Many of the actions of estradiol in the central nervous system (CNS) are mediated via intracellular estrogen receptors (ERs)/transcription factors that interact with steroid response elements on target genes. In addition, there is compelling evidence for membrane-associated ERs in hypothalamic and other CNS neuron; but it is not well understood how estradiol signals via membrane-associated receptors and how these signals impact neuronal excitability and physiological functions. It has been known for some time that estradiol can rapidly alter neuronal activity within seconds, indicating that some cellular effects can occur via membrane initiated events. Thus, estradiol can affect second messenger systems, including calcium mobilization and a plethora of kinases to alter cell excitability and even gene transcription in hypothalamic neurons. Therefore, this minireview will summarize our current knowledge of rapid membrane-initiated and intracellular signaling by estradiol in the hypothalamus, the nature of receptors involved, and how they contribute not only to control of reproduction but other vital homeostatic functions.
Estrogen Neurobiology
17β-estradiol (E2) modulates hypothalamic neuronal excitability that ultimately regulates reproduction, energy balance, temperature, circadian rhythms, and stress. In addition, E2 is involved in neuronal synaptic plasticity in the hippocampus, striatum, and cerebellum (1–3). Obviously, E2 signaling in the hypothalamus is the quintessential function that controls reproduction (4). In females, E2 signaling in the hypothalamus is the basis of positive and negative feedback within the hypothalamic-pituitary-ovarian axis. The endocrine status of gonads is communicated to the brain by circulating E2 that activates hypothalamic circuits that regulate ovulation. E2 both inhibits and stimulates the release of GnRH and LH, as well as FSH, and stimulates sexual behavior. E2 binds to and activates the classical ERα and ERβ but also G protein-coupled metabotropic receptors.
Classically, ERs were defined by their ability to bind estrogens and elicit a specific response (5). They were considered cytosolic receptors that upon E2 binding underwent a conformational change and translocation to the nucleus, where they interacted with DNA to regulate the expression of targeted genes. Subsequently, ERα (ESR1) and ERβ (ESR2) were cloned in the 1980s and 1990s, respectively (6, 7). Although they are the product of different genes, ERα and ERβ share a similar modular structure that binds E2 and have significant sequence homology, especially in their DNA and ligand binding domains. Also, ERα and ERβ interact with other transcription factors, such as Fos and Jun, which bind DNA at the activator protein-1 (AP-1) site, to regulate transcription independent of estrogen-response elements (EREs) (8).
Another parallel line of research developed in the 1970s that implicated E2 in rapid, nongenomic actions in numerous neuronal and nonneuronal cells: E2 membrane signaling rapidly increased levels of cAMP in the uterus (9), altered firing of hypothalamic neurons within seconds (10), and the release of neuropeptides (11). However, the concept of “rapid” nongenomic effects for estrogen signaling was foreign to endocrinologists. Although E2 elicited effects on hypothalamic and hippocampal neurons at subnanomolar concentrations, there did not appear to be identifiable steroid receptors associated with the plasma membrane for mediating these rapid actions (12, 13). This changed in the 1990s, when membrane localization of ERα was documented in pituitary cells and primary cultures of hippocampal CA1 neurons (14, 15). Moreover, Razandi et al (16) discovered that nuclear and membrane receptors were encoded by the same ER genes, and ERα and ERβ were shown to complex with G protein signaling cascades. In addition, several groups had identified membrane ERs (mERs) that were not derived from ERα or ERβ transcripts (17–19), including a bona fide G protein-coupled receptor (GPCR), GPR30/GPER1 (20, 21). It was evident from the investigation of “nongenomic” signaling that although some of these signaling cascades initiated at the membrane were tied to rapid membrane effects on ion channel activity, others led to the regulation of gene transcription, similar to the membrane-to-nucleus signaling described for many neurotransmitters (22). In this light, it has been more accurate to differentiate between membrane-initiated signaling and nuclear-initiated signaling when discussing hormone actions in neurons and nonneural cells (23). Therefore, this minireview will summarize the role of mERs in hypothalamic functions, keeping in mind that similar membrane-initiated actions of E2 have been documented in other brain structures, such as the hippocampus, striatum and cerebellum, CNS structures involved in cognition and motor functions, respectively (1–3).
Nuclear-Initiated Signaling of E2
Early studies used 3H-E2 to explore binding sites in the brain, and these studies revealed that estradiol-concentrating neurons were localized in hypothalamic regions, including the preoptic area (POA), periventricular nucleus (PV), and arcuate nucleus (24–28). Once ERα and ERβ were cloned, their distribution was thoroughly elucidated using in situ hybridization and/or immunocytochemistry (29–39). ERα is robustly expressed in regions such as the POA, bed nucleus stria terminalis, amygdala, PV, ventrolateral part of the ventromedial nucleus of the hypothalamus, and the arcuate nucleus. ERβ is found in many of the same regions, but is more highly expressed in the bed nucleus stria terminalis, POA, PV of the hypothalamus, and supraoptic nuclei, with some notable species differences (32, 33, 39–41). ERα and ERβ are also found in other brain regions, including the cortex, hippocampus, midbrain, and striatum (32, 42). Colocalization studies have identified ERα in hypothalamic neurons containing gamma-Aminobutyric acid (GABA), neurotensin, somatostatin, galanin, dopamine, norepinephrine, neuropeptide Y (NPY), proopiomelanocortin (POMC), and kisspeptin (Kiss1) (33, 43–50). ERβ is expressed in different populations of hypothalamic neurons: GnRH, vasopressin, oxytocin, and nociceptin/orphanin FQ, as well as in midbrain serotonin neurons (51–60). ERα and ERβ are colocalized in neurons expressing corticotropin-releasing hormone and IGF-1, as well as in subpopulations of unidentified hypothalamic neurons (37, 52, 61, 62).
The nuclear-initiated signaling of estradiol via ERα and ERβ exerts diverse effects in a number of tissues that involves gene stimulation as well as gene repression (63–68). In general, the “classical” signaling pathway of E2 involves steroid-dependent formation of nuclear ER homo- or heterodimers and the subsequent binding of this complex with a unique DNA sequence known as an ERE, in E2-responsive gene promoters and enhancers (69–71).
However, many genes in the brain that are estrogen-responsive do not appear to contain ERE sequences (71, 72). There is compelling evidence that ERα and ERβ can regulate transcription of some of these “estrogen-responsive” genes by interacting with other DNA-bound transcription factors, such as specificity protein-1 and AP-1, rather than binding directly to DNA (71, 73, 74). In contrast to ERα, the ligand-induced responses with ERβ at an AP-1 site illustrate the negative transcriptional regulation by estrogens and strong positive regulation by ER antagonists like ICI 164384 (73). In addition, Kiss1 mRNA is differentially regulated by E2 in the anteroventral periventricular preoptic area and arcuate nucleus. Although the positive E2 regulation of Kiss1 mRNA expression in the AVPV is dependent on an ERE-binding site, the down-regulation of Kiss1 mRNA in the arcuate nucleus is via an ERE-independent mechanism (75). Therefore, there are potentially multiple mechanisms for differential regulation of gene expression by E2 via nuclear-initiated signaling.
