as steatosis and inflammation associated with the presence of one of the three additional features: ballooning of hepatocytes, Mallory hyaline, and fibrosis on liver histology. Since these three features are difficult to diagnose non-invasively, NASH is usually a histological diagnosis except in situations where hepatic fibrosis is diagnosed with the help of transient elastography (TE) (Fibroscan) and hence can be a useful non-invasive modality in differentiating patients with simple steatosis and NASH.32 The differentiation between NAFLD and NASH is very important in determining the prognosis, risk of progression, and for assessing the liver-related and cardiovascular morbidity and mortality, occurring more commonly in patients with NASH than in those with NAFLD.33 Since liver biopsy cannot be done in all patients, it can be directed at those likely to have severe disease i.e., NASH. Various parameters such as gender, age, aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio, the presence of diabetes mellitus, and other components of MS can help in predicting severe disease i.e., NASH, hence helping in directing the liver biopsies in these patients (Figure 1). Liver biopsy is helpful not only in differentiating NASH and no NASH but also in further categorizing the patients with NASH into different grades and stages based on different classification systems (Appendix 1).33,34
Figure 1.
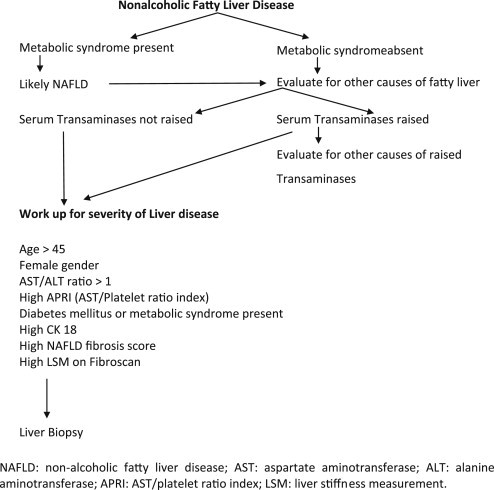
Diagnostic work-up in patients with non-alcoholic fatty liver disease.
Non-alcoholic Steatohepatitis-Related Cirrhosis and Hepatocellular Carcinoma
Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) has also emerged as the most common cause of Cryptogenic cirrhosis (CC) as shown by many studies from the West.35 Like any chronic liver disease, NAFLD/NASH may be asymptomatic in the beginning and may present later as CC or even HCC. It may be difficult in these patients to recognize NAFLD/NASH even on histology because liver fat decreases with increasing fibrosis and the characteristic changes of NAFLD/NASH may not be evident once it goes into the stage of cirrhosis. Data from the West indicate that 50–75% of patients with CC have obesity and diabetes,36 and 33% of patients transplanted for CC show evidence of NAFLD on histology of their explanted livers.
In a study from Mumbai, NASH with/without cirrhosis, and CC occurred more commonly in patients with diabetes than those without it. The incidence of diabetes in CC was 57% versus 30% in non-cryptogenic cirrhosis,37 suggesting the role of diabetes mellitus and NAFLD in causing cirrhosis. In a recent study from Chandigarh, results of surrogate markers of NAFLD in 65 patients with CC were compared with 50 patients with virus-related cirrhosis of comparable age, gender, and severity of liver disease.38 All possible etiologies for cirrhosis including viral, autoimmune, Wilson's disease, and iron overload were excluded. Screening for occult HBV infection [by total antibodies against core antigen (anti-HBc total)] and celiac disease (by anti-tissue transglutaminase antibodies, anti-endomysial antibodies, and duodenal biopsies) was also done in 16 and 10 patients, respectively. Mean BMI was higher in patients with CC (26.06 ± 5.96 kg/m2) in comparison to virus-related cirrhosis (22.12 ± 1.71 kg/m2). A higher number of patients with CC had abnormal waist [38 (58.5%) vs 15 (30%), type 2 diabetes mellitus [26 (40%) vs 5 (10%)], and lower serum high-density lipoprotein (HDL) [35 (53.8%) vs 3 (6%)] in comparison to virus-related cirrhosis, again suggesting NAFLD as the possible etiology in these patients of CC.38 The most convincing data on high prevalence of NAFLD in CC come from a recent study from a large volume liver transplant center in Delhi. Clinicopathological features of NAFLD were explored by the clinical data and by examining the explant livers in living donor liver transplant recipients. Among 103 adult liver transplant recipients with different types of chronic liver disease, 30 had a pre-liver transplant diagnosis of CC. Of the 30 CC cases, 19 (63.3%) were finally labeled as NAFLD-related cirrhosis and showed histological features in several respects different from those reported for the early and established phases of NAFLD.39
Regarding NAFLD as the cause of HCC, in a study from the US, CC accounted for 29% of all HCC, and was the second-most important cause after HCV infection.40 Half of these patients had clinical or histological features of NAFLD.40 Non-alcoholic fatty liver disease accounts for 40% of cases of HCC in UK and is the most common etiology.41 A study from Italy showed that patients with cryptogenic HCC had higher prevalence of obesity and diabetes mellitus and higher triglyceride and cholesterol levels suggesting NAFLD as the underlying etiology.42 Further the occurrence of HCC in non-cirrhotic livers in NASH is a cause of recent concern, and requires more evaluation.42
On the Eastern side, a study from Japan found that 17 out of 320 (5.3%) HCC cases were either associated with NASH or had a cryptogenic etiology.43 Cryptogenic HCC accounted for 5.4% of all HCC in Korea, and this group had higher BMI, triglyceride levels, diabetes mellitus, and hypertension compared to HCC with well-defined etiologies.44 In another study from Korea, 20 out of 36 (56%) patients with CHCC had at least two risk factors for NAFLD.45 At Postgraduate Institute of Medical Education & Research (PGIMER) Chandigarh, India, surrogate markers of NAFLD in 39 patients with CHCC with all possible etiologies for HCC excluded were compared with 39 patients with virus-related HCC (VHCC). Patients with CHCC had a higher BMI (24.35 ± 4 kg/m2 vs 22.5 ± 3.4 kg/m2) and higher prevalence of type 2 diabetes mellitus [15 (38.5%) vs 7 (17.9%)] in comparison to VHCC.38 Higher prevalence of metabolic risk factors if taken as surrogate markers of NAFLD suggests that NAFLD is an important cause of both CC and CHCC in India as well.
Non-hepatic Outcomes in Non-alcoholic Fatty Liver Disease
Two non-hepatic outcomes included in the spectrum of NAFLD include the higher risk of cardiovascular morbidity and mortality in these patients (especially in those with NASH) and higher risk of developing diabetes mellitus in patients with NAFLD. Recently, a lot of data have emerged to suggest the increased atherosclerosis and cardiovascular risk in patients with NAFLD, but it is still a matter of debate whether NAFLD per se predisposes to these abnormalities or the increased atherosclerosis and cardiovascular risk in patients with NAFLD is dependent on the presence of metabolic abnormalities or MS. The presence of subclinical atherosclerosis can be assessed non-invasively by an increase in carotid intima media thickness (CIMT) (structural atherosclerosis) or by endothelial dysfunction studied by flow-mediated vasodilatation (FMD). Increased risk of cardiovascular disease in a population can be estimated by various scoring systems such as the Prospective Cardiovascular Munster study (PROCAM), Adult Treatment Panel III (ATPIII), or Framingham score. In a study, even though Targher et al found that patients with NAFLD had greater CIMT (1.14 ± 0.20 vs 0.82 ± 0.12 mm; P < 0.001) than controls, MS and its individual components were more frequent in those with NAFLD.46 The marked difference in CIMT between the groups also slightly weakened after adjustment for MS components. In a cross-sectional study, McKimmie et al evaluated the association between hepatic steatosis and coronary aortic and carotid artery calcium and CIMT in 623 participants from diabetes heart study. They found a significant association between steatosis and aortic calcium and CIMT which completely disappeared after adjusting for other cardiovascular disease risk factors including visceral obesity.47 On the other hand, Volzke and colleagues found that individuals with fatty liver had higher CIMT and more often had carotid plaques than persons without fatty liver (plaque prevalence rate 76.8% vs 66.6%; P < 0.001). This association persisted even after adjustment for confounding factors.48 Similarly, in the study done by Brea et al, CIMT was found to be higher in NAFLD patients than in age- and sex-matched controls (P < 0.01). Further by logistic regression and adjustment for various confounders, the presence of NAFLD was associated with a higher CIMT independently of MS and all its traits.49 A study from a tertiary care center in India has shown that the increased atherosclerosis and cardiovascular risk in patients with NAFLD is dependent on MS.50
Development of diabetes on long-term follow-up in patients with NAFLD has been shown from both Western and Asian countries. In a study from USA, 22% of non diabetic NAFLD developed diabetes over a 7.6-year follow-up.51 A study from Japan showed that non-diabetic NAFLD patients had a hazard ratio of 4.8 for developing DM even after adjusting for age and BMI compared to controls (8.1% NAFLD patients developed diabetes over 4-year follow-up compared to 1.8% in controls, P < 0.001).52 These non-hepatic outcomes of NAFLD make NAFLD important not only for the hepatologists but also for the cardiologists and endocrinologists.
