Abstract
The definitive treatment for patients with end-stage liver disease is orthotropic transplantation. However, this option is limited by the disparity between the number of patients needing transplantation and the number of available livers. This issue is becoming more severe as the population ages and as the number of new cases of end-stage liver failure increases. Patients fortunate enough to receive a transplant are required to receive immunosuppressive therapy and must live with the associated morbidity. Whole organ engineering of the liver may offer a solution to this liver donor shortfall. It has been shown that perfusion decellularization of a whole allogeneic or xenogeneic liver generates a three-dimensional ECM scaffold with intact macro and micro architecture of the native liver. A decellularized liver provides an ideal transplantable scaffold with all the necessary ultrastructure and signaling cues for cell attachment, differentiation, vascularization, and function. In this review, an overview of complementary strategies for creating functional liver grafts suitable for transplantation is provided. Early milestones have been met by combining stem and progenitor cells with increasingly complex scaffold materials and culture conditions.
Keywords: organ engineering, extracellular matrix, biologic scaffold, decellularization, liver tissue engineering
Abbreviations: BAL, biohybrid artificial liver; ECM, extracellular matrix; PLECM, porcine-liver-derived extracellular matrix; SEC, sinusoidal endothelial cell; SEM, scanning electron microscopy; DAMP, damage associated molecular pattern; HMECs, human microvascular endothelial cells; NPCs, non-parenchymal cells; SDS, sodium dodecyl sulfate; BMC, basement membrane complex; CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
Allogeneic liver transplantation is the “gold standard” for patients with end-stage liver disease but is limited by its high cost and the severe donor organ shortage.1 Both xenotransplantation and hepatocyte transplantation represent alternative therapies, but these approaches have had limited clinical success. Xenotransplantation could provide a limitless supply of donor organs; however, previous attempts have resulted in hyperacute rejection and death. Hepatocyte transplantation offers much promise for correcting nonemergency conditions such as genetic defects of the liver, but low efficiency of engraftment, long-term immunosuppression from the use of allogeneic cells, and a lag time of 48 h for the transplanted hepatocytes to become functional in vivo have limited the clinical success.2,3 Biohybrid artificial liver (BAL) devices provide temporary support for patients waiting for an allogeneic liver transplant, and since the liver can regenerate, the temporary support provided by BAL may allow sufficient time for this process. However, the lack of a reliable cell source combined with the inability of BAL to maintain the functionality of hepatocytes for long periods of time has limited its clinical utility.4
These therapeutic challenges have catalyzed the concept of whole organ engineering using three-dimensional biologic scaffolds composed of extracellular matrix (ECM). Whole organ engineering of the liver is based upon three fundamental concepts: 1) the native ECM of the liver represents an ideal and required substrate for liver regeneration, 2) three-dimensional acellular liver scaffolds retain the three-dimensional macrostructure, the native microvascular network, and the bile drainage system; allowing for complete recellularization of all native cell types, and 3) liver regeneration can be promoted when reseeded three-dimensional acellular liver grafts are placed in the appropriate three-dimensional microenvironment, specifically, in-situ in patients with liver failure. The general approach taken for engineering functional liver tissue with ECM scaffolds can be found in Figure 1.
Figure 1.
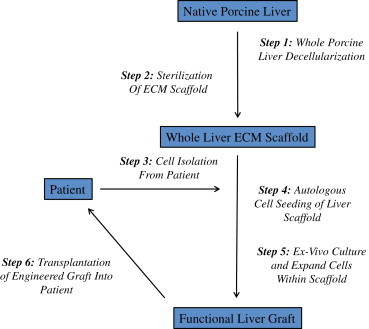
Conceptual overview of the general approach taken for engineering functional liver tissue. Healthy porcine livers would be harvested, decellularized, and sterilized to produce a whole liver ECM scaffold. This scaffold would then be seeded with a population of the patient's cells and cultured ex-vivo until the graft is suitable for transplantation.
Liver ECM represents the secreted product of the resident cells of the liver, and it is therefore logical that L-ECM is the ideal microenvironment in which hepatocytes can maintain their phenotype and functionality. The liver ECM (i.e., stroma) has also been shown to be essential for liver generation following injury. Whole liver decellularization can be accomplished by vascular perfusion with a cocktail of enzymes, proteases, detergents, and hypotonic saline rinses that completely remove all cellular elements while largely maintaining the native composition and ultrastructure of the underlying three-dimensional matrix.5–7 The creation of a functional liver has not been accomplished to date, but several intermediate milestones have been reached by tissue engineers of the heart,8 liver,5–7,9–13 lung,14–17 pancreas,18 and kidney.19,20 By integrating increasingly complex cell combinations, scaffold materials and culture environments, these efforts have successfully recapitulated different aspects of organ development and provided valid lessons that can be applied to future liver tissue engineering work.
Extracellular matrix as a biologic scaffold
Biologic scaffolds composed of allogeneic or xenogeneic ECM have been used in millions of human patients to reconstruct a variety of tissues including the skin,21 body wall,22,23 urinary bladder,24 and rotator cuff,24 among others. The ECM is in a state of dynamic reciprocity with resident cells in response to changes in the microenvironment and has been shown to provide cues that affect cell migration and cell proliferation,25–27 cell differentiation,28–32 and host innate immune response modulation.31,33–35
The creation of an acellular ECM scaffold involves decellularization of a source tissue or organ with the ultimate goal of preserving the native ECM ultrastructure and composition. Reviews of tissue decellularization techniques and their effect upon ECM properties are available,36,37 and new techniques are continually being developed for application to whole organs. The deleterious in vivo effects of ineffective decellularization with the retention of residual cellular material are recognized.38 However, definitive quantitative standards for effective decellularization of whole organs have yet to be established. This topic will be further discussed in section 3.2.
Biologic scaffold materials are typically, but not always, marketed and regulated as surgical mesh devices. These materials are composed of ECM harvested from a variety of allogeneic or xenogeneic tissue sources including dermis (e.g., AlloDerm® Lifecell Corp.), urinary bladder (e.g., MatriStem®, Acell Inc), small intestine (e.g., Biodesign® Cook Biotech Inc.), mesothelium (e.g., Meso BioMatrix™, Kensey Nash Corp.), and pericardium (e.g., Lyoplant®, B. Braun Melsungen AG), among others. Clinical products composed of ECM have been manufactured from many of these tissues and from a variety of species including human, porcine, bovine, and equine. There is evidence that tissue specific bioscaffolds are preferred, or even required, for functional reconstruction of whole organs such as lung and liver. However, tissue specificity of the biologic scaffold does not appear to be a requirement for reconstruction of many tissues such as skeletal muscle,39 esophagus,40–43 and urinary bladder.44,45 The mechanisms by which ECM bioscaffolds facilitate functional and constructive tissue remodeling include positive effects upon cell mitogenesis and chemotaxis,35,37,46,47 cell differentiation,48–52 and modulation of the host innate immune response.34,53–57 It is likely that the three-dimensional ultrastructure, surface topology, surface ligand landscape, and composition of the ECM all contribute to these constructive effects.