Membrane-Initiated Signaling of E2
Selective membrane binding sites for E2 were first identified on endometrial cells (76, 77), and later studies revealed relatively high affinity, specific binding of [3H]-E2 to synaptosomal membranes prepared from the adult rat brain (78). The CNS binding was later corroborated using the membrane impermeant E2–6-[125I] conjugated to BSA (79). Furthermore, competition-binding assays of synaptosomal membranes showed that the hypothalamus exhibited a relatively high-affinity (3nM) binding site for E2 and somewhat lower affinity binding sites in the olfactory bulb and cerebellum (80, 81). The stereospecificity of the binding was demonstrated by displacement of the radiolabeled E2 with cold E2 or E2-BSA, but not by 17α-estradiol or 17α-estradiol-BSA even at micromolar concentrations (80).
In parallel electrophysiological studies, E2 was shown to have acute, rapid membrane-initiated signaling actions in many CNS structures, including the hypothalamus (10, 82–89). Three decades ago, the nature and physiological significance of these actions were a matter of debate, but it is now generally accepted that some of the actions of E2 are too fast to be attributed to the classical nuclear-initiated steroid signaling of ERα or ERβ. We now know that ERα and ERβ can associate with signaling complexes in the plasma membrane (16, 90–94). In addition, many of the rapid effects of E2 can be induced by selective ERα or ERβ ligands, antagonized by the ER antagonist ICI 182780, and abrogated in animals bearing mutations in ERα and/or ERβ genes (64, 90, 95–99). However, it is also evident that E2 can activate bona fide GPCRs, the most notable GPR30 and a putative Gαq-coupled mER (Gαq-mER) (18, 19, 100–105).
Over the years, evidence has been generated in the support of a novel Gαq-mER. Intracellular sharp electrode and whole-cell patch recording from guinea pig and mouse hypothalamic slices were used to characterize this Gαq-mER (13, 18, 19). These 2 independent electrophysiological methods established that E2 acts rapidly and stereospecifically within physiologically relevant concentrations to significantly reduce the potency of μ-opioid and GABAB agonists (ie, heterologous desensitization) to activate G protein-coupled inwardly rectifying K+ (GIRK) channels (Figure 1) (13, 18). Estrogenic desensitization of μ-opioid and GABAB receptors was mimicked by stimulation of adenylyl cyclase with forskolin or by direct protein kinase A (PKA) activation with the nonhydrolyzable cAMP analog Sp-Cyclic 3′,5′-hydrogen phosphorothioate adenosine hydrate, in a concentration-dependent manner (13, 18). Furthermore, selective PKA antagonists blocked the effects of E2. As predicted from the literature on desensitization of GPCRs (106), PKA is downstream in a signaling cascade that is initiated by a Gαq-mER that is linked to activation of phospholipase C (PLC)-protein kinase C (PKC)-PKA (18, 19). E2 does not alter the affinity of the μ-opioid and GABAB ligands for their respective receptors (107). Furthermore, the ER antagonists ICI 164384 and ICI 182780 block the actions of E2 with subnanomolar affinity (Ki = 0.5nM), which is similar to Ki for antagonism of ERα (13, 108). These pharmacological findings clearly argue for a G protein-coupled membrane receptor with high selectivity for E2.
Figure 1.
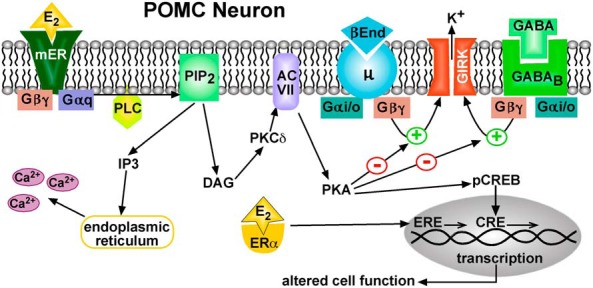
Cellular actions of E2 in POMC neurons. Schematic overview of the E2-mediated modulation of Gαi,o-coupled (μ-opioid and GABAB) receptors via a membrane-associated receptor (mER) in hypothalamic POMC neurons. E2 binds to a mER that is Gαq-coupled to activate phospholipase C (PLC) and catalyzes the hydrolysis of membrane-bound phosphatidylinositol 4,5-biphosphate (PIP2) to inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG). Calcium is released from intracellular stores (endoplasmic reticulum) by IP3, and DAG activates PKCδ. Through phosphorylation, adenylyl cyclase VII (AC VII) activity is up-regulated by PKC. The generation of cAMP activates protein kinase A (PKA), which can uncouple μ-opioid (μ) and GABAB receptors from their signaling pathway through phosphorylation of a downstream effector molecule (eg, G protein-coupled, inwardly rectifying K+, GIRK, channels). PKA can also potentially phosphorylate other channels (eg, canonical transient receptor potential [TRPC] channels) to alter their function (data not shown). In addition, the membrane-initiated E2 signaling through PKA can phosphorylate cAMP-responsive element binding protein (pCREB), which can alter gene transcription through its interaction with the cAMP response element (CRE). Therefore, the rapid membrane-initiated signaling can alter gene expression in an ERE-independent fashion.
The results from these early physiological and pharmacological experiments led to the design of a synthetic diphenylacrylamide compound called STX (18), which is structurally similar to 4-OH tamoxifen, for selectively targeting the Gαq-mER signaling pathway (18). As predicted, STX has greater affinity (∼20-fold) for the Gαq-mER than E2 and most importantly does not bind to ERα or ERβ (19, 109). Furthermore, both STX and E2 activate the Gαq signaling pathway in POMC neurons in mice lacking both ERα and ERβ and in GPR30 knockout mice (19, 110).
Although somewhat controversial as a stand-alone ER (111), some of the effects of E2 in the CNS are thought to be mediated via GPR30 (103, 105, 112). Initial studies showed specific binding of E2 to membranes isolated from ERα−, ERβ−, and GPR30+ (SKBR3) breast cancer cells and E2-mediated stimulation of a Gαs-coupled signaling pathway (113). The signaling pathway is somewhat circuitous and involves E2 activating Gβγ-subunits that promote the release and activation of an epidermal growth factor (EGF) precursor pro-heparin binding EGF (pro-HB-EGF) (20, 114, 115). The active HB-EGF binds to the EGF receptor to facilitate receptor dimerization and downstream activation of MAPK. Although GPR30 protein and mRNA have been localized in several brain regions, including the hypothalamus by immunocytochemical staining and RT-PCR, respectively, definitive binding studies on isolated neural membranes have not been done (103, 116–119). With respect to hypothalamic function, several studies have shown that GPR30 is involved in mediating rapid actions of E2 in monkey placode GnRH neurons (103) and adult mouse GnRH neurons (120) (see below). Therefore, E2 can rapidly alter neuronal excitability through ERα, ERβ, and/or novel GPCRs.