Pathogenesis of non-alcoholic fatty liver disease
The basic defect in the development of hepatic steatosis is the imbalance between import and export of fat to and from the liver. Sources of excess import include excess of dietary intake of fat if compensatory decrease in lipolysis and de-novo synthesis does not take place. Similarly, an excess de-novo fat synthesis in the liver or lipolysis in the peripheral tissues with increased delivery of free fatty acids (FFA) to liver causes steatosis. Reduced β-oxidation of fatty acids and decreased export as very low density lipoprotein (VLDL) also contribute to hepatic steatosis. Insulin resistance with or without full-blown MS is the central mechanism of hepatic steatosis in patients with NAFLD, which in turn develops in the setting of an inappropriate diet, sedentary lifestyle, obesity, and advancing age. Role of genetic variations in predisposing to the development of steatosis by affecting the various steps in the normal metabolism of fat and carbohydrates is also under investigation.
Metabolic Syndrome
Metabolic syndrome is a clinical syndrome characterized by the constellation of various components namely, obesity, type 2 diabetes, dyslipidemia, and hypertension. Isolated obesity and diabetes are rare in the general population. In most cases, they are associated, and hypertension and dyslipidemia may also be present. In 1988, Reaven53 proposed the term ‘syndrome X’ to define the contemporary presence of altered glucose regulation, hypertriglyceridemia, low HDL-cholesterol and hypertension, and a syndrome carrying a high risk of cardiovascular mortality. The MS is probably much wider and most subjects have evidence of additional metabolic disorders (elevated uric acid, impaired fibrinolysis, and endothelial dysfunction). Obesity, namely central obesity, type 2 diabetes, hyperlipidemia, and hypertension are all characterized by elevated insulin concentrations, which predict the development of the metabolic disorder. Accordingly, DeFronzo and Ferrannini54 proposed the term ‘insulin-resistance syndrome’ to define this clustering of diseases. The borders of the syndrome remain difficult to define. The presenting features vary, and new metabolic disorders get added in random order. In general, obesity comes first, followed by hyperlipidemia and diabetes, whereas hypertension is not predictable. The first attempt to define MS came from World Health Organization (WHO) (Appendix 2), experts setting new criteria for the definition of diabetes.55 Their criteria, partly based on the assessment of insulin sensitivity, are scarcely applicable to the general population. New criteria were defined by the European Group for Insulin Resistance in 1999, limiting the syndrome to non-diabetic subjects, but the critical problem of IR was not addressed.56 Only in 2001, the third Report of the National Cholesterol Education Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III (ATPIII)] provided a working definition of MS,57 based on a combination of five categorical and discrete variables, easily measurable in clinical practice. According to ATP III criteria, MS is defined by the presence of at least three out of five components namely, central obesity, diabetes mellitus, hypertension, low HDL, and high triglycerides (Appendix 2).57 The limits for individual components are usually derived from the guidelines of the International Societies or the statements of World Health Organization. A fasting plasma glucose (FPG) of >126 mg/dl on more than one occasion, a random plasma glucose of >200 mg/dl in a symptomatic patient, or a 2 h plasma glucose [post-prandial glucose (PPG)] of >200 mg/dl on glucose tolerance test (GTT) is defined as diabetes mellitus. Fasting plasma glucose of >110 mg/dl and <126 mg/dl is defined as impaired fasting glucose (IFG) and 2 h plasma glucose after ingestion of 75 g oral glucose between 140 mg/dl and 200 mg/dl as impaired glucose tolerance (IGT). In the lipid profile, HDL <40 mg/dl in males and <50 mg/dl in females and serum triglycerides (TG) >150 mg/dl are taken as abnormal.58
Asia–Pacific/Indian Modifications for Metabolic Syndrome
Even at lower BMI, Asians have been found to have a high percentage of body fat compared to white Caucasians and Blacks. At a given percentage of body fat, BMI values of Asians including Asian-Indians were 3 kg/m2 lower than those in white Caucasians. This is partly explained by the body build (trunk to leg-length ratio), low muscularity, adaptation to chronic calorie deprivation, and ethnicity. More importantly, the morbidity and mortality associated with higher body fat occur more frequently at lower BMI in Asians than in white Caucasians. A study from Delhi showed that about 66% of men and 88% of women classified as non-obese based on the international cut-off of BMI had one or more cardiovascular risk factors.59 Based on these data, it has been suggested that the BMI limits for overweight and obesity should be lower for Asians-Indians. Recommendations for BMI are as follows: Normal 18–22.9 kg/m2, overweight 23–24.9 kg/m2, and obesity ≥25 kg/m2. A high prevalence of abdominal obesity is seen in Asians, including Asian-Indians even when the BMI is <25 kg/m2. Similarly, Asians have been found to have more intra-abdominal adipose tissue than Caucasians, in spite of having smaller waists. Lower cut-offs (waist circumference ≥90 cm in males and ≥80 cm in females) are also recommended for identifying abdominal obesity in Asians. Hence, while defining MS in Asian-Indians, it is recommended to use the Asia–Pacific/Indian cut-offs for abdominal obesity.60
Association Between Non-alcoholic Fatty Liver Disease and Metabolic Syndrome
The MS and NAFLD share similar associations such as diabetes, hypertriglyceridemia, and obesity. Since metabolic risk factors are so common in patients with NAFLD, there is evidence now to show that NAFLD may actually be a hepatic manifestation of MS. Nearly 90.0% and 33.0% NAFLD subjects have at least one feature and all features of the MS, respectively, with the presence of MS, increasing the risk of NAFLD increases to 4–11 times,61 making NAFLD less likely to remit as well. Majority of the patients with NAFLD are obese and resultantly get nearly five-fold higher risk of developing steatosis.62 The risk of NAFLD is also high for those with impaired fasting glucose, albeit lower than that with type 2 diabetes mellitus (T2DM).61–63 Typically, NAFLD has been observed to be consistently associated with type 2 diabetes mellitus (28–55%) and dyslipidemia (27–92%). Non-alcoholic fatty liver disease, in the presence of normoglycemia and normal or moderately higher body weight, is characterized by clinical and laboratory data (e.g., impaired insulin sensitivity, abnormalities in lipid metabolism) that do not differ from those with diabetes and obesity. Two other metabolic factors, hypertriglyceridemia and low HDL-cholesterol level, are present in 62% and 54%of NAFLD patients, respectively.64 The prevalence of fatty liver increases by at least two-fold in those who are non-obese, non-diabetic but have a primary hypertension.65
Insulin Resistance
Normally, insulin acts on skeletal muscle, adipocytes, and the liver for maintaining glucose and lipid homeostasis. Insulin maintains this homeostasis by various actions ranging from increased uptake of FFA, conversion to triglycerides and storage along with decreased lipolysis in adipose tissue, uptake of glucose by skeletal muscles, storage of glucose as glycogen in liver with inhibition of glycogenolysis and gluconeogenesis to increased de-novo lipogenesis and reduced oxidation of fatty acids in the liver. The net result of all these actions is to utilize glucose, reduce lipolysis of FFA, and promote storage of fats as triglycerides in the adipose tissue. Insulin resistance is characterized by inappropriately high levels of plasma insulin for the corresponding blood sugar level, due to inability of cells to respond to insulin leading on to high plasma glucose levels, increased lipolysis, and increased delivery of fatty acids to liver; it is characterized by high levels of plasma insulin initially, as a pancreatic compensatory response, to be eventually followed by dwindling levels of insulin, with development of type 2 diabetes.