Innate Host Response to Extracellular Matrix Scaffolds
Macrophages are a heterogenous subset of mononuclear phagocytes that play an important role in the host response to implanted biomaterials. The macrophages participating in the host response following implantation of a biomaterial are exposed to multiple stimuli including cytokines and effector molecules secreted by cells (including other macrophages) active at the implantation site, microbial agents, epitopes associated with the implanted biomaterial, and the degradation products of the biomaterial, among others.58 Similar to the Th1/Th2 paradigm, populations of macrophages can be classified phenotypically and functionally along a spectrum ranging from cytotoxic/pro-inflammatory types (designated as M1 “classically activated” macrophages) or wound healing/anti-inflammatory types (designated as M2 “alternatively activated” macrophages).56 It has been shown that in response to the implantation of ECM scaffolds, cytokine expression is consistent with a predominant M2 and Th2 response. Macrophages are required for scaffold degradation in vivo and determine the overall remodeling outcome.54,57
Extracellular Matrix Degradation
It has been shown that most ECM scaffolds are rapidly degraded in vivo. A previous study showed that 14C labeled ECM scaffolds were 60% degraded at 30 days post implantation and 100% at 90 days post-surgery in a model of canine Achilles tendon repair. During this period, the scaffold was populated and degraded by host cells and resulted in the formation of site-specific functional host tissue.59 The major mechanism of excretion of the degraded scaffold was via hematogenous circulation and elimination by the renal excretion. Recent findings suggest that the degradation products of ECM scaffolds are bioactive.35,37,50,60,61 One of the biologic effects of ECM degradation products is the recruitment of tissue specific stem and progenitor host cells to the site of degradation.35,47,60 Therefore, not only does a biologic scaffold, such as liver ECM, modulate the host innate immune response toward a constructive phenotype,55–57 but a biologic scaffold also has the potential to recruit endogenous progenitor cells to the site which can participate in the reconstitution of functional liver tissue. It is expected that the three-dimensional L-ECM scaffolds will also rapidly degrade and be replaced by new matrix secreted by the seeded hepatocytes.
Liver Specific Extracellular Matrix
As previously stated, tissue specific bioscaffolds are required for constructive remodeling of select tissues, including the liver. This requirement may be due to the selective nature of hepatocytes, which are notorious for losing functional phenotype quickly in culture. The use of complex ECM substrates derived from mammalian tissues for effective hepatocyte culture began more than two decades ago. Early culture models utilized ECM substrates derived from either rat liver or the Engelbreth-House sarcoma mouse tumor (i.e., Matrigel). Rat hepatocytes cultured on type-1 collagen show less cell attachment and survival compared to rat hepatocytes cultured upon a biomatrix derived from solubilized rat liver. This result further demonstrates that liver specific ECM is important in maintaining cells introduced to a decellularized scaffold.
ECM substrates derived from porcine, bovine, and human livers have been used to improve hepatocyte survival, polarity and liver-specific functions in vitro. Lin et al compared rat hepatocytes cultured on ECM biologic scaffolds derived from porcine-liver (PLECM) to well-characterized hepatocyte culture models (type-1 collagen sandwich configuration or a single layer of type-1 collagen).62 Hepatocytes survived up to 45 days on a sheet form of PLECM and several liver-specific functions such as albumin synthesis, urea production, and P-450 IA1 activity were markedly enhanced compared to the growth and metabolism of cells cultured on a single layer of type-1 collagen.
In a previous study, two different biologic substrates were compared for their ability to support primary human hepatocyte function in vitro: porcine-liver-derived extracellular matrix (PLECM) and Matrigel.63 Albumin secretion, hepatic transport activity, and ammonia metabolism were used to determine hepatocyte function. Hepatocytes cultured between two layers of PLECM or Matrigel showed equally high levels of albumin expression and secretion, ammonia metabolism, and hepatic transporter expression and function. In another study, three different acellular ECM scaffolds were investigated in a physiologically relevant in vitro culture model for their ability to maintain hepatic sinusoidal endothelial cell (SEC) phenotype.64 The cell culture model used SECs only or a coculture of SECs with hepatocytes on ECM substrates derived from the liver (L-ECM), bladder (UBM-ECM), or small intestinal submucosa (SIS-ECM). The effect of the ECM substrate upon SEC dedifferentiation was evaluated using scanning electron microscopy (SEM) and confocal microscopy. When SECs alone were cultured on uncoated glass slides, collagen I, UBM-ECM, or SIS-ECM, SECs showed signs of dedifferentiation after 1 day. In contrast, SECs alone cultured on L-ECM maintained their differentiated phenotype for at least 3 days, indicated by the presence of many fenestrations on SEC surface, expression of anti-rat hepatic sinusoidal endothelial cells mouse IgG MoAb (SE-1), and lack of expression of CD31. When SECs were cocultured with hepatocytes on any of the ECM scaffolds, the SECs maintained a near-normal fenestrated phenotype for at least 1 day. However, SEM revealed that the shape, size, frequency, and organization of the fenestrations varied greatly depending on ECM source. At all-time points, SECs cocultured with hepatocytes on L-ECM maintained the greatest degree of differentiation. This study demonstrated that the acellular ECM scaffold derived from the liver maintained SEC differentiation in culture longer than any of the other tested substrate materials and that contact and crosstalk between different cell types of the same tissue may also be beneficial to preserving or promoting proper function. The conclusion of these studies supports the hypothesis that L-ECM provides a preferred substrate and microenvironment for maintaining hepatic cell viability and function.