E2 Signaling in GnRH Neurons
The relatively fast (∼15 min) inhibition of GnRH and LH secretion by systemic administration of E2 to ovariectomized females is compatible with a membrane initiated signaling mechanism. In fact, years ago, it was found that guinea pig GnRH neurons are rapidly hyperpolarized by E2 via activation of an inwardly rectifying K+ conductance in the presence of tetrodotoxin, which blocks fast Na+ channel activity and isolates GnRH neurons from action potential-driven synaptic inputs (86, 121). In mice, physiological concentrations (picomolar) of E2 rapidly augment KATP (also of the inwardly rectifying family) channel activity to hyperpolarize GnRH neurons and inhibit their firing (104). E2 signals via a PKC-PKA pathway, and the selective Gαq-mER ligand STX is also able to mimic the effects of E2 in GnRH neurons (13, 18, 19, 104). Both the effects of E2 and STX are abrogated by the ER antagonist ICI 182780 with a Ki of 0.5nM (104, 122), which is similar to the Ki for antagonism of ERα in peripheral tissues (108). Female mice treated with an E2 implant for 4–7 days continue to exhibit an augmented KATP current, which indicates that the membrane-initiated signaling cascade persist over this time period (123). This would imply that membrane-initiated signaling by E2 is involved in negative feedback regulation of GnRH neurons.
In rhesus macaque vomeronasal explants (the source of immature GnRH neurons), E2 synchronizes Ca2+ oscillations in GnRH neurons to a periodicity of approximately 60 minutes, similar to pulsatile GnRH release in primates (124). Knockdown of ERα and ERβ do not block the E2-induced changes in Ca2+ oscillations (125), and nanomolar concentrations of a membrane-impermeant E2 (E2-dendrimer conjugate), the GPR30 agonist G1 or the Gαq-mER ligand STX stimulate Ca2+ oscillations within 10–20 minutes (103, 105, 126). Therefore, similar to E2, G1 and STX increase the frequency and synchronization of Ca2+ oscillations in rhesus macaque GnRH neurons (103, 105, 125). The effects of E2 (1nM) are eliminated in GnRH neurons transfected with GPR30 small interfering RNA (siRNA), confirming that E2 can signal via GPR30 (103). However, higher concentrations of E2 (10nM) are only partially blocked by GPR30 siRNA transfection, indicating that E2 is also signaling via other receptors (105). Indeed, STX (10nM) increases the Ca2+ oscillations, synchronization, and GnRH release from macaque vomeronasal explants, all of which are not altered by GPR30 siRNA transfection (105). Importantly, the effects of STX are blocked by the ER antagonist ICI 182780 and by a PLC inhibitor (105). This would suggest that E2 signaling via GPR30 and a putative Gαq-mER in rhesus macaque GnRH neurons rapidly increases calcium oscillations and synchronization, and stimulates GnRH release.
In mouse GnRH neurons, E2 acts postsynaptically via ERβ or presynaptically via ERα to rapidly alter GnRH neuronal excitability. Postsynaptically, high physiological (picomolar to nanomolar) concentrations of E2 or the selective ERβ ligand diarylpropionitrile enhance action potential firing by modulating intrinsic after hyperpolarizing and after depolarizing potentials via a PKA-dependent mechanism (127). In addition, E2 rapidly potentiates high-voltage-activated Ca2+ currents (L- and R-type Ca2+ channels) via ERβ and GPR30, suggesting that Ca2+ signaling is also a target for membrane-initiated signaling actions in GnRH neurons (120). In contrast, low physiological (10pM) concentrations of E2 or the selective ERα ligand propylpyrazole triol reduced action potential firing in GnRH neurons by attenuating excitatory GABAergic input (127). Collectively, these findings indicate that the rapid actions of E2 on GnRH neurons involve several membrane-associated receptors (ie, ERα, ERβ, GPR30, and Gαq-mER) that play a critical role in modulating GnRH neuronal excitability.
E2 Signaling and Sexual Receptivity
Female sexual receptivity in rats is controlled by E2 through a hypothalamic circuitry that encompasses the medial preoptic nucleus (MPN), the arcuate nucleus, and the ventromedial nucleus of the hypothalamus (89, 128). Although long considered a nuclear-mediated event, recent evidence indicates that membrane-initiated signaling of E2 is important for sexual behavior in female rats (see Refs. 129–131 for review). One of the more prominent pathways involves E2 activating an ERα complex that transactivates metabotropic glutamate type 1a receptors (mGluR1as) in the arcuate nucleus. mGluR1a receptors through a Gαq-mediated signaling cascade excite NPY/agouti-related peptide (AgRP) neurons, which indirectly excite POMC neurons (89). The MPN-projecting POMC neurons release the peptide β-endorphin that activates μ-opioid receptors, which subsequently internalize in MPN neurons (132–134). This transient activation/internalization of μ-opioid receptors in the MPN appears to be necessary for full expression of sexual receptivity measured 30–48 hours after E2 treatment (135). Infusion of a membrane-impermeant E2 or a mGluR1a agonist into the arcuate nucleus stimulates μ-opioid receptor internalization (93). More recently, Christensen and Micevych (136) showed that infusions of STX into arcuate nucleus activate and internalize μ-opioid receptors in the MPN and augment sexual receptivity in female rats given a “subbehavioral” dose of E2. These actions are blocked by arcuate infusions of an mGluR1a antagonist, which suggests that Gαq-mER can collaborate with metabotropic glutamate receptors to promote sexual receptivity in female rats.
E2 Signaling in POMC and NPY/AgRP Neurons
Energy homeostasis
Hypothalamic POMC and NPY/AgRP neurons reside within the arcuate nucleus and compose a critical circuit for regulating energy homeostasis (137). Selective optogenetic stimulation of NPY/AgRP neurons evokes intense feeding (138), and ablation of these neurons in adults causes starvation (139–141). Similar genetic approaches have revealed that POMC cells have the exact opposite actions in the control of energy homeostasis (19, 138, 142–145). Moreover, these 2 populations of neurons regulate feeding through sensing circulating levels of metabolic hormones, thereby altering their firing frequency and the release of peptide and/or amino acid neurotransmitters onto target neurons in the paraventricular nucleus (PVN) and other hypothalamic nuclei (137).
POMC and NPY/AgRP neurons are also sensitive to circulating estrogens. Experiments dating back 30–40 years determined that the anorectic effects of E2 in rodents are mediated through CNS sites of action because direct injections of E2 into the arcuate/ventromedial nucleus were effective to reduce food intake, body weight and increase wheel running activity in females (146–148). Additional experiments demonstrated that E2 up-regulates the expression of the peptide β-endorphin in POMC neurons in ovariectomized female guinea pigs (149, 150). Furthermore, postmortem studies showed that there is a decrease in hypothalamic β-endorphin expression associated with weight gain in postmenopausal women who abstained from hormone replacement therapy (151). More recently it has been shown that E2 signaling via ERα is a critical component in the regulation of energy homeostasis (152). In rodents, hypoestrogenic states are clearly associated with decreased activity and an increase in body weight (19, 148, 153–159). In humans, a loss-of-function mutation in ERα, reported in a case history from 1 male individual, resulted in a clear metabolic phenotype with expression of type 2 diabetes, hyperinsulinemia, and obesity (160). However, global reinstatement of an ERα that is lacking the ERE targeting domain is sufficient for “rescuing” the metabolic deficits in mice (161). These findings suggest an important role for non-ERE-mediated E2 signaling, albeit this could be via other transcriptional activity (162) or membrane-initiated signaling of ERα. Indeed, a point mutation in ERα (Cysteine 451 to Alanine), which precludes palmitoylation and membrane trafficking of ERα globally, creates an obese phenotype with excessive visceral fat deposition in mice fed a normal chow diet (163). Moreover, brain-specific knockout of ERα causes hyperphagia and hypometabolism (142, 164), and selective knockdown of ERα in POMC neurons recapitulates the hyperphagic phenotype in female mice (142). However, one must be cautious in interpreting global and conditional ERα gene deletion experiments, because ERα is a transcription factor affecting the expression of hundreds of genes important for cell signaling, and many of these genes are essential for membrane initiated actions of E2 that contribute to POMC excitability and hence control of energy homeostasis (72). With this caveat in mind, it appears that the arcuate nucleus and specifically POMC neurons are major targets for the anorectic actions of estrogens via ERα signaling, which underscores their importance in the control of energy homeostasis. In addition, POMC neurons are also involved in the rewarding aspects of food intake (165, 166).