Non-alcoholic fatty liver disease patients with or without MS have been shown to have higher IR compared to controls. Though glucose clamp studies are the ideal method of studying IR, most studies in patients with NAFLD have used HOMA-IR (Appendix 3), even though simpler methods such as insulin tolerance test (ITT) have been shown to be comparable to HOMA-IR in the Indian context.66 Studies have demonstrated that NAFLD is associated with higher IR compared to controls, even after excluding overweight and obese subjects and that IR increases with increasing degree of steatosis. A comparison between patients of different ethnicities has shown that Indians have higher IR in comparison to Caucasians and other races.11
Data from India support the higher prevalence of IR in patients with NAFLD present in 83–98% of patients.16,17,67,68 A study from Kolkata has also shown higher HOMA-IR levels in non-obese patients with NAFLD (though they used the International criteria of obesity) in comparison to non-obese controls without NAFLD.28
Insulin resistance in NAFLD is predominantly peripheral occurring at the skeletal muscle and adipose tissue. Peripheral IR in the skeletal muscle causes reduced glucose uptake leading to hyperglycemia. In the adipose tissue, IR impairs the anti-lipolytic action of insulin leading to increased release of FFA. Elevated plasma concentrations of insulin, glucose, and fatty acids then promote hepatic fatty acid and triglyceride uptake, de-novo lipid synthesis (via the SREBP) (sterol-regulatory element-binding protein) and C/EBP (CCAAT/enhancer-binding protein) and impair β-oxidation of fatty acids by negative feedback. Insulin resistance also increases intra-hepatocytic fatty acids by increasing glycolysis and decreasing apolipoprotein B-100 thereby blocking the export of VLDL. The development of IR in NAFLD is probably related to the imbalance between pro-insulin (adiponectin) and anti-insulin (TNF-α) cytokines particularly those secreted from adipose tissue (adipokines). Alterations in several molecules, including FFA, TNF-α, membrane glycoprotein PC-1, and leptin interfere with the insulin signaling pathway. Free fatty acids are both the result and cause of IR. Excess FFA cause hepatic IR by down regulating insulin receptor substrate-1 (IRS-1) signaling and by activation of the inhibitor kappa-β kinase (IKK-β)/nuclear factor kappa β (NF-κβ) pathway.
Other Pathogenic Mechanisms in Non-alcoholic Fatty Liver Disease
Other than the IR and MS, the mechanisms involved in the pathogenesis of NAFLD/NASH include the role of lipotoxicity, oxidative stress and cytokines, serum and liver iron overload, innate and adaptive immunity, small intestinal bacterial overgrowth, and finally the polymorphisms of the genes involved in lipid accumulation, oxidative stress, and hepatic fibrosis.
Diagnosis of non-alcoholic fatty liver disease
Diagnosis of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis
Diagnosis of NAFLD requires a detailed history to exclude the intake of significant amount of alcohol and other secondary causes of fatty liver as mentioned in the section on “Definitions and Spectrum” (Figure 1). Most patients with non-cirrhotic NAFLD are asymptomatic in the beginning with incidental detection of raised liver enzymes or fatty liver on ultrasound. Some patients are detected to have fatty liver on ultrasound and raised enzymes during work-up for dyspeptic symptoms, malaise or fatigability, or work-up for other illness. Anthropometry may reveal overweight, obesity, or central obesity; mild hepatomegaly may be an important sign in around half of these patients but signs of liver failure are absent unless the patient has progressed to cirrhosis or HCC. Serum biochemistry shows either normal or mildly elevated AST and ALT with ALT more than AST.
Diagnostic modalities are thus directed first to confirm the presence of fatty liver and secondly to grade the severity of liver disease (Figure 1). The diagnosis of fatty liver is usually made on ultrasound with exclusion of other causes of fatty liver and raised liver enzymes if present. Ultrasound is an accurate, reliable imaging technique for the detection of fatty liver, as compared with histology, with a sensitivity of 84.8% and a specificity of 93.6% for detecting ≥20–30% steatosis.69 Computed tomography scan and MRI really do not add much and are as good as ultrasound for detecting fat in the liver.69 Magnetic resonance spectroscopy is better in detecting fat but none of these modalities can detect the degree of inflammation and fibrosis, hence are not good in differentiating between only steatosis and histological NASH. Since ultrasound is easily available, not requiring much of expertise, inexpensive without any radiation risk, it should be the first modality to assess the presence and grading of hepatic steatosis and for the severity of liver disease. On abdominal ultrasound, the physician should look for the liver echogenicity and its comparison with that of kidney and spleen, Vascular blurring, and deep attenuation of ultrasound signal.
Fibroscan (transient elastography) is a new non-invasive modality in detecting liver fibrosis and its role is still being evolved in various liver diseases including NAFLD. Many patients with fatty liver as evident on conventional imaging may turn out to have significant fibrosis on Fibroscan32,70 and may be subjected to a liver biopsy (Figure 1).
All patients of NAFLD irrespective of the liver enzyme elevation should undergo a detailed physical examination and anthropometry including, height, weight, BMI, waist circumference, and waist–hip ratio for the assessment of overweight and central and overall obesity (Figure 1). These patients should be further evaluated with liver function tests (LFTs) and for the presence of other components of MS namely hypertension, IGT, serum triglycerides, and HDL. In addition, all patients should also be screened for hepatitis B surface antigen (HBsAg) and antibodies to hepatitis C virus (anti-HCV). Further work-up including autoimmune markers, celiac disease work-up, serum iron profile, and serum ceruloplasmin should be done only in patients with raised liver enzymes depending on the age of the patient71 (Figure 1). Even though NAFLD is very common in patients with DM, the presence of diabetes in patients of NAFLD presenting with raised transaminases is not very common when evaluated by FPG and post-prandial plasma glucose. If facilities are available, patients can be tested for IR by the simple method of measuring the HOMA-IR, otherwise can be subjected to only 2-h glucose tolerance test (2-h GTT).