Methods of liver decellularization and scaffold processing
Isolating the extracellular matrix from an intact liver requires a process that removes cellular material, while preserving the ultrastructure, composition, and ligand landscape of the underlying matrix. Minimal damage to vascular structures is essential for eventual re-endothelialization, anastomosis, and in vivo implantation of three-dimensional liver scaffolds. Removal of cells from their integrin-bound anchors and intercellular adhesion complexes while maintaining extracellular matrix surface topography and resident ligands is challenging. A combination of physical, ionic, chemical, and enzymatic methods are typically used to accomplish decellularization. Organs, such as the heart,65–67 liver,7,9 kidney,19 pancreas,18 and lung,14 have been decellularized by using each organ's vascular network to deliver decellularizing solutions. This method leaves the organ semitransparent in appearance while retaining the ultrastructure of the whole organ with intact vascular basement membranes and architecture. Re-endothelialization of the denuded vascular network is necessary to support blood flow and to prevent thrombosis. Reviews of tissue decellularization techniques and their effect upon ECM properties are available.29,30
The most effective protocol for the decellularization of a liver will depend upon characteristics of the source liver, including species (e.g., porcine vs. non-human primate), age (e.g., neonatal vs. adult), and lipid content (e.g., high vs. low fat diet). Regardless of the processing method used, preservation of the native hepatic matrix composition and ultrastructure is the primary objective. All methods used for decellularization are inherently disruptive with unavoidable adverse effects upon the native architecture and key proteins and/or growth factors. However, the extent of disruption can be minimized with careful consideration of the method used.
Current Methods of Whole Liver Decellularization
In 2008, perfusion decellularization was reported as a technique to generate acellular whole-organ scaffolds from cadaveric organs.66 In this approach, decellularizing agents are delivered via the native vasculature of the organ and are thereby equally distributed across the entire mass of the organ. By applying physiologic perfusion pressures, decellularization solutions can effectively permeate the organ via arteries, arterioles and capillaries and remove cellular debris via the venous system, thereby minimizing their retention within the scaffold. The minimum exposure of the source organ to the decellularization solutions lowers the risk of the chemical or physical alterations of ECM proteins and growth factor loss, thus facilitating the generation of a more biocompatible scaffold for organ engineering. Although previous studies utilizing perfusion decellularization have reported a combination of SDS, Triton X-100 and PBS perfusion, the ideal detergent recipe must be tailored to the specifics of the harvested liver (i.e., species and age). There have been several published methods for generating an acellular liver scaffold. An overview of these methods can be found in Table 1. Perfusion decellularization of whole liver generates an acellular ECM scaffold with intact three-dimensional anatomical structures and patent vasculature conduits that can be re-endothelialized. Decellularized liver scaffolds have been shown to be free of significant DNA content and nuclear fragments, while retaining major ECM proteins (collagen I, III, laminin, fibronectin and glycosaminoglycans).
Table 1.
Methods of Whole Liver Decellularization.
| Author | Species | Protocol time | Temp (°C) | Perfusion inlet | Detergents used | Flow rate or pressure | Protocol overview |
|---|---|---|---|---|---|---|---|
| Shupe et al11 | Rat | 6 h | Room | Inferior Vena Cava | 1% Triton X-100 2% Triton X-100 3% Triton X-100 0.1% SDS |
5 ml/min |
|
| Uygun et al9 | Rat | 4 days | 4° | Portal Vein | 0.01% SDS 0.1% SDS 1% SDS 1% Triton X-100 |
1 ml/min |
|
| Soto-Gutierrez et al7 | Rat | 2 days | −80°Room 37° Room |
Inferior Vena Cava | 3% Triton X-100 | 8 ml/min |
|
| Bao et al10 | Rat | 2 days | 4° | Portal Vein | 1% SDS 0.5% SDS 0.25 SDS 1% Triton X-100 |
25 mmHg |
|
| Barakat et al6 | Porcine | – | Room 4° | Portal Vein | 0.25% SDS 0.5% SDS |
80 mmHg |
|
| Zhou et al5 | Murine | 6 h | 37° | Portal vein | 1% SDS 1% Triton X-100 |
5 ml/min |
|
| Baptista et al14 | Rat & Ferret | – | Room | Portal vein | 1% Triton X-100 | 5 ml/min |
|
| Mirmalek-Sani et al12 | Porcine | – | 4° Room 4° |
Hepatic Artery | 1% Triton X-100 2% Triton X-100 3% Triton X-100 0.1% SDS |
50 ml/min |
|
| Nari et al15 | Rabbit | 2 days | −80° Room | Inferior Vena Cava | 3% Triton X-100 0.1% SDS |
6-10 ml/min |
|
| Wang et al16 | Murine | 6 h | Room | Portal Vein | 1% SDS 1% Triton X-100 |
5 ml/min |
|
| Ren et al17 | Rat | 3.5 h | 37° | Portal Vein | 1% SDS | 5 ml/min |
|
| 1% Triton X-100 |
|
Criteria for Decellularization
Following whole liver decellularization, the organ usually assumes a pale or translucent quality. However, macroscopic appearance alone is insufficient to determine the extent of decellularization. While there is no universal consensus on criteria for adequate decellularization, standard metrics are beginning to emerge. Three relatively stringent criteria have been proposed to establish sufficient decellularization: specifically, the remaining ECM scaffold must have 1) less than 50 ng of dsDNA per mg of dry weight, 2) DNA fragments less than 200 bp in length, and 3) no visible nuclear material in histologic analysis with DAPI or H&E.30
Failure to completely decellularize a tissue leads to negative outcomes upon in vivo implantation, including a pro-inflammatory response with associated M1 macrophages and subsequent fibrosis. Such a reaction is likely caused in part by damage associated molecular pattern (DAMP) molecules and can lead to seroma formation, sterile abscess formation, and chronic inflammation. A recently published study determined the association between decellularization efficacy and host response by qualitative and quantitative methods.68 ECM devices containing significantly more cellular material showed a predominantly M1 pro-inflammatory macrophage response while ECM device containing less DNA resulted in a macrophage response predominantly of an M2 phenotype. Other studies have shown that a scaffold that contains cellular material promotes a clear M1 phenotype macrophage response at 3 days; whereas, the equivalent acellular scaffold promotes a strong M2 phenotype.36 Rieder et al have shown that decellularization can reduce the chemotactic potential of heart valve tissue for macrophages but does not inhibit the activation of macrophages although they did not study macrophage polarization.60 Ariganello et al have shown that in vitro exposure of a macrophage cell line to decellularized tissue elicited lower esterase and phosphatase activity consistent with a subdued inflammatory response comparable to the M2 phenotype.35
The Effects of Detergents on Extracellular Matrix Scaffolds
The choice of detergent used for whole liver decellularization is an important factor because the recellularization process will be dependent on the integrity of the remaining substrate. Each detergent, depending on its chemical characteristics, has unique and distinct effects on ECM composition and structure. Less harsh detergents, such as Triton X-100 or other non-ionic detergents are preferred for maintaining the native ECM structure and composition compared to detergents such as SDS, which can denature essential ligands and proteins within the ECM.
