Abstract
Malignancy masquerading as liver abscess, and presenting with fever, is mainly described in patients with colorectal cancers with liver metastasis. Primary liver tumors such as hepatocellular carcinoma or intrahepatic cholangiocarcinoma presenting as non-resolving liver abscess is extremely uncommon and carries a dismal prognosis. We present a rare case of non-resolving liver abscess as a presenting manifestation of intrahepatic cholangiocarcinoma.
Keywords: liver abscess, cholangicarcinoma, liver tumor, liver cancer, hepatocellular carcinoma
Abbreviations: AFP, alphafetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA 19-9, cancer antigen 19-9; CEA, carcinoembryonic antigen; CECT, contrast enhanced computerized tomography; CK, cytokeratin; CT, computerized tomography; ESR, erythrocyte sedimentation rate; FDG, fludeoxyglucose; FNAC, fine needle aspiration cytology; GGT, gamma-glutamyl transpeptidase; Hb, hemoglobin; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IU, international units; IVC, inferior vena cava; PET, positron emission tomography; RHV, right hepatic vein; SAP, serum alkaline phosphatase; UGI, upper gastrointestinal; USG, ultrasonography; WBC, white blood count
Febrile illness due to amoebic or pyogenic liver abscesses is frequently seen in tropical countries. Malignancy masquerading as liver abscess, and presenting with fever, is mainly described in patients with colorectal cancers with liver metastasis1 and neuroendocrine tumors.2 Fever maybe due to tumor necrosis getting liquefied and transforming into abscess, or due to release of cytokines, or due to paraneoplastic manifestation. Primary liver tumor such as hepatocellular carcinoma or intrahepatic cholangiocarcinoma presenting as liver abscess is extremely uncommon.3,4 We report here a case of intrahepatic cholangiocarcinoma which masqueraded as a non resolving liver abscess.
Case report
A 57-year-old male, known case of type-2 diabetes mellitus, on oral hypoglycaemic agents for last 10 years, presented to us with 2-month history of low-grade intermittent fever, not associated with chills and rigor. The fever used to be in the range of 100°–101 °F, not associated with any chills, rigors, or night sweats, and used to occur daily. There was no history of weight loss, pain abdomen, cough, jaundice, pruritus or any clinical feature of cholangitis. Prior to admission to our hospital, he had been investigated for the cause of fever at another medical center. Urine, blood cultures, Widal, typhoid serology and amoebic serology had been negative. His radiological investigations in the form of USG and CT abdomen (Figures 1 and 2) showed right lobe hypoechoic and hypodense, multi-septate lesion of size 7 cm × 6 cm with irregular shaggy margins consistent with liver abscess. There was no evidence of any central or segmental intrahepatic biliary radical dilatation and the common bile duct was normal in size. Based on these findings, he was treated as a case of liver abscess, and received third generation cephalosporins plus antiprotozoal for 2 weeks. However, there was no abatement in his fever range, intensity or frequency. Hence, he was referred to our hospital.
Figure 1.
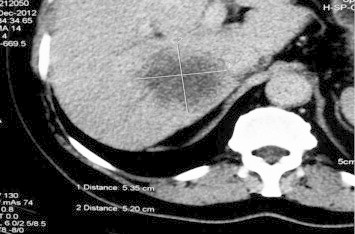
CT abdomen.
Figure 2.
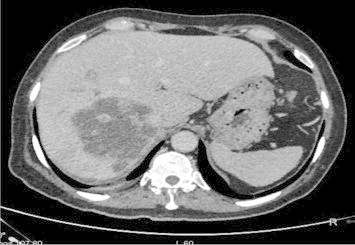
CT angiography delayed phase.
At presentation to our hospital his physical examination revealed pallor, a 2 cm tender hepatomegaly, but no splenomegaly. His investigations were as follows: Hb 10.9 g/dL, WBC 8.1 × 103/μL, platelets 140 × 103/μL, ESR 86 mm at 1 h, serum creatinine 0.53 mg/dL, serum bilirubin 0.3 mg/dL, AST 24 IU/L, ALT 32 IU/L, SAP 186 IU/L, and GGT 214 IU/L. Urine routine and microscopy examination was normal. Blood and urine cultures were sterile. Serum procalcitonin level was 0.28 ng/ml, Widal test was negative, amoebic and hydatid serologies were negative. His virology studies for HBV, HCV and HIV were negative. Mantoux test was negative. His USG abdomen revealed right lobe liver abscess (in segment 6 and 7) of size 8.5 cm × 7 cm with septations and irregular shaggy margins. There was no evidence of any intrahepatic biliary radical dilatation and common bile duct was normal. His diagnostic USG-guided aspiration from abscess cavity done on two occasions revealed thick purulent material, negative for Gram stain, acid-fast stain, and fungal stain. The culture of the purulent material was sterile with cytology showing abundant pus cells only.