Severity of NAFLD can be assessed either non-invasively by using various biomarkers used either singly or in combination, or with the help of imaging and liver biopsy. Since serum biomarkers are not available routinely, are costly, and lack standardization, the severity assessment is usually based on imaging and liver biopsy. As mentioned earlier, ultrasound, CT scan, and MRI including MRS though are good for hepatic steatosis, cannot pick up ballooning or Mallory hyaline and are poor in detecting hepatic fibrosis unless there is frank cirrhosis. Only non-invasive imaging modality which can help picking up hepatic fibrosis is tissue elastography done with various techniques [Fibroscan, acoustic radiation forced impulse (ARFI), and magnetic resonance elastography (MRE)]. Since all forms of elastography are poorly available and are expensive, the only useful modality in assessing the severity of liver disease in patients with NAFLD is liver biopsy. Since liver biopsy is an invasive procedure and is not free of complications, it should be directed at patients who are likely to be benefitted the most from this procedure.71 The risk factors for severe histological disease include gender, age, AST/ALT ratio, the presence of diabetes mellitus, and other components of MS.71 Cytokeratin 18 (CK 18), NAFLD fibrosis score and the presence of MS are helpful in predicting NASH and fibrosis in patients with NAFLD.71 Since the treatment of patients with NAFLD is still evolving, there is need to study the efficacy of various drugs in a randomized controlled manner (Figure 1). All patients being evaluated in such clinical trials should also be subjected to paired liver biopsies before and after the drug has been given. Even though a definite diagnosis of NAFLD/NASH can be made only on histology, convincing these patients for a liver biopsy is difficult due to the slowly progressive nature of the disease and lack of specific treatment. At a tertiary care center, liver biopsy could be done in 43 out of 127 patients of NAFLD who presented with persistent raised ALT. But on comparison, clinical characteristics of 43 biopsy-proven patients were similar to 84 non-biopsy-proven patients.17 A recent study from Delhi described typical histological features of NAFLD as the presence of macrovesicular steatosis, lobular neutrophilic inflammation with additional presence of Mallory bodies, ballooning degeneration, lipogranuloma, and pericellular fibrosis.72 Such liver damage predominates in perivenular regions i.e., zone 3 of hepatic acinus. In a separate study, female gender, BMI, waist–hip ratio, hypercholesterolemia, and LDL levels were found as independent predictors of disease severity in patients with NASH and to influence the decision to biopsy.73 A recent study from coastal India found that the insulin resistance and dyslipidemia rather than the glycemic status were the determinant factors that had positive correlation with higher histopathological grades of NAFLD.74 Overall liver histology is mild at presentation in Indian patients presenting with raised transaminases with mild-to-moderate degree of inflammation and mild-to-moderate stage of fibrosis. Histological NASH is present only in half of them and cirrhosis at presentation is uncommon.16,17,67
Diagnosis of Non-Alcoholic Steatohepatitis Related Cirrhosis and Hepatocellular Carcinoma
As mentioned in the section of spectrum of NAFLD, NAFLD/NASH is an important cause of CC and HCC. Since liver histology is not very helpful in making the diagnosis of NASH-related cirrhosis or HCC (liver fat decreases with increasing fibrosis), the diagnosis of NAFLD/NASH as a cause of CC and HCC is usually based on the presence of metabolic risk factors. Once all other possible etiologies are excluded, the diagnosis of NAFLD/NASH-related cirrhosis and HCC can be made if there is the presence of two or more components of MS. Many patients tend to lose weight with the development of cirrhosis and HCC; hence, a history of overweight or obesity may be sufficient to include this risk factor as a component of MS. Similarly, it is advisable to calculate BMI or waist circumference when these patients are free of ascites or pedal edema or make appropriate reductions in body weight and waist when calculating the overweight/obesity and central obesity in these patients. Many patients with cirrhosis of liver tend to develop cirrhosis-related DM which needs to be differentiated from the long-standing type 2 DM when including this risk factor as a component of MS.
Cardiology and Endocrine Evaluation
Even though patients with NAFLD, especially those with severe liver disease (NASH), are prone to increased atherosclerosis and its consequences including the cardiovascular morbidity and mortality, routine cardiovascular evaluation in all patients with NAFLD cannot be recommended. The cardiovascular evaluation however can be suggested selectively in those detected to have NAFLD at an old age, having metabolic risk factors. Detailed cardiovascular evaluation is recommended in patients with NASH-related cirrhosis and NASH-related HCC before they are subjected to liver transplantation. Other than the screening for DM (preferably by doing the 2 h GTT), IR and dyslipidemia, screening for other endocrine disorders associated with NAFLD (hypothyroidism, hypothalamic and pituitary dysfunction) is not recommended routinely in patients with NAFLD. Women with NAFLD and MS can however be screened for polycystic ovarian syndrome (PCOS).
Treatment of non-cirrhotic Non-Alcoholic Fatty Liver Disease
Therapeutic modalities in patients with NAFLD have been applied according to the risk factors of NAFLD. Different treatment modalities for NAFLD include lifestyle modifications such as weight loss and exercise, treatment of risk factors such as control of DM and control of hyperlipidemia, insulin-sensitizing agents such as biguanides, thiazolidinediones, antioxidants, and cytoprotective agents (Figure 2, Tables 2 and 3).
Figure 2.
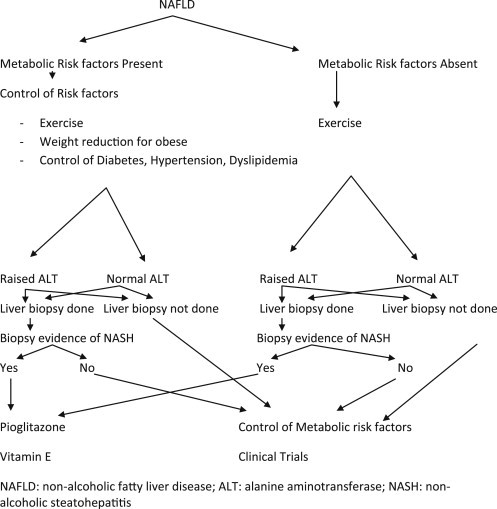
Management algorithm in patients with non-alcoholic fatty liver disease.
Table 2.
Clinical Trials of Lifestyle Modifications in Patients with Non-alcoholic Fatty Liver Disease.
| Author | Study design | N | Outcome |
|---|---|---|---|
| Palmer and Schaffner, 199075 | 1-arm intervention with low calorie diet (25–30 kcal/kg) and low-impact aerobics for 1 year | 39 | Improved liver enzymes in those who lost weight vs those who did not |
| Ueno et al 199776 | Nonrandomized controlled trial of diet and exercise vs no treatment | 25 | Improved steatosis in treated group |
| Samaha et al 200377 | Obese patients placed on low carbohydrate vs low fat restricted diet | 132 | Low carbohydrate group—greatest weight loss, improved insulin resistance in nondiabetics, reduced triglycerides |
| Huang et al 200578 | 1 year dietary counseling in biopsy-proven NASH | 23 | NASH improved in 9 of 15 patients |
| Dansinger et al 200579 | Randomized trial of overweight/obese placed on diets: Atkins (carbohydrate restriction); zone (macronutrient balance); weight watchers (calorie restriction); Ornish (fat restriction) | 160 | Reduced body weight (3–4.8 kg) |
| Bhat et al 201268 | 6 month intervention to study the effect of regular aerobic exercise on insulin resistance, serum aminotransferase and liver histology in NAFLD patients | 60 | Lifestyle modification improves IR resulting in improvement in ALT and liver histology in NAFLD patients |
| Sreenivasa Baba 200665 | To study the effect of regular aerobic exercise on serum aminotransferase levels in patients with NASH | 65/94 patients of NASH | Moderate intensity aerobic exercise helps in normalizing ALT levels in patients with NASH |
| Duseja et al16 | Lifestyle modifications + UDCA for 6 months | 100 | 74% patients achieved complete biochemical response and 10% partial biochemical response |
NASH: non-alcoholic steatohepatitis; NAFLD: non-alcoholic fatty liver disease; ALT: alanine aminotransferase.
Table 3.
Clinical Trials of Various Drugs in Non-alcoholic Fatty Liver Disease.
| Drugs | Author and year | Type of study | Duration (months) | S. amino-transferase | Histology |
|---|---|---|---|---|---|
| Metformin | Marchesini et al 200180 | Pilot open label | 4 | + | ND |
| Uygun et al 200481 | Open label | 6 | + | NS | |
| Nair et al 200482 Bugianesi et al 200583 | Open label Open label | 12 12 | + + | + + | |
| Haukeland et al 200984 | Double-blind, placebo-controlled | 6 | NS | NS | |
| Duseja et al 200785 | Prospective study | 6 | + | ND | |
| Pioglitazone | Promrat et al 200486 | Pilot open label | 12 | + | + |
| Belfort et al 200687 | Placebo controlled trial | 6 | + | + | |
| Sanyal et al 200488 | RCT | 24 | + | ND | |
| Sharma et al 200989 | RCT | 6 | + | + | |
| Vitamin E | Hasegawa et al 200189 | Open label | 12 | + | + |
| Sanyal et al 201090 | Open label | 24 | + | + | |
| Madan et al 2005108 | Retrospective study (vitamin E + UDCA + lifestyle) | 6 | + | ND | |
| UDCA | Lindor et al 200492 | Double-blind placebo control trial | 24 | NS | NS |
| Laurin et al 199693 | Pilot open label | 12 | + | + | |
| Singh et al 200794 | Prospective study (high dose) | 6 | + | + | |
| Ratziu et al 201195 | RCT (high dose) | 12 | + | ND | |
| Duseja et al 200716 | Prospective study | 6 | + | ND | |
| Betaine | Abdelmalek et al 200196 | Pilot study | 12 | + | + |
| Losartan | Yokohama et al 200497 | Open label | 12 | + | + |
| Pentoxifylline | Adams et al 200498 | Pilot open label study | 12 | + | + |
| Buranawui 200799 | RCT | 6 | + | ND | |
| Satapathy et al 2007100 | Pilot open label study | 6 | + | + | |
| Lee et al 2008101 | RCT | 3 | + | ND | |
| S-adenosyl-methionine | Baranovsky et al 2010102 | RCT | 4 | + | ND |
| Atorvastatin | Gomez et al 2006103 | Open label | 12 | + | ND |
UDCA: ursodeoxycholic acid; RCT: randomized controlled trial; (+): improvement; (−): no improvement; NS: not significant; ND: not done.