The ability of STX to robustly mimic the rapid effects of E2 on POMC neuronal activity led to the hypothesis that the putative Gαq-mER may also have a role in the control of energy homeostasis. Peripheral administration of STX was found to mimic the effects of E2 in controlling energy homeostasis (Figure 2) (19, 167, 168). Both E2 and STX reduce food intake, body weight gain, and abdominal fat accumulation after ovariectomy. The reduced food intake was shown to correlate primarily with reduced meal frequency in both E2- and STX-treated ovariectomized guinea pigs (168).
Figure 2.
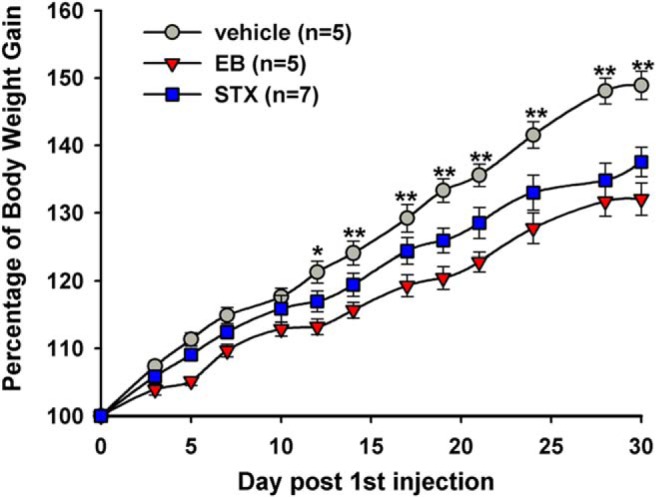
E2 and STX attenuate the body weight gain in female guinea pigs after ovariectomy. Ovariectomized female guinea pigs were given bidaily sc injections of vehicle, E2 benzoate (EB), or STX starting on day 0 (1 week after ovariectomy). Both EB and STX significantly decreased body weight gain (two-way ANOVA overall significant effect, P < .001), and post hoc Newman-Keuls analysis revealed daily significant differences between EB and vehicle-treated, and STX and oil-treated groups (**, P < .01). (Reproduced from Roepke et al [167].)
We recently discovered a complementary Gαq-mER-mediated anorexigenic pathway in NPY/AgRP neurons. E2 and the Gαq-mER ligand STX rapidly enhance (sensitize) the GABAB receptor agonist baclofen activation of GIRK channels, and this effect is blocked by the ER antagonist ICI 182780 (169). We also identified an ERα-mediated signaling pathway that opposes the Gαq-mER signaling cascade: activating ERα with the selective agonist propylpyrazole triol rapidly suppresses GABAB-mediated activation of GIRK channels in NPY/AgRP neurons (169). In gonadectomized mice, the “nonselective” ligand E2 can either enhance or suppress GABAB-mediated currents (169). However, coadministering phosphatidylinositol 3 kinase (PI3K) inhibitors, specifically a selective inhibitor of p110β, results in E2 enhancing the GABAB receptor-mediated response. Thus, ERα via PI3K attenuates (desensitizes) the GABAB receptor-mediated response in NPY/AgRP neurons. Physiologically, the effects of E2 could depend on the relative expression of ERα vs Gαq-mER in these orexigenic cells. NPY/AgRP neurons may serve a metabolic function when Gαq-mER expression predominates, whereas they may serve a reproductive function when more ERα is expressed (Figure 3) (see Ref. 170 for review).
Figure 3.
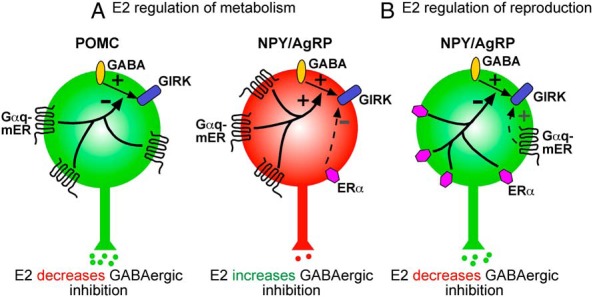
Effects of E2 and STX on hypothalamic POMC and NPY/AgRP neurons. Summary of how E2 excites POMC neurons and inhibits NPY/AgRP neurons via a Gαq-mER-mediated signaling pathway to regulate metabolism (A). Activation of the Gαq pathway attenuates the coupling of the GABAB receptor to GIRK channel activation in POMC neurons, thereby increasing the excitability, but enhances the GABAB receptor activation of GIRK channels in NPY/AgRP neurons, thereby decreasing their excitability. In NPY/AgRP neurons, ERα is also associated with the membrane, and its activation leads to attenuation of the GABAB receptor coupling to GIRK channels (B), which is hypothesized to be involved in the control of reproduction (see text for more details).
Systemic treatment with STX, similar to E2, regulates gene transcription in the arcuate nucleus, and many of the genes are involved in the control of neuronal excitability (eg, the T-type calcium channel subunit CaV3.1) and intracellular signaling in arcuate neurons (167). For example, the PI3K regulatory subunits are regulated by E2 and STX: PI3K p55γ mRNA is increased by E2 treatment (171) and PI3K p85α mRNA is up-regulated by STX (167). Therefore, the putative Gαq-mER may also function in the estrogenic control of energy homeostasis through direct excitation of POMC and inhibition of NPY/AgRP neurons through a Gαq signaling cascade to alter gene transcription in these anorexigenic and orexigenic neurons, respectively. Indeed, the electrophysiological effects of STX in NPY/AgRP neurons are consistent with the finding that STX down-regulates arcuate NPY mRNA expression in ovariectomized female guinea pigs (167).
What are the source of these high picomolar (Ki of ICI 164384 = 0.5nM) (13) concentrations of E2 to cause these rapid effects on POMC and NPY/AgRP neurons? Although circulating levels of E2 are in the low to high picomolar range, there is now compelling evidence that E2 (peak levels > 1000 pg/mL) is locally synthesized and released from the mediobasal hypothalamus of rhesus macaques (172). Neuroestrogen production has also been measured in the hippocampus, cerebellum, and brainstem (see Ref. 173 for review). Therefore, not only ovarian estrogens but locally produced E2 could activate the Gαq-mER signaling cascade to provide continued excitation of POMC neurons and inhibition of NPY/AgRP neurons. However, food intake is depressed during the preovulatory phase of the menstrual cycle in humans, monkeys, and guinea pigs that correlates with peak levels of circulating E2 (see Ref. 174 for review); and ovariectomy (or menopause) often leads to increased food intake and weight gain, which argues for a substantial role of ovarian E2 feedback in the hypothalamic control of energy homeostasis.