Detergents commonly used in the decellularization of organs and tissues include Triton X-100,70 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS),18 deoxycholic acid, and sodium dodecyl sulfate (SDS).66,10 Detergents can solubilize cell membranes and dissociate DNA from proteins, making them attractive for the decellularization process. Ionic detergents can be more effective for cellular removal than non-ionic and zwitterionic detergents.75 However, subjecting tissue to harsh detergents, such as SDS, can disrupt the ECM structure,76 eliminate growth factors,77 and/or denature essential proteins.78
In a recent study, four detergents commonly used for decellularization of tissues and organs were systematically evaluated and compared for their effect on the basement membrane complex (BMC).79 The ability of the resulting scaffold to support human microvascular endothelial cells (HMECs) in vitro was determined. This study is relevant because the success of whole liver engineering will be critically dependent upon the re-endothelialization of the organ's vasculature. The detergents investigated were 3% Triton X-100, 4% sodium deoxycholate, 8 mM CHAPS, and 1% SDS. Results were as follows:
Collagen Content
Scaffolds treated with 3% Triton X-100, 8 mM CHAPS and 4% sodium deoxycholate retained a soluble collagen content similar to that of the non-detergent water control. Treatment with 1% SDS resulted in a significant loss of detectable soluble collagen. This finding suggests that detergent treatment with SDS resulted in either a decrease in soluble collagen present or modification of the molecular structure of this collagen to the point of insolubility.
GAG Content
Scaffolds treated with 3% Triton X-100, 4% sodium deoxycholate and 8 mM CHAPS retained GAG similar to that of the water control, while scaffolds treated with 1% SDS retained a smaller amount of detectable GAG than the water control.
Elastin Content
Scaffolds treated with Triton X-100 and sodium deoxycholate retained elastin fibers: whereas, CHAPS had no visible elastin fibers, and SDS had only a small amount of thin fragmented fibers.
Fiber Network Analysis
Differences in scaffold surface fiber organization and evidence of collagen fiber denaturation were apparent from both SEM inspection and the results of automated image algorithms. SDS and CHAPS caused marked alterations of collagen fiber architecture while Triton X-100 and sodium deoxycholate were better tolerated and showed the surface of the BMC maintained an appearance that more closely resembled that of the no-detergent control. These structural changes and the associated changes in the ligand landscape provide insight into the results of the cell seeding experiments. When HMECs were cultured on basement membrane exposed to the chosen detergents, clear differences were seen in cell morphology, confluence, infiltration depth, and integrin β-1 expression.
Extracellular Matrix-Cell Interactions
HMECs cultured on the BMC prepared with 3% Triton X-100 had a similar level of confluence, infiltration depth, and phenotype compared to cells cultured on scaffolds treated with type I water (control). These HMECs were characterized by a flat morphology. HMECs cultured on the BMC prepared with 8 mM CHAPS were less confluent, had a greater infiltration depth, and an atypical phenotype compared to HMECs cultured on the control. HMECs cultured on scaffolds prepared with 4% sodium deoxycholate were less confluent, had a similar infiltration depth, and an atypical phenotype compared to cells cultured on a no-detergent control. HMECs cultured on scaffolds prepared with 1% SDS had a similar percentage of confluence, similar infiltration depth, but a less normal phenotype compared to cell cultured on a no-detergent control.
HMECs cultured on the BMC prepared with 8 mM CHAPS and 1% SDS had a lower number of cells stain positive for integrin β-1 compared to HMECs cultured on the BMC not subjected to a detergent. HMECs cultured on the BMC prepared with 3% Triton X-100 and 4% sodium deoxycholate had a similar percentage of cells expressing integrin β-1 compared to cells cultured on the no-detergent control tissue. The percent of cells positive for Ki67 was below 3% for all groups, and no significant differences were seen when comparing to the control.
This study shows that each detergent, depending on its chemical characteristics, has distinct effects on ECM composition and structure. Less disruptive detergents, such as Triton X-100 or other non-ionic detergents are preferred for maintaining the native ECM structure and composition compared to more harsh detergents, such as SDS, which can denature essential ligands and proteins within the BMC. The disruption or denaturation of the native BMC architecture can negatively impact the interaction of cells with the scaffold. The results of this study can aid in the formulation of tissue and organ decellularization protocols such that the native biological activity of the resulting extracellular matrix scaffold is maximally preserved.
Enzymes
There have been several published methods for generating biologic scaffolds composed of ECM, each of which describes a unique and specific recipe of enzymes and detergents to be used on the source tissue. Trypsin is an enzyme commonly used in the decellularization process because it is a well-characterized protease naturally found in the digestive tract of many vertebrates. As a protease, trypsin disrupts cell adhesion molecules (i.e., integrins) and cleaves peptide bonds with remarkable specificity.
Trypsin can disrupt both cell–cell and cell-ECM bonds as well as cleave cell surface proteins. In conjunction with EDTA, an ion chelating agent that disrupts cell–cell cadherin adhesions, trypsin is a powerful decellularization agent. Perfusing an organ with a solution of trypsin/EDTA is an effective initial step in the process of whole organ decellularization. However, the perfusion with trypsin is time dependent and must be performed at low concentrations to preserve the proteins within the ECM to the maximum extent possible. The combination of trypsin followed by a detergent is typically necessary to achieve complete decellularization.
Sterilization of Whole Liver Scaffolds
Recellularization and implantation of a whole liver scaffold will obviously require sterility. Gamma irradiation, e-beam, glutaraldehyde, ethylene oxide and peracetic acid have all been used as methods of sterilization and have been extensively evaluated for their effect on bioscaffold mechanical and biological integrity. In addition to sterilization, glutaraldehyde effectively crosslinks ECM proteins. Other chemical agents, such as carbodiimide and genipin, are also crosslinking agents, which can be used prior to sterilization. Chemical crosslinking of ECM proteins (e.g., collagen) stabilizes and strengthens the ECM structure and severely inhibits in vivo degradation. However, sterilization techniques that also crosslink must be avoided because crosslinked ECM scaffolds have been shown to elicit a foreign body response very similar to non-degradable synthetic polymer scaffolds (e.g., polypropylene).54 This response is predominantly M1 in nature, causing fibrosis leading to chronic inflammation.34
Whole Liver Extracellular Matrix Scaffold Recellularization
There have been several published methods for cell seeding a whole liver ECM scaffold. An overview of these methods can be found in Table 2. The methods employed in recellularization of whole-organ scaffolds are typically adaptations of techniques from a wide range of procedures including traditional cell culture, tissue-engineering methods, cell-transplantation therapies, and isolated-organ perfusion. The recellularization process can be considered in two major steps. The first is cell seeding, in which the goal is distribution of appropriate cell types to all areas of the three-dimensional liver scaffold. The second is perfusion culture, which is typically utilized to prepare the cells for in vivo function by exposing them to physiological conditions.
Table 2.