Lifestyle Modifications/Weight Reduction
All patients with NAFLD irrespective of their body weight should be advised lifestyle modifications in the form of regular exercise and those with overweight and obesity are advised weight reduction (Figure 2, Table 2). Regular exercise has been shown to improve the insulin sensitivity even without weight reduction. Further, regular exercise programme can be helpful for improving the cardiovascular profile as well. The exercise regimen should consist of brisk walking, jogging, or rhythmic aerobic exercises for a minimum of 45 min, 5 days per week, to achieve a target heart rate of 60–70% of the maximal heart rate. Initial weight reduction in patients with overweight and obesity should be 10% of the body weight to be reduced in 6–8 months. Various dietary regimens are available but severe hypo caloric diets are not recommended in NAFLD. Overall, patients with overweight and obesity need to create a negative balance by consuming fewer calories and burn more calories by regular exercises. In a study by Duseja et al, efficacy of lifestyle modifications and ursodeoxycholic acid (UDCA) in improving the liver enzymes was studied in 100 patients with NAFLD.16 After 6 months of lifestyle modification and UDCA, 74 (74%) patients achieved a biochemical response (64 patients—complete biochemical response, 10 patients—partial biochemical response). In two separate studies published from Lucknow, lifestyle modification given for six months improved IR, serum ALT and liver histology in patients with NAFLD.68,104
Though there is lack of data from India, data from the West show that bariatric surgery is an effective modality for weight reduction and for improving liver histology in obese patients with NAFLD. As highlighted earlier, patients with NAFLD in India presenting to the hepatologists do not have very high BMI so as to qualify for bariatric surgery. More patients may be benefitted with this modality, if BMI cut-offs are reduced in Indian patients with NAFLD.
Pharmacotherapy Therapy of Non-alcoholic Fatty Liver Disease
The pharmacological treatment of patients with NAFLD is still evolving. No single therapy for NAFLD has clearly been proven effective, especially, in favorably modifying the course of the disease. Of the various drugs used in patients with NAFLD (Figure 2, Table 3), pioglitazone and vitamin E have been found to be the most useful and are recommended for nondiabetic patients with histological NASH. Indian data are available for the use of UDCA, metformin, pioglitazone, vitamin E, and pentoxifylline in patients with NAFLD.
Ursodeoxycholic Acid
Ursodeoxycholic acid is the non-hepatotoxic epimer of chenodeoxycholic acid. Ursodeoxycholic acid replaces endogenous bile acids, which are potential hepatotoxins, has membrane stabilizing and cytoprotective effects on mitochondria as well as immunological effects. It is believed that by decreasing bile acids, UDCA protects against hepatocyte injury and decreases oxidative stress in patients with NAFLD. Ursodeoxycholic acid has been used in the treatment of some hepatobiliary diseases for nearly two decades. Thus, unlike other medications evaluated for patients with NAFLD, there are abundant data on the safety of long-term use of UDCA in patients with liver disease. Ina RCT conducted by Lindor et al, it was observed that 2 years of therapy with UDCA at a dose of 13–15 mg/kg/day, although safe and well tolerated, is not better than placebo for patients with NASH.92
Open-label pilot studies have evaluated the therapeutic benefits of UDCA in adults with NASH. In one of these studies, 24 patients received UDCA in a regimen of 13–15 mg/kg/day for 12 months.93 This therapy led to a significant improvement in hepatic aminotransferases levels and the degree of hepatic steatosis compared to baseline. In a study from north India, lifestyle modifications and UDCA (300 mg twice a day) given for 6 months achieved a biochemical response in 74 out of 100 patients.16 High-dose UDCA (23–28 mg/kg) or placebo failed to improve the overall histology in 185 patients with NASH in comparison with placebo except the lobular inflammation.105 Ratziu et al also used slightly higher doses of UDCA (28–35 mg/kg) in a RCT of 126 patients for 1 year.95 This treatment led to a decrease in ALT and improved fibrotest results. There was no repeat liver biopsy at the end of this study. In a pilot study by Singh et al, high dose UDCA (600 mg b.d.) for a mean duration of 6 months was found to improve both biochemical parameters and liver histology in diagnosed cases of NASH.94
Metformin
In a randomized controlled study, metformin, added to a lipid and calorie-restricted diet, significantly reduced IR and mean hepatic aminotransferase levels, but no definite improvement was reported on liver histology.81 These data were not confirmed in an observational, open-label study of 1 year of metformin treatment. Hepatic aminotransferase levels improved during the initial 3 months, along with improved insulin sensitivity, but then returned to pretreatment levels and histology did not change.82
By contrast, in a randomized study controlled against either vitamin E or a prescriptive diet, metformin significantly increased the rate of hepatic aminotransferase normalization after correction for age, gender, basal aminotransferases, and change in BMI (OR 5.98; 95% CI 2.05–17.45) and improved IR.83 In a sub-sample of metformin-treated patients, a second liver biopsy showed an improvement in steatosis, as well as in fibrosis and necro-inflammation, but no repeated histology was available for the control arm.83
In an Indian study by Duseja et al, 25 patients with NAFLD who were non-responders to lifestyle modifications were prescribed metformin for 6 months; all of them achieving a partial biochemical response, with a reduction in ALT levels with complete normalization of ALT in 14 (56%) of patients.85 Some recent open-label randomized studies have shown no benefits of metformin on liver steatosis, assessed histologically or by CT, aminotransferase levels, or markers of insulin resistance, and inflammation in comparison to placebo or lifestyle intervention.84 Hence, presently, the role of metformin in treatment of patients with NAFLD remains uncertain.71
Thiazolidinediones
In one of the earlier, 1-year of pioglitazone improved insulin sensitivity and hepatic aminotransferase levels, reduced liver size and hepatic fat content, and improved histology.86 In another controlled study, all patients treated with either pioglitazone or vitamin E had normalized hepatic aminotransferase levels. However, the combination therapy had more impact on histology as well as metabolic disturbances (hyperinsulinemia, IR, high FFA levels), and metabolic changes predicted the improvement in hepatic steatosis.88 While treating NAFLD with Pioglitazone, weight-gain, edema, and worsening of pre-existing congestive heart failure should be kept in mind as potential adverse effects. In the Placebo Vs Pioglitazone Vs Vitamin E study for the treatment of non-diabetic patients with nonalcoholic steatohepatitis (PIVENS) study, though there was an improvement in NASH (47% vs 21%, P = 0.05) on taking pioglitazone at 30 mg/day for 12 months, primary end points of necro-inflammation and fibrosis were not affected however, significant benefits of pioglitazone were observed for some of the secondary outcomes.91 In a RCT Sharma et al from India compared efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis and found that pioglitazone group had better improvement in both metabolic factors and liver histology in patients with NASH compared to pentoxifylline.89 Based on the available data, though pioglitazone is best recommended in non-diabetic patients with biopsy-proven NASH, its long-term safety profile is yet to be determined.71
Antioxidants
Of all the anti-oxidant drugs, vitamin E (α-tocopherol) is the best studied and is recommended in non-diabetic patients with biopsy-proven NASH, however long-term safety profile of vitamin E is yet to be fully established.