During the hospital course he received multiple antibiotics and antifungals, including extended-spectrum penicillins, carbapenems, teicoplanin, and fluconazole. The reason for using these high-end antibiotics in spite of negative blood and urine cultures and normal procalcitonin was history of diabetes, recent hospitalization, twice needling of the liver lesion, non-response to third generation cephalosporins and metronidazole for two weeks; thus raising the suspicion of either healthcare-associated infection or infection with drug-resistant organisms. With history of diabetes mellitus and non-response to multiple antibiotics fungal infection was also suspected, hence antifungal was also used. However his fever did not abate.
In view of non-resolution of fever and no response to broad-spectrum antibiotics, with persistently negative cultures, and negative abscess culture and cytology; we ordered a triphasic CECT of whole abdomen keeping in mind the differential diagnosis of a necrotic liver malignancy or liver metastasis. CT scan revealed a multi-septate hard single hypodense lesion of size 9.7 cm × 8.7 cm × 8.4 cm, in right lobe liver suggestive of liver abscess with compression of right portal vein and encasement of intrahepatic IVC and RHV thrombosis (Figure 3). There was no evidence of intrahepatic biliary radical dilatation and common bile duct was normal. His tumor markers were: AFP 2.7 IU/mL, CA 19-9 926.1 IU/mL, CEA 31.21 ng/mL. On the basis of the imaging showing IVC encasement, right portal vein compression and right hepatic vein thrombosis, laboratory reports showing elevated CA19.9 in absence of cholangitis, elevated CEA and non improvement of fever the possibilities kept were necrotic primary malignancy or metastasis from the gastrointestinal tract. His UGI endoscopy and colonoscopy done to search for any primary malignancy were normal. On the basis of findings of triple-phase CECT abdomen and prior two USG FNAC's from abscess ‘cavity’ revealing negative cytology we decided to do a USG guided FNAC and biopsy from the ‘wall’ of the abscess in the right lobe of liver. To our surprise the abscess wall FNAC showed round tumor cells with moderately pleomorphic nuclei, prominent nucleoli with mitotic figures and scant cytoplasm suggestive of adenocarcinoma. The Trucut biopsy from the abscess wall revealed round tumor cells with moderately pleomorphic nuclei, prominent nucleoli with mitotic figures, and scant cytoplasm consistent with features of adenocarcinoma infiltrating the portal tracts and right hepatic vein invasion. There was abundant surrounding desmoplasia (Figure 4). Immunohistochemistry showed positivity for CK7 (marker for biliary origin) (Figure 5) and negativity for CK20 (hepatocellular origin). Hence the lesion was reported to be moderately differentiated cholangiocarcinoma. Subsequently a FDG PET CT (Figure 6) was done to look for systemic metastasis, which revealed FDG avid intrahepatic mass lesion with no extrahepatic, or lymph nodal spread and no extrahepatic vascular involvement. There was no evidence of any distant metastasis.
Figure 3.
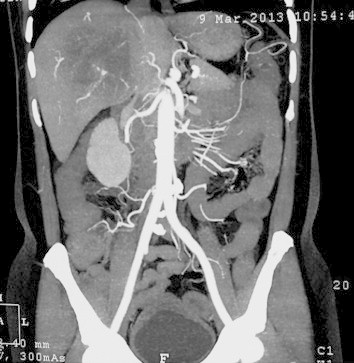
RHV thrombosis with intrahepatic IVC encasement.
Figure 4.
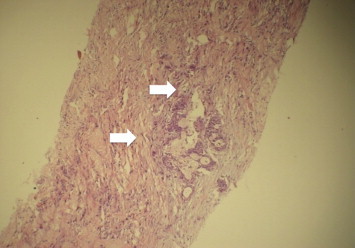
Tumor cells with abundant surrounding desmoplasia.
Figure 5.
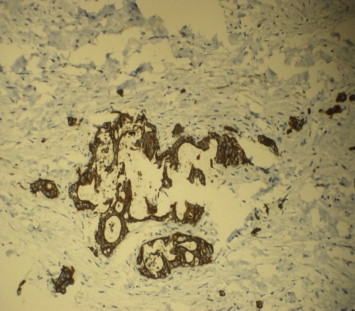
Tumor cells with CK7 positivity.
Figure 6.
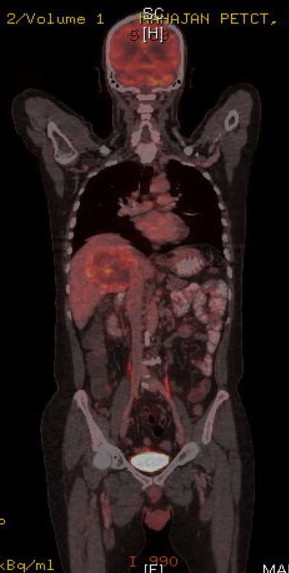
Localized disease on PET CT with no extra hepatic vascular spread.