Thermoregulation
Another critical homeostatic function controlled by the hypothalamus and modulated by circulating estrogens is the maintenance of core body temperature (Tc) (175). In fact, approximately 80% of perimenopausal/postmenopausal women experience hot flashes (176), characterized by periods of sweating and peripheral vasodilation, and are often associated with increased environmental temperature (177). A hot flash can be characterized as an exaggerated heat dissipation response initiated by the preoptic temperature-sensitive neurons. Although the mechanisms generating this response are not well understood, repeated observations have found that most hot flashes are preceded by elevation in Tc independent of peripheral vasoconstriction or elevated metabolic rate (178–180). Therefore, it has been postulated that elevated Tc may serve as one trigger of menopausal hot flashes (177, 181). There is compelling evidence for a reduction in the incidence of hot flashes in hypoestrogenic females with E2 treatment (181–183). The specific cellular mechanisms underlying actions of estrogens are unknown. However, E2 lowers Tc and in addition reduces hot flashes by raising the Tc sweating threshold (181–183). The estrogenic reduction in the naloxone-induced rise in tail skin temperature of morphine-dependent ovariectomized rats has been the “industry standard” for a hot flash model (184–186). However, the morphine-dependence, naloxone-precipitated withdrawal rat model has a number of problems related to the adverse autonomic reactions independent of an elevation in core and skin temperature. But the model does highlight a potential role of opioids (eg, from POMC neurons) directly or indirectly on preoptic temperature-sensitive neurons, given that μ-receptors are highly expressed in the POA and warm-sensitive neurons are responsive to μ-opioid agonists (187–189).
We established a guinea pig “hot flash” model based on the hypothesis that the expression of vasomotor symptoms in menopausal women is due to an elevation in Tc and a reduced thermo-neutral zone in Tc (178–180). In the ovariectomized female guinea pig, both E2 and STX significantly reduce Tc compared with animals receiving vehicle injections (Figure 4) (168). Although the cellular target for E2 modulation of Tc has not been identified, one potential mechanism is the direct action of E2 on thermosensitive (GABAergic) neurons in the medial POA of the hypothalamus (190–192). Previous studies in ovariectomized female guinea pigs have shown that medial preoptic GABAergic (putative thermosensitive) neurons respond to acute E2 treatment via a Gαq-mER signaling pathway that attenuates GABAB inhibitory input, leading to increased neuronal excitability (193). Activation of these medial preoptic GABA neurons are responsible for evoking vasomotor responses in rodents (194) underlying heat dissipation responses (ie, vasodilatation, sweating) as seen in women experiencing hot flashes.
Figure 4.
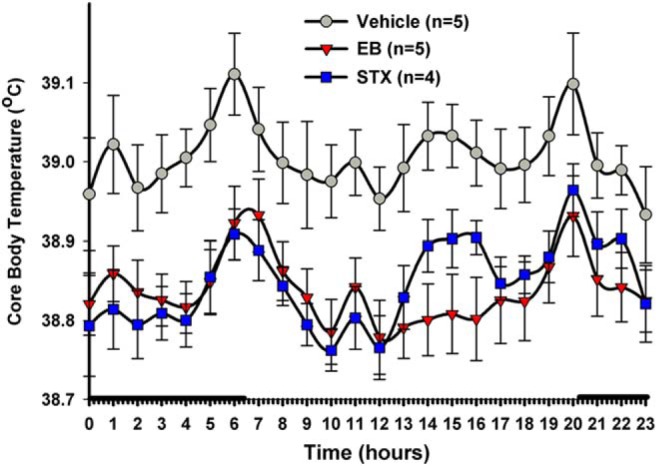
E2 and STX reduce core body temperature (Tc) of ovariectomized female guinea pigs. Similar to E2 benzoate (EB)-treated females (8 μg/kg), STX (6 mg/kg) significantly decreased Tc compared with the propylene glycol vehicle (PPG). One week after ovariectomy, females were given daily sc injections of ligands at 10 am on M, W, and F. The data are presented as the mean ± SEM for each hour of the day averaged over 3 weeks of temperature probe recordings (ie, 3 weeks of temperature probe [SubCue DataLogger] measurements were collapsed down to a 24-h period). The data were analyzed using a two-way ANOVA (P < .05, F = 4.787, df = 2) with post hoc Newman-Keuls multiple comparison tests. All data points for both STX and EB were significantly different from vehicle (P < .01) except for the STX 2 pm to 4 pm hour time points (P < .05). The solid bar above the x-axis represents lights off or nighttime. (Reproduced from Roepke et al [168].)
Bone metabolism
Recently, STX has been found to mimic E2's effects on bone density in the ovariectomized female guinea pigs (168). Although E2 has direct effects on the osteoclast/osteoblast cells involved in bone remodeling (195, 196), E2 can reduce bone loss, a hallmark of hypoestrogenic states, in part, through the preautonomic nuclei in the hypothalamus. It is known that the preautonomic PVN neurons drive sympathetic activity and are involved in bone remodeling via a β2-adrenergic receptor-dependent mechanism (197, 198). There is an increase in bone formation and a decrease in bone reabsorption in β2-adrenergic receptor knockout mice (199). Unlike wild-type mice, gonadectomy of β2-adrenergic receptor knockout mice does not alter bone mass or bone resorption parameters, indicating that increased sympathetic activity may be responsible for the bone loss in hypoestrogenic states (199). The preautonomic PVN neurons receive a robust μ-opioid receptor inhibitory input from POMC neurons (200); therefore, E2 via Gαq-mER can affect their excitability via inhibitory POMC input. We found that STX, like E2, preserves cancellous bone density after ovariectomy, which supports a potential role of this hypothalamic signaling pathway in maintenance of bone density (168). However, STX, like E2, may also have peripheral effects (eg, directly on osteoblasts), which cannot be ruled out. Certainly, the decreased PVN output would dampen the sympathetic nervous system activity that controls bone remodeling via the β2-adrenergic receptor activity in the osteoblast cells (198).
Conclusions
Based on decades of research, it is now clear that E2 can act much like a metabotropic (GPCR) neurotransmitter to alter neuronal excitability and vital autonomic functions controlled by the hypothalamus. Signals initiated by E2 at the plasma membrane can trigger multiple intracellular signaling cascades (eg, MAPK, PI3K, and PKC) that result in the phosphorylation of hundreds of proteins that ultimately affect not only cell excitability but also gene transcription. For example, E2 turns on adenylyl cyclase activity and increase cAMP levels in POMC neurons within minutes (13). cAMP activates PKA, which in turn phosphorylates K+ channels and/or calcium channels to alter their activity. PKA can also phosphorylate cAMP-responsive element binding protein to elicit new gene transcription. In contrast to the anorexigenic POMC neurons, engagement of the putative Gαq-mER in orexigenic NPY/AgRP neurons elicits the opposite response, ie, an increase in K+ channel activation by the GABAB receptor (Figure 3) and down-regulation of NPY mRNA expression. Thus, E2 can act on hypothalamic neurons in a cell-specific manner to generate the appropriate physiological responses by eliciting a combination of rapid changes in membrane excitability accompanied by slower alterations in gene expression. A future challenge is to identify the putative Gαq-mER, its interaction with other metabotropic receptors (eg, mGluR1a) and its physiological functions not only in the hypothalamus but throughout the CNS.