A study reported the results of treatment with α-tocopherol in children with NAFLD. Vitamin E (400–1200 IU/day orally) was given for 4–10 months and led to a significant improvement in hepatic aminotransferases.106 In another study, α-tocopherol (300 mg/day) was given for 1 year to patients with liver biopsy-proven NASH and those with a clinical diagnosis of NAFLD.107 Hepatic aminotransferases improved significantly compared to baseline, whereas the degree of steatosis, inflammation, and fibrosis improved or remained unchanged in the patients with NASH in whom post-treatment liver biopsy was performed. Vitamin E therapy (800 IU daily) for 96 weeks, as compared with placebo, was associated with a significantly higher rate of improvement in histological features in NASH (43% vs 19%, P = 0.001). In a randomized study comparing Vitamin E with Metformin in children with NAFLD (The Treatment of Nonalcoholic Fatty Liver Disease in Children [TONIC] trial), it was observed that neither vitamin E nor metformin was superior to placebo in attaining the primary outcome of sustained reduction in ALT level in patients with pediatric NAFLD.107 However, in the PIVENS trial, Vitamin E was found to be superior to placebo for the treatment of nonalcoholic steatohepatitis in adults without diabetes. In 2005, Madan et al from New Delhi compared lifestyle interventions, lifestyle interventions + UDCA and lifestyle interventions + UDCA + vitamin E for management of NAFLD. They reported that all patients in lifestyle interventions + UDCA + vitamin E normalized their ALT levels and it was significantly higher than that seen in other two groups.108
Pentoxifylline
Pentoxifylline is a methylxanthine compound inhibiting the production of cytokines (mainly tumor necrosis factor-α) involved in the pathogenesis of NASH. In a pilot study, Adams et al tested its effectiveness in NASH patients in 2004.98 Twenty patients of NASH were administered pentoxifylline for 12 months. It was observed that aminotransferase levels among patients with NASH improved but it also cautioned for more trials to overcome the side-effect profile.98 In a study conducted in India by Satapathy et al, nine patients (mean age 31.6 ± 7.2 years) of histologically proven NASH were administered, pentoxifylline 400 mg t.d. for 12 months, and it was found that aminotransferases improved significantly, and steatosis/lobular inflammation reduction was observed in 5 patients, Brunt's down staging in 6 patients, and reduction in fibrosis stage in 4 patients.100
Statin Use in Patients with Non-alcoholic Fatty Liver Disease
Statins if required for the treatment of dyslipidemia can be used safely in patients with NAFLD even in the presence of raised transaminases.71 Patients with dyslipidemia and type 2 diabetes benefit with statin therapy of a cardio-protective effect greater than those without diabetes. The statin therapy did not increase the number of negative hepatic events and it has been observed that the liver enzyme levels were normalized under statin treatment for patients with NAFLD.103 But more prospective randomized studies are still needed to support it, and also taking into account the differences between the members of statin class because different statins might have different effects in NAFLD.
Alcohol Use in Patients with Non-alcoholic Fatty Liver Disease
Heavy alcohol consumption has many harmful effects including those on liver and should be discouraged regardless of whether an individual has NAFLD or not. However, emerging epidemiological data suggest that light-to-moderate wine drinking may have favorable effects from a liver standpoint.109 But most studies are cross-sectional in nature and have utilized surrogates such as aminotransferases and liver imaging. Furthermore, it is not clear if cardiovascular and metabolic benefits of light-to-moderate alcohol consumption observed in general population are extended to those with NAFLD and NASH. There are emerging studies to suggest that even light alcohol consumption may increase the risk of cancers (e.g., breast and colon). Until further data from rigorously conducted prospective studies become available, we believe that individuals with NAFLD should avoid alcohol consumption of any type or amount.71
Treatment of NASH Related Cirrhosis and Hepatocellular Carcinoma
Treatment of patients with NASH-related cirrhosis and HCC is no different from other causes and hence will not be discussed further in this paper.
Role of Cardiologists and Endocrinologists
Non-alcoholic fatty liver disease is a lifestyle disease with underlying genetic predisposition, and is associated with significant morbidity and mortality, related not only to liver damage and higher risk of hepatocellular cancer, but also to long-term occurrence of DM and cardiovascular disease.40,50 Management of patients with NAFLD can be improved with a multidisciplinary team approach. Endocrinologists could be involved to implement lifestyle changes, achieve weight loss in overweight and obese individuals, improve insulin sensitivity, optimize treatment, improve the management of hyperglycemia and DM, and cardiologists could be involved to improve the management of hypertension and dyslipidemia. As mentioned earlier, use of statins is safe in patients with NAFLD, even in those with elevated transaminases. Further, as suggested earlier, cardiovascular evaluation should be done in selected patients and if found to have CAD should be treated accordingly. Cardiologists have an important role to play in this regard and also for treatment of cardiovascular risk factors. Lifestyle modifications suggested by cardiologists to improve the cardiovascular profile will help in improving the hepatic manifestations of NAFLD as well.
Conclusions
Non-alcoholic fatty liver disease is a significant health issue in India. It is now becoming clear that NAFLD is closely associated with marked metabolic derangements, mainly in the form of insulin resistance and the metabolic syndrome, which are important determinants of T2DM and CVD. A multi-disciplinary approach involving not only the hepatologists but also the internists, endocrinologists, and the cardiologists may be beneficial in managing patients with NAFLD. Diagnostic work-up in patients with NAFLD should include the assessment of severity of liver disease at the onset in addition to exclusion of other causes of fatty liver and raised transaminases. A highly individualized approach for the lifestyle modifications based on a thorough assessment of individual metabolic and nutritional status is recommended as the first line of treatment. Pharmacological treatment in NAFLD is still evolving and requires randomized controlled trials with histological end points in a large number of patients. Of the various drugs, pioglitazone and vitamin E are recommended for the non-diabetic patients who has biopsy-proven NASH.
Conflicts of interest
All authors have none to declare.
Acknowledgment
We acknowledge the contribution of Dr. Sushant Dahiya of Elsevier Singapore Pte Ltd, Singapore in preparing this manuscript.
Appendix.
1). Appendix 1. Non-alcoholic Fatty Liver Disease Activity Score (NAS)
| Item | Score | Extent |
|---|---|---|
| Steatosis | 0 | <5% |
| 1 | 5–33% | |
| 2 | >33–66% | |
| 3 | >66% | |
| Hepatocyte ballooning | 0 | None |
| 1 | Few balloon cells | |
| 2 | Many balloon cells/prominent ballooning | |
| Labor inflammation | 0 | No foci |
| 1 | <2 foci/200× | |
| 2 | 2–4 foci/200× | |
| 3 | >4 foci/200× |
Table Components of Non-alcoholic Fatty Liver Disease Activity Score (Adapted from Kleiner et al 2005).NAS: Non-alcoholic fatty liver disease activity score.
2). Appendix 2. Metabolic Syndrome Definitions
| Criteria | WHO (1998) | NCEP ATP III (2001) | Modified NCEP ATP III (2004) | IDF (2005) | Possible definition for south Asiansa |
|---|---|---|---|---|---|
| Fasting glucose (mg/dl) | DM, IGT, IFG or insulin resistance with ≥2 of the following: | ≥3 of the following: ≥110 | ≥3 of the following: ≥100, or T2DM or treatment | Central obesity (see below) and ≥2 of the following: ≥100 or T2DM diagnosis | Fasting hyperinsulinemia and ≥2 of the following: IFG, IGT, T2DM or treatment |
| Obesity | Central obesity (WHR >0.90 in males or >0.85 in females) and/or BMI >30 kg/m2 | Waist circumference >102 cm in males or >88 cm in females | Waist circumference >102 cm in males or >88 cm in females | Waist circumference >90 cm in males or >80 cm in females | Waist circumference >87 cm in males or >82 cm in females and/or BMI >23 kg/m2 |
| BP (mm or Hg) | ≥140/90 | ≥130/85, or treatment | ≥130/85, or treatment | ≥130/85, or treatment | ≥130/85, or treatment |
| Triglyceride (mg/dl) | ≥150 | ≥150, or treatment | ≥150, or treatment | ≥150, or treatment | ≥150, or treatment |
| HDL (mg/dl) | <35 in males or <39 in females | <40 in males and <50 in females | <40 in males and <50 in females | <40 in males and <50 in females, or treatment | <40 in males and <50 in females, or treatment |
| Others | Microalbuminuria (urinary albumin excretion rate ≥20 mg/min or albumin:creatinine ratio ≥30 mg/g) | Non-alcoholic fatty liver disease Subscapular skinfold thickness >18 mm |
Lower cut-offs may be probably necessary for BP and lipid levels than that mentioned for South Asians which requires further studies to be defined. WHO: World Health Organization; NCEP: National Cholesterol Education Program; ATP: Adult Treatment Panel; IDF: International Diabetes Federation; DM: diabetes mellitus; IGT: impaired glucose tolerance; IFG: impaired fasting glucose; BP: blood pressure; HDL: high-density lipoprotein; WHR: waist-hip ratio; BMI: body mass index.