Published Methods for Cell Seeding a Whole Liver ECM Scaffold.
| Author | ECM scaffold source species | Cell types | Number of cells | Method of cell delivery | Culture duration | Implantation site | Duration of graft survival |
|---|---|---|---|---|---|---|---|
| Baptista et al14 | Ferret | Human fetal liver cells Human umbilical vein endothelial cells |
7.0 × 10ˆ7 3.0 × 10ˆ7 |
Portal vein perfusion | 7 days | n/a | n/a |
| Bao et al10 | Rat | Rat Hepatocytes | 1.0 × 10ˆ8 | Portal vein perfusion | 6 h | In series with native liver via end-to-end anastomosis with the portal vein | 72 h |
| Barakat et al6 | Porcine | Human fetal stem cells Human fetal liver cells |
3.5 × 10ˆ8 1.0 × 10ˆ9 |
Portal and hepatic vein perfusion | 3, 7, 13 days | Into the infrahepatic space by using the recipient portal vein and infrahepatic inferior vena cava as an inflow and outflow, respectively | 2 h |
| Soto-Gutierrez et al7 | Rat | Mouse hepatocytes | 5.0 × 10ˆ7 | Injection or portal vein perfusion | 7 days | n/a | n/a |
| Uygun et al9 | Rat | Rat hepatocytes | 5.0 × 10ˆ7 | Portal vein perfusion | 14 days | Heterotopic, following unilateral nephrectomy | 8 h |
The first challenge in recellularization of decellularized liver scaffolds is its repopulation with an appropriate mixture and number of cells as well as directing each cell type to necessary niches within the scaffold to match the native distribution. In addition, non-parenchymal cells such as fibroblasts and endothelial cells enhance the functional phenotype of the hepatocytes and contribute to the organization of the cellular architecture of the liver.
Endothelial cells are necessary to provide a non-thrombogenic barrier for the decellularized liver matrix and assure that blood flow in vivo is confined to the vascular spaces and that the parenchymal cells are protected from the shear stress created by the flow. A major advantage of whole-organ scaffolds is the presence of intact vascular networks, but full utilization of this vascular system to direct flow within the tissue in vivo requires adequate endothelialization. Functional long-term endothelialization of a liver bioscaffold has yet to be demonstrated, although initial attempts have been promising in showing endothelial cell attachment. Strategies to improve the endothelialization of three-dimensional organ constructs are under investigation.
Delivery of Cells Within a Decellularized Scaffold
Seeding techniques currently employed in recellularization of whole-organ grafts are essentially adaptations of the approaches employed in cell-transplantation therapies. Cells are either injected directly into the organ or injected into the circulation with the expectation that the cells will home to the injury site. These techniques include intramural injection of cells or infusion of cells into the vasculature followed by continuous perfusion.
In the report for whole-rat heart recellularization, recellularization occurred with 50–75 × 106 neonatal cardiac cells delivered in five injections of 200 μl each into the anterior left ventricle with a seeding efficiency of approximately 50%.66 This approach resulted in approximately 34% of recellularization proximal to the injection sites with decreasing percentages distally. Furthermore, 2 × 107 endothelial cells were placed by direct infusion into the aorta, which yielded 550.7 ± 99 cells per mm2 on the endocardial surface and 264.8 ± 49.2 cells per mm2 within the vascular tree after 7 days of perfusion culture.
A study conducted by Soto-Gutierrez et al compared three different methods for hepatocyte seeding of a three-dimensional liver scaffold. The three evaluated methods were1 direct parenchymal injection,2 multistep infusion, or3 continuous perfusion. The three-dimensional liver matrix reseeded with the multistep infusion of hepatocytes generated ∼90% of cell engraftment and supported liver-specific functional capacities of the engrafted cells, including albumin production, urea metabolism, and cytochrome P-450 induction.7
Cell Type and Source
Hepatocytes are the parenchymal cells of the liver and account for approximately 80% of all liver cells.80 Hepatocytes perform most of the functions of the liver and work in concert with a number of other cell types to perform the functions essential for survival of the individual. The non-parenchymal cells (NPCs) of the liver include sinusoidal endothelial cells, Kupffer cells, and stellate cells.80 It is likely the addition of liver-derived NPCs will be necessary in the recellularization of a three-dimensional liver scaffold. Co-cultivation of primary human hepatocytes with other cells types can maintain liver-specific functions for several weeks in vitro.81 Liver-derived NPCs as well as non-hepatic endothelial cells, epithelial cells, and fibroblasts have been cocultured with hepatocytes to maintain hepatocyte morphology and a variety of synthetic, metabolic, and detoxification functions of the liver. Bhatia et al have provided an extensive summary of studies used to preserve hepatocyte-specific functions in vitro through coculture with other cells.82
The cell source used to seed three-dimensional ECM scaffolds is an open and unanswered question. Availability of human donor livers for hepatocyte isolation is inadequate: thus, alternatives to autologous primary hepatocytes should be considered. Induced pluripotent stem cell populations are attractive due to their typically rapid and extensive proliferation and their potential to be used without immunosuppressant drugs. However, many questions regarding induced pluripotent stem cells remain unanswered. The in vitro differentiation state of stem cells that leads to optimal survival in vivo is unknown. It is believed that early-stage progenitor cells may be more efficient than fully differentiated liver cells because they can give rise not only to hepatocytes but also to other cell types (non-parenchymal liver cells) including bile duct epithelial cells required for the formation of the sophisticated hepatic anatomy.
Construction of a Vascular Network
It has previously been shown that the basement membrane and elastin fibers of the vascular network of the decellularized liver matrix were intact following whole liver decellularization.7 A corrosion cast of the liver matrix was created and showed the existence and preservation of the entire vascular system (portal vein, hepatic artery vasculature, central vein and biliary tract) similar to normal liver. This vascular network integrity is an important feature for subsequent endothelialization. These denuded vascular conduits must be re-endothelialized to prevent coagulation. Prevention of blood coagulation is typically difficult to achieve and failure to do so hinders long-term in vivo testing of engineered livers. Blood contact is normally restricted to the endothelium, and the coagulation cascade is initiated if blood is exposed to tissue collagen through direct and indirect interactions of collagen with platelet glycoprotein surface receptors. Unless the graft has been fully endothelialized to conceal collagen, coagulation will occur when the graft is exposed to circulating blood. Coagulation can be prevented in vivo by covering the vascular bed in the decellularized liver matrix with endothelial cells. In practice, achieving near-perfect endothelial cell coverage of the vasculature in the scaffold is challenging. A potential solution is to deposit heparin throughout the vasculature of the scaffold.