Acknowledgments
We thank previous and current members of their laboratories who contributed to the work described here, especially Dr Anna Malyala, Dr Jian Qiu, Dr Troy A. Roepke, and Dr Chunguang Zhang. We also thank Ms Martha A. Bosch for her skilled assistance with the illustrations and manuscript preparation.
This work was supported by National Institutes of Health Grants NS 038809, NS 043330, and DK 068098.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related peptide
- AP-1
- activator protein-1
- CNS
- central nervous system
- E2
- 17β-estradiol
- EGF
- epidermal growth factor
- ER
- estrogen receptor
- ERE
- estrogen-response element
- GABA
- gamma-Aminobutyric acid
- GIRK
- G protein-coupled inwardly rectifying K+
- GPCR
- G protein-coupled receptor
- Gαq-mER
- Gαq-coupled mER
- Kiss1
- Kisspeptin
- mER
- membrane ER
- mGluR1a
- metabotropic glutamate type 1a receptor
- MPN
- medial preoptic nucleus
- NPY
- neuropeptide Y
- PI3K
- phosphatidylinositol 3 kinase
- PKA
- protein kinase A
- PKC
- protein kinase C
- PLC
- phospholipase C
- POA
- preoptic area
- POMC
- proopiomelanocortin
- PV
- periventricular nucleus
- PVN
- paraventricular nucleus
- siRNA
- small interfering RNA
- STX
- synthetic diphenylacrylamide compound
- Tc
- core body temperature.
References
- 1. Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. [DOI] [PubMed] [Google Scholar]
- 2. Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hedges VL, Ebner TJ, Meisel RL, Mermelstein PG. The cerebellum as a target for estrogen action. Front Neuroendocrinol. 2012;33:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen EV, DeSombre ER. Estrogen-receptor interaction. Science. 1973;182:126–134. [DOI] [PubMed] [Google Scholar]
- 6. Walter P, Green S, Greene G, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;23:7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kushner PJ, Agard DA, Greene GL, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. [DOI] [PubMed] [Google Scholar]
- 9. Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar DK, Fink G. Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol. 1980;86:511–524. [DOI] [PubMed] [Google Scholar]
- 12. Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lagrange AH, Ronnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–612. [DOI] [PubMed] [Google Scholar]
- 14. Pappas TC, Gametchu B, Yannariello-Brown J, Collins TJ, Watson CS. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine. 1994;2:813–822. [Google Scholar]
- 15. Clarke CH, Norfleet AM, Clarke MS, Watson CS, Cunningham KA, Thomas ML. Perimembrane localization of the estrogen receptor α protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology. 2000;71:34–42. [DOI] [PubMed] [Google Scholar]
- 16. Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocinol. 1999;13:307–319. [DOI] [PubMed] [Google Scholar]
- 17. Toran-Allerand CD, Guan X, MacLusky NJ, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu J, Bosch MA, Tobias SC, et al. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 21. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. [DOI] [PubMed] [Google Scholar]
- 22. Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2001;98:2808–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. [DOI] [PubMed] [Google Scholar]
- 24. Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. [DOI] [PubMed] [Google Scholar]
- 25. Sar M, Stumpf WE. Cellular locatization of progestin and estrogen in guinea pig hypothalamus by autoradiography. In: Stumpf WE, Grant LD, eds. Anatomical Neuroendocrinology. Munich, Germany: S. Karger-Basal; 1975:142–152. [Google Scholar]
- 26. Warembourg M. Radioautographic localization of estrogen-concentrating cells in the brain and pituitary of the guinea pig. Brain Res. 1977;123:357–362. [DOI] [PubMed] [Google Scholar]
- 27. Sar M. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science. 1984;223:938–940. [DOI] [PubMed] [Google Scholar]
- 28. Tardy J, Pasqualini JR. Localization of [3H]-estradiol and gonadotropin-releasing hormone (GnRH) in the hypothalamus of the fetal guinea-pig. Exp Brain Res. 1983;49:77–83. [DOI] [PubMed] [Google Scholar]
- 29. Sar M, Parikh I. Immunohistochemical localization of estrogen receptor in rat brain, pituitary and uterus with monoclonal antibodies. J Steroid Biochem. 1986;24:497–503. [DOI] [PubMed] [Google Scholar]
- 30. DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol. 1991;305:591–612. [DOI] [PubMed] [Google Scholar]
- 31. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. [DOI] [PubMed] [Google Scholar]
- 32. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. [DOI] [PubMed] [Google Scholar]
- 33. Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. [DOI] [PubMed] [Google Scholar]
- 34. Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor β (ERβ) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERα mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. [DOI] [PubMed] [Google Scholar]
- 35. Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor β (ERβ) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Mol Brain Res. 2000;76:191–204. [DOI] [PubMed] [Google Scholar]
- 36. Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 37. Gréco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ERβ and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. [DOI] [PubMed] [Google Scholar]
- 38. Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-α distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. [DOI] [PubMed] [Google Scholar]
- 39. Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-β distribution in the human hypothalamus: similarities and differences with ER α distribution. J Comp Neurol. 2003;466:251–277. [DOI] [PubMed] [Google Scholar]
- 40. Mitra SW, Hoskin E, Yudkovitz J, et al. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. [DOI] [PubMed] [Google Scholar]
- 41. Warembourg M, Leroy D. Comparative distribution of estrogen receptor α and β immunoreactivities in the forebrain and the midbrain of the female guinea pig. Brain Res. 2004;1002:55–66. [DOI] [PubMed] [Google Scholar]
- 42. Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. [DOI] [PubMed] [Google Scholar]
- 43. Flügge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. [DOI] [PubMed] [Google Scholar]
- 44. Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. [DOI] [PubMed] [Google Scholar]
- 45. Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. [DOI] [PubMed] [Google Scholar]
- 46. Herbison AE. Somatostatin-immunoreactive neurones in the hypothalamic ventromedial nucleus possess oestrogen receptors in the male and female rat. J Neuroendocrinol. 1994;6:323–328. [DOI] [PubMed] [Google Scholar]
- 47. Horvath TL, Leranth C, Kalra SP, Naftolin F. Galanin neurons exhibit estrogen receptor immunoreactivity in the female rat mediobasal hypothalamus. Brain Res. 1995;675:321–324. [DOI] [PubMed] [Google Scholar]
- 48. Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y and β-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. [DOI] [PubMed] [Google Scholar]
- 49. Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. [DOI] [PubMed] [Google Scholar]
- 50. Hu L, Wada K, Mores N, Krsmanovic LZ, Catt KJ. Essential role of G protein-gated inwardly rectifying potassium channels in gonadotropin-induced regulation of GnRH neuronal firing and pulsatile neurosecretion. J Biol Chem. 2006;281:25231–25240. [DOI] [PubMed] [Google Scholar]
- 51. Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-β messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. [DOI] [PubMed] [Google Scholar]
- 52. Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen recetpros colocalize in female rat brain. Neuroscience. 2000;99:751–760. [DOI] [PubMed] [Google Scholar]
- 53. Hrabovszky E, Shughrue PJ, Merchenthaler I, et al. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. [DOI] [PubMed] [Google Scholar]
- 54. Hrabovszky E, Steinhauser A, Barabás K, et al. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. [DOI] [PubMed] [Google Scholar]
- 55. Kalló I, Butler JA, Barkovics-Kalló M, Goubillon ML, Coen CW. Oestrogen receptor β-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol. 2001;13:741–748. [DOI] [PubMed] [Google Scholar]