3). Appendix 3. Homeostasis Model Assessment–Insulin Resistance
HOMA = fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5.
Level suggesting insulin resistance ≥1.8–2.0.
References
- 1.Anjana R.M., Pradeepa R., Deepa M. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdiaDIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . 5th ed. International Diabetes Federation; Brussels, Belgium: 2011. Diabetes Atlas. [Google Scholar]
- 3.Gupta R. Trends in hypertension epidemiology in India. J Hum Hypertens. 2004;18:73–78. doi: 10.1038/sj.jhh.1001633. [DOI] [PubMed] [Google Scholar]
- 4.Mohan V., Seedat Y.K., Pradeepa R. The rising burden of diabetes and hypertension in southeast asian and african regions: need for effective strategies for prevention and control in primary health care settings. Int J Hypertens. 2013;2013:409083. doi: 10.1155/2013/409083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misra A., Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5:2708–2733. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra A., Vikram N.K. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20:482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Wasir J.S., Misra A. The metabolic syndrome in Asian-Indians: impact of nutritional and socio-economic transition in India. Metab Syndr Relat Disord. 2004;2:14–23. doi: 10.1089/met.2004.2.14. [DOI] [PubMed] [Google Scholar]
- 8.Misra A., Misra R. Asian Indians and insulin resistance syndrome: global perspective. Metab Syndr Relat Disord. 2003;1:277–283. doi: 10.1089/1540419031361390. [DOI] [PubMed] [Google Scholar]
- 9.Misra A., Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 10.Luthra A., Nigam P., Misra A. Metabolic correlates of non-alcoholic fatty liver disease and management issues: a perspective for Asian Indians. Diabetes Metab Syndr Res Rev. 2007;1:279–285. [Google Scholar]
- 11.Bajaj S., Nigam P., Luthra A. A case-control study on insulin resistance, metabolic co-variates & prediction score in non-alcoholic fatty liver disease. Indian J Med Res. 2009;129:285–292. [PubMed] [Google Scholar]
- 12.Petersen K.F., Dufour S., Feng J. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen K.F., Dufour S., Hariri A. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duseja A., Aggarwal R. APOC3 and PNPLA3 in non-alcoholic fatty liver disease: need to clear the air. J Gastroenterol Hepatol. 2012;27:848–851. doi: 10.1111/j.1440-1746.2012.07103.x. [DOI] [PubMed] [Google Scholar]
- 15.Duseja A. Nonalcoholic fatty liver disease in India - is it different? Trop Gastroenterol. 2006;27:142–146. [PubMed] [Google Scholar]
- 16.Duseja A., Das A., Das R. The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci. 2007;52:2368–2374. doi: 10.1007/s10620-006-9136-y. [DOI] [PubMed] [Google Scholar]
- 17.Duseja A., Das A., Dhiman R.K. Indian patients with nonalcoholic fatty liver disease presenting with raised transaminases are different at presentation. World J Gastroenterol. 2007;13:649–650. doi: 10.3748/wjg.v13.i4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duseja A. Nonalcoholic fatty liver disease in India – a lot done, yet more required! Indian J Gastroenterol. 2010;29:217–225. doi: 10.1007/s12664-010-0069-1. [DOI] [PubMed] [Google Scholar]
- 19.Chitturi S., Farrell G.C., George J. Non-alcoholic steatohepatitis in the Asia-Pacific region: future shock? J Gastroenterol Hepatol. 2004;19:368–374. doi: 10.1111/j.1440-1746.2003.03252.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y.J., Li Y.Y., Nie Y.Q. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., Xu C., Yu C. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol. 2009;50:1029–1034. doi: 10.1016/j.jhep.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Akahoshi M., Amasaki Y., Soda M. Correlation between fatty liver and coronary risk factors: a population study of elderly men and women in Nagasaki, Japan. Hypertens Res. 2001;24:337–343. doi: 10.1291/hypres.24.337. [DOI] [PubMed] [Google Scholar]
- 23.Lai S.W., Ng K.C. Which anthropometric indices best predict metabolic disorders in Taiwan? South Med J. 2004;97:578–582. doi: 10.1097/00007611-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Lee K., Sung J.A., Kim J.S., Park T.J. The roles of obesity and gender on the relationship between metabolic risk factors and non-alcoholic fatty liver disease in Koreans. Diabetes Metab Res Rev. 2009;25:150–155. doi: 10.1002/dmrr.924. [DOI] [PubMed] [Google Scholar]
- 25.Singh S.P., Nayak S., Swain M. Prevalence of non-alcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 26.Amarapurkar D., Kamani P., Patel N. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163. [PubMed] [Google Scholar]
- 27.Mohan V., Farooq S., Deepa M. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Das K., Das K., Mukherjee P.S. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 29.Duseja A., Bhansali A., Bhadada S. Nonalcoholic fatty liver disease in patients with recent onset type 2 diabetes mellitus (abstract) J Gastroenterol Hepatol. 2004;19(suppl l):A402. [Google Scholar]
- 30.Gupte P., Amarapurkar D., Agal S. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 31.Duseja A., Chawla Y. Nonalcoholic fatty liver disease in India – how much? How soon? Trop Gastroenterol. 2005;26:1–3. [PubMed] [Google Scholar]
- 32.Wong V.W., Vergniol J., Wong G.L. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 33.Matteoni C.A., Younossi Z.M., Gramlich T. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 34.Kleiner D.E., Brunt E.M., Van Natta M. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 35.Clark J.M., Diehl A.M. Nonalcoholic fatty liver disease: an under-recognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell S.H., Oelsner D.H., lezzoni J.C. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 37.Amarapurkar D., Das H.S. Chronic liver disease in diabetes mellitus. Trop Gastroenterol. 2002;23:3–5. [PubMed] [Google Scholar]
- 38.Duseja A., Sharma B., Kumar A. Nonalcoholic fatty liver in a developing country is responsible for significant liver disease. Hepatology. 2010;52:2248–2249. doi: 10.1002/hep.23838. [DOI] [PubMed] [Google Scholar]
- 39.Nayak N.C., Vasdev N., Saigal S., Soin A.S. End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol. 2010;41:425–430. doi: 10.1016/j.humpath.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Marrero J.A., Fontana R.J., Su G.L. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 41.Das D., Chattopadhyay D., Aslam T. NAFLD and the changing face of hepatocellular cancer (HCC) [Abstract] Gut. 2011;60(suppl 1):A243. [Google Scholar]
- 42.Bugianesi E., Leone N., Vanni E. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 43.Abe H., Yoshizawa K., Kitahara T. Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol. 2008;43:967–974. doi: 10.1007/s00535-008-2264-8. [DOI] [PubMed] [Google Scholar]
- 44.Oh K.C., Park S.H., Park J.C. Is the prevalence of cryptogenic hepatocellular carcinoma increasing in Korea? Korean J Gastroenterol. 2005;45:45–51. [PubMed] [Google Scholar]
- 45.Song H.Y., Lee H.K., Lee J.S. Risk factors of cryptogenic hepatocellular carcinoma in patients with low body mass index or without metabolic syndrome. Korean J Intern Med. 2012;27:47–52. doi: 10.3904/kjim.2012.27.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 47.McKimmie R.L., Daniel K.R., Carr J.J. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volzke H., Robinson D.M., Kleine V. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastronterol. 2005;11:1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brea A., Mosquera D., Martín E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 50.Guleria A., Duseja A., Kalra N. Patients with non-alcoholic fatty liver disease (NAFLD) have an increased risk of atherosclerosis and cardiovascular disease. Trop Gastroenterol. 2013;34:74–82. doi: 10.7869/tg.2012.101. [DOI] [PubMed] [Google Scholar]
- 51.Adams L.A., Lymp J.F., St Sauver J. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Shibata M., Kihara Y., Taguchi M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–2944. doi: 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- 53.Reaven G. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 54.DeFronzo R.A., Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization Department of Non-communicable Disease Surveillance . World Health Organization; Geneva: 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. [Google Scholar]