Challenges and future directions
The key limiting step for successful implementation of an engineered liver is the establishment a functional non-thrombotic vascular network throughout. Several groups have attempted the implantation of a seeded ECM construct, only to have it thrombose shortly after implantation. Furthermore, delivering all cell types to their native spatial location and recapitulating the liver's complex cellular organizations is challenging. The liver is composed of several distinct cell types, including hepatocytes, sinusoid endothelial, Kupffer, stellate and biliary epithelial cells. These cells function in a highly sophisticated and integrated manner, which is difficult to engineer in vitro. However, it is believed that liver regeneration can be promoted when seeded three-dimensional acellular liver grafts are placed in the appropriate three-dimensional microenvironment, specifically, in-situ in patients with liver failure. Studies aimed at successful implantation of an engineered liver graft in pre-clinical large animal models are in progress.
Conflicts of interest
All authors have none to declare.
References
- 1.Fuhrman C., Jougla E., Nicolau J., Eilstein D., Delmas M.C. Deaths from chronic obstructive pulmonary disease in France, 1979–2002: a multiple cause analysis. Thorax. 2006;61(11):930–934. doi: 10.1136/thx.2006.061267. PubMed PMID: 16738039; PubMed Central PMCID: PMC2121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonavita A.G., Quaresma K., Cotta-de-Almeida V. Hepatocyte xenotransplantation for treating liver disease. Xenotransplantation. 2010;17(3):181–187. doi: 10.1111/j.1399-3089.2010.00588.x. Epub 2010/07/20. PubMed PMID: 20636538. [DOI] [PubMed] [Google Scholar]
- 3.Fiegel H.C., Kaufmann P.M., Bruns H. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med. 2008;12(1):56–66. doi: 10.1111/j.1582-4934.2007.00162.x. Epub 2007/11/21. PubMed PMID: 18021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diekmann S., Bader A., Schmitmeier S. Present and future developments in hepatic tissue engineering for liver support systems: state of the art and future developments of hepatic cell culture techniques for the use in liver support systems. Cytotechnology. 2006;50(1–3):163–179. doi: 10.1007/s10616-006-6336-4. Epub 2008/11/13. PubMed PMID: 19003077; PubMed Central PMCID: PMC3476010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Lessa N., Estrada D.C. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl. 2011;17(4):418–427. doi: 10.1002/lt.22270. Epub 2011/03/30. PubMed PMID: 21445925; PubMed Central PMCID: PMC3079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barakat O., Abbasi S., Rodriguez G. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173(1):e11–e25. doi: 10.1016/j.jss.2011.09.033. Epub 2011/11/22. PubMed PMID: 22099595. [DOI] [PubMed] [Google Scholar]
- 7.Soto-Gutierrez A., Zhang L., Medberry C. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17(6):677–686. doi: 10.1089/ten.tec.2010.0698. Epub 2011/03/08. PubMed PMID: 21375407; PubMed Central PMCID: PMC3103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampey B.P., Stewart B.J., Petersen D.R. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J Biol Chem. 2007;282(3):1925–1937. doi: 10.1074/jbc.M610602200. Epub 2006/11/17. PubMed PMID: 17107949; PubMed Central PMCID: PMC2956423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uygun B.E., Soto-Gutierrez A., Yagi H. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–820. doi: 10.1038/nm.2170. Epub 2010/06/15. PubMed PMID: 20543851; PubMed Central PMCID: PMC2930603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao J., Shi Y., Sun H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 2011;20(5):753–766. doi: 10.3727/096368910X536572. Epub 2010/11/09. PubMed PMID: 21054928. [DOI] [PubMed] [Google Scholar]
- 11.Shupe T., Williams M., Brown A., Willenberg B., Petersen B.E. Method for the decellularization of intact rat liver. Organogenesis. 2010;6(2):134–136. doi: 10.4161/org.6.2.11546. Epub 2010/10/05. PubMed PMID: 20885860; PubMed Central PMCID: PMC2901817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirmalek-Sani S.H., Sullivan D.C., Zimmerman C., Shupe T.D., Petersen B.E. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol. 2013;183(2):558–565. doi: 10.1016/j.ajpath.2013.05.002. Epub 2013/06/12. PubMed PMID: 23747949; PubMed Central PMCID: PMC3730770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang R., Stern M.M., Smith L. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011;32(29):7042–7052. doi: 10.1016/j.biomaterials.2011.06.005. Epub 2011/07/05. PubMed PMID: 21723601. [DOI] [PubMed] [Google Scholar]
- 14.Song J.J., Kim S.S., Liu Z. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92(3):998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion – 6. Epub 2011/08/30. PubMed PMID: 21871290. [DOI] [PubMed] [Google Scholar]
- 15.Ott H.C., Clippinger B., Conrad C. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16(8):927–933. doi: 10.1038/nm.2193. Epub 2010/07/16. PubMed PMID: 20628374. [DOI] [PubMed] [Google Scholar]
- 16.Petersen T.H., Calle E.A., Zhao L. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–541. doi: 10.1126/science.1189345. Epub 2010/06/26. PubMed PMID: 20576850; PubMed Central PMCID: PMC3640463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortiella J., Niles J., Cantu A. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16(8):2565–2580. doi: 10.1089/ten.tea.2009.0730. Epub 2010/04/23. PubMed PMID: 20408765. [DOI] [PubMed] [Google Scholar]
- 18.Goh S.K., Bertera S., Olsen P. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34(28):6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. Epub 2013/06/22. PubMed PMID: 23787110; PubMed Central PMCID: PMC3748589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan D.C., Mirmalek-Sani S.H., Deegan D.B. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33(31):7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. PubMed PMID: 22841923. [DOI] [PubMed] [Google Scholar]
- 20.Song J.J., Guyette J.P., Gilpin S.E. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–651. doi: 10.1038/nm.3154. Epub 2013/04/16. PubMed PMID: 23584091; PubMed Central PMCID: PMC3650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathare D.B., Jadhav A.S., Shingare M.S. Validated chiral liquid chromatographic method for the enantiomeric separation of pramipexole dihydrochloride monohydrate. J Pharm Biomed Anal. 2006;41(4):1152–1156. doi: 10.1016/j.jpba.2006.02.024. Epub 2006/04/04. PubMed PMID: 16580170. [DOI] [PubMed] [Google Scholar]