- 56. Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadortropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. [DOI] [PubMed] [Google Scholar]
- 57. Herbison AE, Skynner MJ, Sim JA. Lack of detection of estrogen receptor-α transcripts in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2001;142:492–493. [DOI] [PubMed] [Google Scholar]
- 58. Gundlah C, Lu NZ, Mirkes SJ, Bethea CL. Estrogen receptor β (ERβ) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res. 2001;91:14–22. [DOI] [PubMed] [Google Scholar]
- 59. Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor β in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. [DOI] [PubMed] [Google Scholar]
- 60. Hrabovszky E, Kalló I, Steinhauser A, et al. Estrogen receptor-β in oxytocin and vasopressin neurons of the rat and human hypothalamus: immunocytochemical and in situ hybridization studies. J Comp Neurol. 2004;473:315–333. [DOI] [PubMed] [Google Scholar]
- 61. Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. [DOI] [PubMed] [Google Scholar]
- 62. Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-α in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. [DOI] [PubMed] [Google Scholar]
- 63. Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. [DOI] [PubMed] [Google Scholar]
- 64. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. [DOI] [PubMed] [Google Scholar]
- 65. Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. [DOI] [PubMed] [Google Scholar]
- 66. Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177. [DOI] [PubMed] [Google Scholar]
- 67. Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cylcin G2 promoter. J Biol Chem. 2006;281:16272–16278. [DOI] [PubMed] [Google Scholar]
- 68. Kininis M, Chen BS, Diehl AG, et al. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O'Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46:163–167. [DOI] [PubMed] [Google Scholar]
- 70. Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. [DOI] [PubMed] [Google Scholar]
- 71. Gruber CJ, Gruber DM, Gruber IM, Wieser F, Huber JC. Anatomy of the estrogen response element. Trends Endocrinol Metab. 2004;15:73–78. [DOI] [PubMed] [Google Scholar]
- 72. Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen-regulated hypothalamic genes. Neurochem Res. 2004;29:1189–1200. [DOI] [PubMed] [Google Scholar]
- 73. Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. [DOI] [PubMed] [Google Scholar]
- 74. Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun. 2003;303:660–668. [DOI] [PubMed] [Google Scholar]
- 75. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. [DOI] [PubMed] [Google Scholar]
- 77. Pietras RJ, Szego CM. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979;11:1471–1483. [DOI] [PubMed] [Google Scholar]
- 78. Towle AC, Sze PY. Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem. 1983;18:135–143. [DOI] [PubMed] [Google Scholar]
- 79. Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17β-estradiol-[125I]bovine serum albumin conjugate. J Steroid Biochem Mol Biol. 1997;62:327–336. [DOI] [PubMed] [Google Scholar]
- 80. Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. [DOI] [PubMed] [Google Scholar]
- 81. Ramirez VD, Zheng J. Membrane sex-steroid receptors in the brain. Front Neuroendocrinol. 1996;17:402–439. [DOI] [PubMed] [Google Scholar]
- 82. Kelly MJ, Moss RL, Dudley CA. The effects of microelecrophoretically applied estrogen, cortisol and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res. 1977;30:53–64. [DOI] [PubMed] [Google Scholar]
- 83. Kelly MJ, Moss RL, Dudley CA, Fawcett CP. The specificity of the response of preoptic-septal area neurons to estrogen: 17α-estradiol versus 17β-estradiol and the response of extrahypothalamic neurons. Exp Brain Res. 1977;30:43–52. [DOI] [PubMed] [Google Scholar]
- 84. Kelly MJ, Moss RL, Dudley CA. The stereospecific changes in the unit activity of preoptic-septal neurons to microelectrophoresed estrogen. In: Ryall RW, Kelly JS, eds. Iontophoresis and Transmitter Mechanisms in the Mammalian Central Nervous System. 1st ed New York, NY: Elsevier/North-Holland Biomedical Press; 1978:113–116. [Google Scholar]
- 85. Kelly MJ, Moss RL, Dudley CA. The effects of ovarectomy on preoptic-septic area neurons to microelectrophoresed estrogen. Neuroendocrinology. 1978;25:204–211. [DOI] [PubMed] [Google Scholar]
- 86. Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. [DOI] [PubMed] [Google Scholar]
- 87. Kelly MJ, Rønnekleiv OK. Rapid membrane effects of estrogen in the central nervous system. In: Pfaff DW, ed. Hormones, Brain and Behavior. 3rd ed San Diego, CA: Academic Press; 2002:361–380. [Google Scholar]
- 88. Rønnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. [DOI] [PubMed] [Google Scholar]
- 89. Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. [DOI] [PubMed] [Google Scholar]
- 92. Szego EM, Barabás K, Balog J, et al. Estrogen induces estrogen receptor α-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych PE. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-α trafficking. J Neurosci. 2009;29:15323–15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dubal DB, Zhu H, Yu J, et al. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. [DOI] [PubMed] [Google Scholar]
- 98. Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. [DOI] [PubMed] [Google Scholar]
- 101. Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. [DOI] [PubMed] [Google Scholar]
- 102. Toran-Allerand CD. Estrogen and the brain: beyond ER-α, ER-β and 17β-estradiol. Ann NY Acad Sci. 2005;1052:136–144. [DOI] [PubMed] [Google Scholar]
- 103. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-protein coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;3:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang C, Kelly MJ, Rønnekleiv OK. 17β-estradiol rapidly increases ATP-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kenealy BP, Keen KL, Rønnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011;152:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. [DOI] [PubMed] [Google Scholar]
- 107. Cunningham MJ, Fang Y, Selley DE, Kelly MJ. μ-opioid agonist-stimulated [35S]GTPγS binding in guinea pig hypothalamus: effects of estrogen. Brain Res. 1998;791:341–346. [DOI] [PubMed] [Google Scholar]
- 108. Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biocheml. 1988;30:263–266. [DOI] [PubMed] [Google Scholar]
- 109. Tobias SC, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. ChemMedChem. 2006;1:565–571. [DOI] [PubMed] [Google Scholar]
- 110. Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lebesgue D, Traub M, De Butte-Smith M, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLos One. 2010;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- 114. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-Mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. [DOI] [PubMed] [Google Scholar]
- 115. Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. [DOI] [PubMed] [Google Scholar]
- 116. Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–910. [DOI] [PubMed] [Google Scholar]
- 117. Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chem Biol. 2006;2:207–212. [DOI] [PubMed] [Google Scholar]
- 118. Brailoiu E, Dun SL, Brailoiu GC, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. [DOI] [PubMed] [Google Scholar]
- 119. Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265–266:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lagrange AH, Rønnekleiv OK, Kelly MJ. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology. 1995;136:2341–2344. [DOI] [PubMed] [Google Scholar]
- 122. Rønnekleiv OK, Zhang C, Kelly MJ. Estradiol and kisspeptin modulation of gonadotropin-releasing hormone (GnRH) neuronal excitability. In: Armstong WE, Tasker JG, eds. Neurophysiology of Neuroendocrine Neurons. Hoboken, NJ: John Wiley and Sons, Ltd; 2014:301–321. [Google Scholar]
- 123. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca2+ oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19:5898–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor α and estrogen receptor β. Steroids. 2011; 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology. 2008;149:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Blaustein JD, Erskine MS. Feminine sexual behavior: cellular integration of hormonal and afferent information in the rodent forebrain. In: Pfaff D, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, Brain and Behavior. 1st ed San Diego, CA: Academic Press; 2002:139–214. [Google Scholar]