- 56.Balkau B., Charles M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 57.National Cholesterol Education Program (NCEP) Expert Panel On Detection, Evaluation, And Treatment Of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 58.American Diabetes Association Standards of medical care in Diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 59.Vikram N.K., Pandey R.M., Misra A. Non-obese (body mass index <25 kg/m2) Asian Indians with normal waist circumference have high cardiovascular risk. Nutrition. 2003;19:503–509. doi: 10.1016/s0899-9007(02)01083-3. [DOI] [PubMed] [Google Scholar]
- 60.Misra A., Misra R., Wijesuriya M., Banerjee D. The metabolic syndrome in South Asians: continuing escalation & possible solutions. Indian J Med Res. 2007;125:345–354. [PubMed] [Google Scholar]
- 61.Almeda-Valdés P., Cuevas-Ramos D., Aguilar-Salinas C.A. Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol. 2009;8:S18–S24. [PubMed] [Google Scholar]
- 62.Paschos P., Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 63.Bugianesi E., McCullough A., Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 64.Athyros V.G., Bouloukos V.I., Pehlivanidis A.N. The prevalence of the metabolic syndrome in Greece: the MetS-Greece Multicentre Study. Diabetes Obes Metab. 2005;7:397–405. doi: 10.1111/j.1463-1326.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 65.Marchesini G., Bugianesi E., Forlani G. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 66.Duseja A., Thumburu K.K., Das A. Insulin tolerance test is comparable to homeostasis model assessment for insulin resistance in patients with nonalcoholic fatty liver disease. Indian J Gastroenterol. 2007;26:170–173. [PubMed] [Google Scholar]
- 67.Madan K., Batra Y., Gupta S.D. Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–3405. doi: 10.3748/wjg.v12.i21.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhat G., Baba C.S., Pandey A. Life style modification improves insulin resistance and liver histology in patients with non-alcoholic fatty liver disease. World J Hepatol. 2012;4:209–217. doi: 10.4254/wjh.v4.i7.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernaez R., Lazo M., Bonekamp S. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Ledinghen V., Vergniol J., Foucher J. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 71.Chalasani N., Younossi Z., Lavine J.E. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 72.Singh D.K., Rastogi A., Sakhuja P., Gondal R., Sarin S.K. Comparison of clinical, biochemical and histological features of alcoholic steatohepatitis and non-alcoholic steatohepatitis in Asian Indian patients. Indian J Pathol Microbiol. 2010 Jul–Sep;53:408–413. doi: 10.4103/0377-4929.68246. [DOI] [PubMed] [Google Scholar]
- 73.Singh D.K., Sakhuja P., Malhotra V., Gondal R., Sarin S.K. Independent predictors of steatohepatitis and fibrosis in Asian Indian patients with non-alcoholic steatohepatitis. Dig Dis Sci. 2008 Jul;53:1967–1976. doi: 10.1007/s10620-007-0074-0. [DOI] [PubMed] [Google Scholar]
- 74.Das S., Singh S.P., Parida P.K., Mallik R.N. Non-alcoholic fatty liver disease in subjects with type 2 diabetes mellitus and non-diabetics with special reference to insulin resistance and hepatic histopathological changes. Diabetes Metab Syndr: Clin Res Rev. 2010;4:226–229. [Google Scholar]
- 75.Palmer M., Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–1413. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 76.Ueno T., Sugawara H., Sujaku K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 77.Samaha F.F., Iqbal N., Seshadri P. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 78.Huang M.A., Greenson J.K., Chao C. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 79.Dansinger M.L., Gleason J.A., Griffith J.L. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 80.Marchesini G., Brizi M., Bianchi G. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 81.Uygun A., Kadayifci A., Isik A.T. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 82.Nair S., Diehl A.M., Wiseman M. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 83.Bugianesi E., Gentilcore E., Manini R. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 84.Haukeland J.W., Konopski Z., Eggesbø H.B. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 85.Duseja A., Das A., Dhiman R.K. Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol. 2007;6:222–226. [PubMed] [Google Scholar]
- 86.Promrat K., Lutchman G., Uwaifo G.I. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 87.Belfort R., Harrison S.A., Brown K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 88.Sanyal A.J., Mofrad P.S., Contos M.J. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 89.Sharma B.C., Kumar A., Garg V. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis. J Clin Exp Hepatol. 2012;2:333–337. doi: 10.1016/j.jceh.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hasegawa T., Yoneda M., Nakamura K. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Ailment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 91.Sanyal A.J., Chalasani N., Kowdley K.V., NASH CRN Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindor K.D., Kowdley K.V., Heathcote E.J. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 93.Laurin J., Lindor K.D., Crippin J.S. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 94.Singh S.P., Das H.S., Panda C.R. A pilot study of high dose of ursodeoxycolic acid (UDCA) in the treatment of nonalcoholic steatohepatitis [NASH] Indian J Gastroenterol. 2007;26(suppl 2):A90. [Google Scholar]
- 95.Ratziu V., de Ledinghen V., Oberti F., FRESGUN A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 96.Abdelmalek M.F., Sanderson S.O., Angulo P. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology. 2009;50:1818–1826. doi: 10.1002/hep.23239. [DOI] [PubMed] [Google Scholar]
- 97.Yokohama S., Yoneda M., Haneda M. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222–1225. doi: 10.1002/hep.20420. [DOI] [PubMed] [Google Scholar]
- 98.Adams L.A., Zeine C.O., Angulo P., Lindol K.D. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 99.Buranawui W., Thung-u-thaisri P., Pramoolsinsap C. Pentoxifylline for treatment of non-alcoholic fatty liver disease (NAFLD): a randomized, placebo-controlled study [Abstract s2161] Gastroenterology. 2006:A330. [Google Scholar]
- 100.Satapathy S.K., Sakhuja P., Malhotra V. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634–638. doi: 10.1111/j.1440-1746.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y.M., Sutedja D., Wai C.T. A randomized controlled pilot study of pentoxifylline in patients with non-alcoholic steatohepatitis (NASH) Hepatol Int. 2008;2:196–201. doi: 10.1007/s12072-008-9058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baranovsky AYu, Raykhelson K.L., Marchenko N.V. S-adenosylmethionine (Heptral®) in treatment of patients with non-alcoholic steatohepatitis. Clin Perspect Gastroenterol Hepatol. 2010;1:3–10. [Google Scholar]
- 103.Gomez-Dominguez E., Gisbert J.P., Moreno-Monteagudo J.A. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 104.Sreenivasa Baba C., Alexander G., Kalyani B. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21(1 Pt 1):191–198. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 105.Leuschner U.F., Lindenthal B., Herrmann G. High dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 106.Lavin J.E. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 107.Lavine J.E., Schwimmer J.B., Van Natta M.L. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madan K., Batra Y., Gupta D.S. Vitamin E-based therapy is effective in ameliorating transaminasemia in nonalcoholic fatty liver disease. Indian J Gastroenterol. 2005;24:251–255. [PubMed] [Google Scholar]
- 109.Dunn W., Xu R., Schwimmer J.B. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. 2008;47:1947–1954. doi: 10.1002/hep.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]