- 22.Jadhav J.P., Govindwar S.P. Biotransformation of malachite green by Saccharomyces cerevisiae MTCC 463. Yeast. 2006;23(4):315–323. doi: 10.1002/yea.1356. Epub 2006/03/18. PubMed PMID: 16544273. [DOI] [PubMed] [Google Scholar]
- 23.Solaroglu I., Cahill J., Jadhav V., Zhang J.H. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37(4):1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. Epub 2006/03/04. PubMed PMID: 16514095. [DOI] [PubMed] [Google Scholar]
- 24.Suryawanshi N.P., Bhutey A.K., Nagdeote A.N., Jadhav A.A., Manoorkar G.S. Study of lipid peroxide and lipid profile in diabetes mellitus. Indian J Clin Biochem. 2006;21(1):126–130. doi: 10.1007/BF02913080. Epub 2006/03/01. PubMed PMID: 23105583; PubMed Central PMCID: PMC3453770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner N.J., Badylak S.F. Biologic scaffolds for musculotendinous tissue repair. Eur Cells Mater. 2013;25:130–143. doi: 10.22203/ecm.v025a09. PubMed PMID: 23329468. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani F., Trinchieri A., Castelnuovo C., Romano A.L., Pisani E. Reconstructive urethroplasty using porcine acellular matrix. Eur Urol. 2003;44(5):600–602. doi: 10.1016/s0302-2838(03)00212-4. PubMed PMID: 14572761. [DOI] [PubMed] [Google Scholar]
- 27.Crisan M., Yap S., Casteilla L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. PubMed PMID: 18786417. [DOI] [PubMed] [Google Scholar]
- 28.Londono R., Jobe B.A., Hoppo T., Badylak S.F. Esophagus and regenerative medicine. World J Gastroenterol. 2012;18(47):6894–6899. doi: 10.3748/wjg.v18.i47.6894. PubMed PMID: 23322986; PubMed Central PMCID: PMC3531672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. Epub 2011/02/08. PubMed PMID: 21296410; PubMed Central PMCID: PMC3084613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert T.W., Sellaro T.L., Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. Epub 2006/03/08. PubMed PMID: 16519932. [DOI] [PubMed] [Google Scholar]
- 31.Valentin J.E., Badylak J.S., McCabe G.P., Badylak S.F. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. PubMed PMID: 17142418. [DOI] [PubMed] [Google Scholar]
- 32.Beattie A.J., Gilbert T.W., Guyot J.P., Yates A.J., Badylak S.F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15(5):1119–1125. doi: 10.1089/ten.tea.2008.0162. Epub 2008/10/08. PubMed PMID: 18837648; PubMed Central PMCID: PMC2789572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smart N.J., Marshall M., Daniels I.R. Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon. 2012;10(3):159–171. doi: 10.1016/j.surge.2012.02.006. Epub 2012/03/23. PubMed PMID: 22436406. [DOI] [PubMed] [Google Scholar]
- 34.Valentin J.E., Stewart-Akers A.M., Gilbert T.W., Badylak S.F. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15(7):1687–1694. doi: 10.1089/ten.tea.2008.0419. Epub 2009/01/08. PubMed PMID: 19125644; PubMed Central PMCID: PMC2792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brennan E.P., Tang X.H., Stewart-Akers A.M., Gudas L.J., Badylak S.F. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2(8):491–498. doi: 10.1002/term.123. Epub 2008/10/29. PubMed PMID: 18956412; PubMed Central PMCID: PMC2706581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrawal V., Johnson S.A., Reing J. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A. 2010;107(8):3351–3355. doi: 10.1073/pnas.0905851106. Epub 2009/12/08. PubMed PMID: 19966310; PubMed Central PMCID: PMC2840465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reing J.E., Zhang L., Myers-Irvin J. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15(3):605–614. doi: 10.1089/ten.tea.2007.0425. Epub 2008/07/26. PubMed PMID: 18652541. [DOI] [PubMed] [Google Scholar]
- 38.Keane T.J., Londono R., Turner N.J., Badylak S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33(6):1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. Epub 2011/12/06. PubMed PMID: 22137126. [DOI] [PubMed] [Google Scholar]
- 39.Mase V.J., Jr., Hsu J.R., Wolf S.E. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. doi: 10.3928/01477447-20100526-24. Epub 2010/07/09. PubMed PMID: 20608620. [DOI] [PubMed] [Google Scholar]
- 40.Nieponice A., McGrath K., Qureshi I. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69(2):289–296. doi: 10.1016/j.gie.2008.04.022. Epub 2008/07/29. PubMed PMID: 18657808. [DOI] [PubMed] [Google Scholar]
- 41.Badylak S.F., Vorp D.A., Spievack A.R. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128(1):87–97. doi: 10.1016/j.jss.2005.03.002. Epub 2005/06/01. PubMed PMID: 15922361. [DOI] [PubMed] [Google Scholar]
- 42.Badylak S.F., Hoppo T., Nieponice A. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17(11–12):1643–1650. doi: 10.1089/ten.tea.2010.0739. Epub 2011/02/11. PubMed PMID: 21306292; PubMed Central PMCID: PMC3098955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badylak S., Meurling S., Chen M., Spievack A., Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35(7):1097–1103. doi: 10.1053/jpsu.2000.7834. Epub 2000/08/05. PubMed PMID: 10917304. [DOI] [PubMed] [Google Scholar]
- 44.Boruch A.V., Nieponice A., Qureshi I.R., Gilbert T.W., Badylak S.F. Constructive remodeling of biologic scaffolds is dependent on early exposure to physiologic bladder filling in a canine partial cystectomy model. J Surg Res. 2010;161(2):217–225. doi: 10.1016/j.jss.2009.02.014. Epub 2009/07/07. PubMed PMID: 19577253. [DOI] [PubMed] [Google Scholar]
- 45.Kropp B.P., Badylak S., Thor K.B. Regenerative bladder augmentation: a review of the initial preclinical studies with porcine small intestinal submucosa. Adv Exp Med Biol. 1995;385:229–235. doi: 10.1007/978-1-4899-1585-6_28. Epub 1995/01/01. PubMed PMID: 8571835. [DOI] [PubMed] [Google Scholar]
- 46.Li F., Li W., Johnson S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11(3–4):199–206. doi: 10.1080/10623320490512390. PubMed PMID: 15370297. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal V., Tottey S., Johnson S.A. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17(19–20):2435–2443. doi: 10.1089/ten.tea.2011.0036. Epub 2011/05/14. PubMed PMID: 21563860; PubMed Central PMCID: PMC3179613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crapo P.M., Medberry C.J., Reing J.E. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33(13):3539–3547. doi: 10.1016/j.biomaterials.2012.01.044. Epub 2012/02/22. doi: S0142-9612(12)00090-7 [pii]10.1016/j.biomaterials.2012.01.044. PubMed PMID: 22341938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeQuach J.A., Yuan S.H., Goldstein L.S., Christman K.L. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A. 2011;17(21–22):2583–2592. doi: 10.1089/ten.tea.2010.0724. Epub 2011/09/03. PubMed PMID: 21883047; PubMed Central PMCID: PMC3204197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tottey S., Corselli M., Jeffries E.M. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17(1–2):37–44. doi: 10.1089/ten.tea.2010.0188. Epub 2010/07/27. PubMed PMID: 20653348; PubMed Central PMCID: PMC3011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voytik-Harbin S.L., Brightman A.O., Kraine M.R., Waisner B., Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67(4):478–491. Epub 1998/01/24. 4<478::AID-JCB6>3.0.CO;2-P [pii]. PubMed PMID: 9383707. [PubMed] [Google Scholar]