As the lesion was deemed resectable on the basis of triphasic CECT and FDG PET, he was planned for a curative resection and a right hemi-hepatectomy. Intraoperative findings revealed an approximately 10 cm × 8 cm hard space occupying lesion in right lobe of liver, predominately in segment 6 and 7 with RHV thrombosis with tumor adherent to intrahepatic IVC with no extrahepatic peritoneal deposits. Rest of abdominal viscera was normal. So in view of intra operative findings revealing a significantly large portion of intrahepatic IVC being adherent to the mass lesion which would entail a very difficult and technically demanding long segment IVC graft reconstruction; decision was taken to do away with a curative resection in the form of conventional right hemi hepatectomy. Hence, a partial right hepatectomy with debulking of maximum possible portion of the lesion without any IVC reconstruction was performed. Post-operative specimen biopsy was consistent with moderately differentiated cholangiocarcinoma with extensive desmoplasia and resected margins positive for tumor with thrombosis in the hepatic veins. Repeat USG abdomen and hepatic vein Doppler showed no extension of thrombus to the IVC or residual portion of the right hepatic vein. Postoperative course was uneventful and his fever subsided. Patient was discharged in stable condition with plan to start chemotherapy once his nutritional status, performance status improved and sutures healed. His post operative follow up was available for 6 months during which he received adjuvant chemotherapy and imaging in the form of USG and CT did not show any local increase in the lesion or vascular involvement. The patient succumbed at around eight months post surgery due to acute coronary syndrome.
Discussion
This is a rare case of an elderly male presenting with persistent non resolving fever, abnormally high SAP and GGT, negative cultures, negative amoebic serology and non resolving liver abscess on imaging which turned out to be an intrahepatic cholangiocarcinoma masquerading as a liver abscess.
Intrahepatic cholangiocarcinoma masquerading as liver abscess is a very rare entity with only a few case reports to date reported in world literature.3,4 There has been no case reported till date from India. Malignancies may present as liver abscess secondary to obstruction of the biliary system or obstruction at the porta hepatis due to lymph nodes or lesion itself. But this mode of presentation is uncommon as malignancy is generally diagnosed much before this stage. Other reason for abscess formation may be tumor necrosis and subsequent superadded infection and transformation to an abscess.5 Primary presentation of malignancy as liver abscess is predominantly reported with metastatic colorectal cancers or neuroendocrine tumors. Intrahepatic cholangiocarcinoma or hepatocellular carcinoma presenting primarily as liver abscess is extremely rare. The presenting clinical manifestations such as persistent fever with or without chills and rigors, right-upper-quadrant abdominal pain, nausea, vomiting, weight loss and diarrhea are non specific and may be similar to pyogenic or amoebic liver abscess thus delaying the diagnosis of malignancy. Pyogenic/amoebic liver abscesses are generally accompanied by insignificant rise in tumor markers such as AFP, CA19.9. Raised AFP or CA19-9 with rising trend of SAP or GGT may thus serve as a diagnostic clue towards differentiating a malignancy from a pyogenic or amoebic liver abscess but are not specific.
Thus the learning lesson from this case is that a malignancy should be suspect when a liver abscess is non-resolving; especially when the patient is elderly, having persistent fever with no response to multiple courses of antibiotics, persistently rising SAP or GGT trend, elevated AFP/CA19.9 in absence of biliary obstruction, repeatedly negative abscess aspirate cultures, negative amoebic serology and non liquefaction of liver abscess on imaging. In such cases efforts should be directed towards obtaining an early CECT abdomen and tissue diagnosis in the form of FNAC or trucut biopsy from the abscess wall to rule out malignancy for a possible early diagnosis as in general the outcome of cholangiocarcinoma, presenting as liver abscess, is dismal.
Conflicts of interest
All authors have none to declare.
References
- 1.Giuliani A., Caporale A., Demoro M., Scimò M., Galati F., Galati G. Silent colon carcinoma presenting as a hepatic abscess. Tumori. 2007 Nov–Dec;93:616–618. doi: 10.1177/030089160709300618. [DOI] [PubMed] [Google Scholar]
- 2.Lee S.H., Kim K.A., Lee J.S. A case of primary neuroendocrine carcinoma of liver presenting with liver abscess. Korean J Gastroenterol. 2006 Oct;48:277–280. [PubMed] [Google Scholar]
- 3.Jan Y.Y., Yeh T.S., Chen M.F. Cholangiocarcinoma presenting as pyogenic liver abscess: is its outcome influenced by concomitant hepatolithiasis. Am J Gastroenterol. 1998 Feb;93:253–255. doi: 10.1111/j.1572-0241.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 4.Li C., Li G., Miao R. Primary liver cancer presenting as pyogenic liver abscess: characteristics, diagnosis, and management. J Surg Oncol. 2012 Jun 1;105:687–691. doi: 10.1002/jso.22103. [DOI] [PubMed] [Google Scholar]
- 5.Choi D., Lim H.K., Kim M.J. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860–1867. doi: 10.2214/ajr.184.6.01841860. [DOI] [PubMed] [Google Scholar]