- 129. Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brains. Front Neuroendocrinol. 2008;29:238–257. [DOI] [PubMed] [Google Scholar]
- 130. Micevych P, Dewing P. Membrane-initiated estradiol signaling regulating sexual receptivity. Front Endocrinol. 2011;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Micevych PE, Kelly MJ. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opoid receptor immunoreactivity in the medial preoptic nucleas and medial amygdala. J Neurosci. 1998;18:3967–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sinchak K, Shahedi K, Dewing P, Micevych PE. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. NeuroReport. 2005;15:1697–1700. [DOI] [PubMed] [Google Scholar]
- 136. Christensen A, Micevych P. A novel membrane estrogen receptor activated by STX induces female sexual receptivity through an interaction with mGluR1a. Neuroendocrinology. 2013;97:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. [DOI] [PubMed] [Google Scholar]
- 138. Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. [DOI] [PubMed] [Google Scholar]
- 140. Arroyo A, Kim B, Rasmusson RL, Yeh J. Hyperpolarization-activated cation channels are expressed in rat hypothalamin gonadotropin-releasing hormone (GnRH) neurons and immortalized GnRH neurons. J Soc Gynecol Invest. 2006;13:442–450. [DOI] [PubMed] [Google Scholar]
- 141. Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xu Y, Nedungadi TP, Zhu L, et al. Distinct Hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kavalali ET, Chung C, Khvotchev M, et al. Spontaneous neurotransmission: an independent pathway form neuronal signaling? Physiology. 2011;26:45–53. [DOI] [PubMed] [Google Scholar]
- 144. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Med. 2007;13:89–94. [DOI] [PubMed] [Google Scholar]
- 145. Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors of POMC neurons in the regulation of sex-specific energy homeostasis. Physiol Behav. 2010;100:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendocrinology. 1969;4:309–320. [DOI] [PubMed] [Google Scholar]
- 147. Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–892. [PubMed] [Google Scholar]
- 148. Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. [DOI] [PubMed] [Google Scholar]
- 149. Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. [DOI] [PubMed] [Google Scholar]
- 150. Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic β-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendocrinology. 1995;61:695–703. [DOI] [PubMed] [Google Scholar]
- 151. Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: effects of age and sex. J Comp Neurol. 1998;401:65–88. [DOI] [PubMed] [Google Scholar]
- 152. Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142:4751–4757. [DOI] [PubMed] [Google Scholar]
- 153. Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–349. [DOI] [PubMed] [Google Scholar]
- 154. Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–558. [DOI] [PubMed] [Google Scholar]
- 155. McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 β: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–657. [DOI] [PubMed] [Google Scholar]
- 156. Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. [DOI] [PubMed] [Google Scholar]
- 158. Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. [DOI] [PubMed] [Google Scholar]
- 159. Clegg DJ, Brown LM, Zigman JM, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. [DOI] [PubMed] [Google Scholar]
- 160. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. [DOI] [PubMed] [Google Scholar]
- 161. Park CJ, Zhao Z, Glidewell-Kenney C, et al. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest. 2011;121:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hewitt SC, Li L, Grimm SA, et al. Novel DNA motif binding activity observed in vivo with an estrogen receptor α mutant mouse. Mol Endocrinol. 2014;28:899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell. 2014;29:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J Neurosci. 2002;22:8251–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Appleyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid β-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. [DOI] [PubMed] [Google Scholar]
- 167. Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Roepke TA, Bosch MA, Rick EA, et al. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305:E632–E640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab. 2007;18:48–50. [DOI] [PubMed] [Google Scholar]
- 171. Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. [DOI] [PubMed] [Google Scholar]
- 172. Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol. 2012;33:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. [DOI] [PubMed] [Google Scholar]
- 175. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–177. [DOI] [PubMed] [Google Scholar]
- 177. Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. [DOI] [PubMed] [Google Scholar]
- 178. Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in meopausal women. J Clin Endocrinol Metab. 1995;80:2354–2358. [DOI] [PubMed] [Google Scholar]
- 179. Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–337. [DOI] [PubMed] [Google Scholar]
- 180. Freedman RR. Hot flashes: behavorial treatments, mechanisms, and relation to sleep. Am J Med. 2005;118:1245–1305. [DOI] [PubMed] [Google Scholar]
- 181. Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77:487–490. [DOI] [PubMed] [Google Scholar]
- 182. Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73:1238–1245. [DOI] [PubMed] [Google Scholar]
- 183. Brooks EM, Morgan AL, Pierzga JM, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor fuction in postmenopausal women. J Appl Physiol. 1997;97:477–484. [DOI] [PubMed] [Google Scholar]
- 184. Simpkins JW, Katovich MJ, Song IC. Similarities between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32:1957–1966. [DOI] [PubMed] [Google Scholar]
- 185. Merchenthaler I, Funkhouser JM, Carver JM, Lundeen SG, Ghosh K, Winneker RC. The effect of estrogens and antiestrogens in a rat model for hot flush. Maturitas. 1998;30:307–316. [DOI] [PubMed] [Google Scholar]
- 186. Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene Acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146:3999–4008. [DOI] [PubMed] [Google Scholar]
- 187. Zhou L, Hammer RP., Jr Gonadal steroid hormones upregulate medial preoptic μ-opioid receptors in the rat. Eur J Pharmacol. 1995;278:271–274. [DOI] [PubMed] [Google Scholar]
- 188. Yakimova KS, Sann H, Pierau FK. Neuronal basis for the hyperthermic effect of mu-opioid agonists in rats: decrease in temperature sensitivity of warm-sensitive hypothalamic neurons. Neurosci Lett. 1996;218:115–118. [DOI] [PubMed] [Google Scholar]
- 189. Petersen SL, LaFlamme KD. Progesterone increases levels of μ-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Mol Brain Res. 1997;52:32–37. [DOI] [PubMed] [Google Scholar]
- 190. Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol. 2001;537(pt 2):521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. [DOI] [PubMed] [Google Scholar]
- 193. Wagner EJ, Ronnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001;21:2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA. 2010;107:8848–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z. 17 β-estradiol-BSA conjugates and 17 β-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem. 2001;81:413–429. [DOI] [PubMed] [Google Scholar]
- 196. Endoh H, Sasaki H, Maruyama K, et al. Rapid activation of MAP Kinase by estrogen in the bone cell line. Biochem Biophys Res Commun. 1997;235:99–102. [DOI] [PubMed] [Google Scholar]
- 197. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. [DOI] [PubMed] [Google Scholar]
- 199. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. [DOI] [PubMed] [Google Scholar]
- 200. Wuarin J-P, Dudek FE. Direct effects of an opioid peptide selective for μ-receptors: intracellular recordings in the paraventricular and supraoptic nuclei of the guinea pig. Neuroscience. 1990;36:291–298. [DOI] [PubMed] [Google Scholar]