- 52.Agrawal V., Kelly J., Tottey S. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng Part A. 2011;17(23–24):3033–3044. doi: 10.1089/ten.tea.2011.0257. Epub 2011/07/12. PubMed PMID: 21740273; PubMed Central PMCID: PMC3226059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–1842. doi: 10.1089/ten.tea.2007.0264. Epub 2008/10/28. PubMed PMID: 18950271. [DOI] [PubMed] [Google Scholar]
- 54.Badylak S.F., Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–116. doi: 10.1016/j.smim.2007.11.003. Epub 2007/12/18. PubMed PMID: 18083531; PubMed Central PMCID: PMC2605275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown B.N., Valentin J.E., Stewart-Akers A.M., McCabe G.P., Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. Epub 2009/01/06. PubMed PMID: 19121538; PubMed Central PMCID: PMC2805023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown B.N., Londono R., Tottey S. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8(3):978–987. doi: 10.1016/j.actbio.2011.11.031. Epub 2011/12/15. PubMed PMID: 22166681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown B.N., Ratner B.D., Goodman S.B., Amar S., Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. Epub 2012/03/06. PubMed PMID: 22386919; PubMed Central PMCID: PMC3727238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner A.B., Lee S.K., Woods E.C., Acharya A.P. Biomaterials-based modulation of the immune system. Biomed Res Int. 2013;2013:732182. doi: 10.1155/2013/732182. Epub 2013/10/31. PubMed PMID: 24171170; PubMed Central PMCID: PMC3793288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert T.W., Stewart-Akers A.M., Simmons-Byrd A., Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89(3):621–630. doi: 10.2106/JBJS.E.00742. Epub 2007/03/03. PubMed PMID: 17332112. [DOI] [PubMed] [Google Scholar]
- 60.Badylak S.F. An assay to quantify chemotactic properties of degradation products from extracellular matrix. Methods Mol Biol. 2013 doi: 10.1007/7651_2013_37. Epub 2013/10/25. PubMed PMID: 24155230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brennan E.P., Reing J., Chew D. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12(10):2949–2955. doi: 10.1089/ten.2006.12.2949. Epub 2007/05/24. PubMed PMID: 17518662; PubMed Central PMCID: PMC3056877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin P., Chan W.C., Badylak S.F., Bhatia S.N. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10(7–8):1046–1053. doi: 10.1089/ten.2004.10.1046. Epub 2004/09/15. PubMed PMID: 15363162. [DOI] [PubMed] [Google Scholar]
- 63.Sellaro T.L., Ranade A., Faulk D.M. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16(3):1075–1082. doi: 10.1089/ten.tea.2008.0587. Epub 2009/10/23. PubMed PMID: 19845461; PubMed Central PMCID: PMC2863084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sellaro T.L., Ravindra A.K., Stolz D.B., Badylak S.F. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13(9):2301–2310. doi: 10.1089/ten.2006.0437. Epub 2007/06/15. PubMed PMID: 17561801. [DOI] [PubMed] [Google Scholar]
- 65.Weymann A., Loganathan S., Takahashi H. Development and evaluation of a perfusion decellularization porcine heart model–generation of 3-dimensional myocardial neoscaffolds. Circ J. 2011;75(4):852–860. doi: 10.1253/circj.cj-10-0717. official journal of the Japanese Circulation Society. PubMed PMID: 21301134. [DOI] [PubMed] [Google Scholar]
- 66.Ott H.C., Matthiesen T.S., Goh S.K. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. PubMed PMID: 18193059. [DOI] [PubMed] [Google Scholar]
- 67.Wainwright J.M., Czajka C.A., Patel U.B. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16(3):525–532. doi: 10.1089/ten.tec.2009.0392. PubMed PMID: 19702513; PubMed Central PMCID: PMC2945869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keane T.J., Londono R., Carey R.M. Preparation and characterization of a biologic scaffold from esophageal mucosa. Biomaterials. 2013;34(28):6729–6737. doi: 10.1016/j.biomaterials.2013.05.052. Epub 2013/06/20. PubMed PMID: 23777917; PubMed Central PMCID: PMC3727430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baptista P.M., Siddiqui M.M., Lozier G. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–617. doi: 10.1002/hep.24067. Epub 2011/01/29. PubMed PMID: 21274881. [DOI] [PubMed] [Google Scholar]
- 75.Hudson T.W., Liu S.Y., Schmidt C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9–10):1346–1358. doi: 10.1089/ten.2004.10.1641. Epub 2004/12/14. PubMed PMID: 15588395. [DOI] [PubMed] [Google Scholar]
- 76.Courtman D.W., Pereira C.A., Kashef V. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28(6):655–666. doi: 10.1002/jbm.820280602. Epub 1994/06/01. PubMed PMID: 8071376. [DOI] [PubMed] [Google Scholar]
- 77.Brown B., Lindberg K., Reing J., Stolz D.B., Badylak S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(3):519–526. doi: 10.1089/ten.2006.12.519. Epub 2006/04/04. PubMed PMID: 16579685. [DOI] [PubMed] [Google Scholar]
- 78.Kasimir M.T., Rieder E., Seebacher G. Comparison of different decellularization procedures of porcine heart valves. Int J Artif Organs. 2003;26(5):421–427. doi: 10.1177/039139880302600508. Epub 2003/06/28. PubMed PMID: 12828309. [DOI] [PubMed] [Google Scholar]
- 79.Faulk D.M., Carruthers C.A., Warner H.J. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10(1):183–193. doi: 10.1016/j.actbio.2013.09.006. Epub 2013/09/24. PubMed PMID: 24055455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161(III-XIII):1–151. doi: 10.1007/978-3-642-56553-3. PubMed PMID: 11729749. [DOI] [PubMed] [Google Scholar]
- 81.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog. 1998;14(3):378–387. doi: 10.1021/bp980036j. Epub 1998/06/17. PubMed PMID: 9622518. [DOI] [PubMed] [Google Scholar]
- 82.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. Epub 1999/11/02. PubMed PMID: 10544172. [DOI] [PubMed] [Google Scholar]


