Abstract
Type 2 diabetes mellitus (T2DM) is characterized by pancreatic islet failure due to loss of β-cell secretory function and mass. Studies have identified a link between a variance in the gene encoding melatonin (MT) receptor 2, T2DM, and impaired insulin secretion. This genetic linkage raises the question whether MT signaling plays a role in regulation of β-cell function and survival in T2DM. To address this postulate, we used INS 832/13 cells to test whether activation of MT signaling attenuates proteotoxicity-induced β-cell apoptosis and through which molecular mechanism. We also used nondiabetic and T2DM human islets to test the potential of MT signaling to attenuate deleterious effects of glucotoxicity and T2DM on β-cell function. MT signaling in β-cells (with duration designed to mimic typical nightly exposure) significantly enhanced activation of the cAMP-dependent signal transduction pathway and attenuated proteotoxicity-induced β-cell apoptosis evidenced by reduced caspase-3 cleavage (∼40%), decreased activation of stress-activated protein kinase/Jun-amino-terminal kinase (∼50%) and diminished oxidative stress response. Activation of MT signaling in human islets was shown to restore glucose-stimulated insulin secretion in islets exposed to chronic hyperglycemia as well as in T2DM islets. Our data suggest that β-cell MT signaling is important for the regulation of β-cell survival and function and implies a preventative and therapeutic potential for preservation of β-cell mass and function in T2DM.
Pancreatic β-cell failure is a key feature of type 2 diabetes mellitus (T2DM) characterized by β-cell secretory dysfunction and loss of β-cell mass (1, 2). The pathophysiology of β-cell dysfunction in T2DM is complex, but it largely manifests as the loss of glucose-stimulated insulin secretion (GSIS) (1, 3). β-Cell loss in T2DM patients has been attributed to increased β-cell apoptosis (2, 4, 5). Recent genome-wide association scan studies have reported that a variance in the gene encoding melatonin (MT) receptor 2 (MT-2) is associated with an increased risk of β-cell failure and T2DM (6). In particular, the MT-2 variant appears to carry the strongest inhibitory effect on GSIS (studied in isolated human islets) compared with 43 other glycemia-associated genetic loci (7). This somewhat unexpected genetic linkage raises a question whether β-cell MT receptor signaling plays a role in the regulation of β-cell function and survival and consequent pathogenesis of T2DM.
The pathogenesis of T2DM per se is also associated with impaired MT production and secretion (8–11). MT is a hormone produced and secreted from the endocrine cells in the pineal gland and exhibits nocturnal production and secretion pattern. Nocturnal MT secretion is significantly reduced in T2DM patients, particularly when populations are weight matched (8). Interestingly, diurnal secretion of other circadian-driven hormones (eg, cortisol) in T2DM remains intact, thus suggesting a specific defect in MT secretion/production axis (8). Studies in animal models of T2DM also report impaired nocturnal MT secretion upon induction of hyperglycemia (9, 10). This apparent reduction in nocturnal MT secretion is associated with a decreased pineal gland size, loss of MT production, and up-regulation of inhibitory α-adrenoreceptors (10). Taken together, loss of MT secretion in T2DM further supports a potential role of MT in pathophysiology of T2DM. This premise is supported by a recent observation demonstrating that loss of nocturnal MT secretion is associated with a higher risk of developing T2DM (11).
Diurnal activation of MT receptor signaling is implicated in the regulation of organisms' circadian rhythms and entrainment of circadian clocks, an observation recently extended to β-cells (12). In addition to its known circadian functions, MT signaling is also involved in the regulation of diverse physiological processes, including metabolic control (13, 14). MT's actions are mediated by two high-affinity Gi/0 receptors (MT-1 and MT-2) with transduction pathways exhibiting a classic Gi-coupled receptor pattern of activation (15). Importantly, the MT receptor transduction pathway varies based on the duration of exposure to MT, ie, acute (minutes) vs persistent (hours). This distinction is particularly important, given that physiological exposure of cells to MT occurs throughout the duration of the dark phase of the circadian cycle (16). Studies show that acute exposure to MT inhibits adenylate cyclase (AC) activity and attenuates cAMP production leading to decreased activation of downstream targets such as protein kinase A (PKA) and cAMP responsive element binding protein (CREB) (15). On the other hand, persistent activation of the MT receptor leads to sensitization and enhancement of AC activity and increased activation of the cAMP-PKA-CREB cascade (17), phenomenon shown to be present in β-cells (18).
Given increased insights into the importance of MT receptor signaling in pathophysiology of T2DM, we hypothesized that the persistent activation of MT signaling (with duration intentionally designed to recapitulate typical nightly exposure to MT) will be beneficial for the regulation of β-cell function and survival in T2DM. To address this hypothesis, we used INS 832/13 cells and isolated human islets from control and T2DM patients to explore the potential of β-cell MT signaling to enhance activation of the cAMP-PKA-CREB pathway and enhance β-cell survival and β-cell secretory function in the context of molecular stress associated with T2DM.
Materials and Methods
Cell culture
The rat insulinoma cell line INS 832/13 was kindly provided by Dr C. Newgard (Duke University, Durham, NC). INS 832/13 cells were grown in RPMI 1640 medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 100 IU/mL penicillin, and 100 mg/mL streptomycin (Invitrogen), 10% heat-inactivated fetal bovine serum (Gemini), and 50 mM β-mercaptoethanol (Sigma) at 37°C in a humidified 5% CO2 atmosphere.
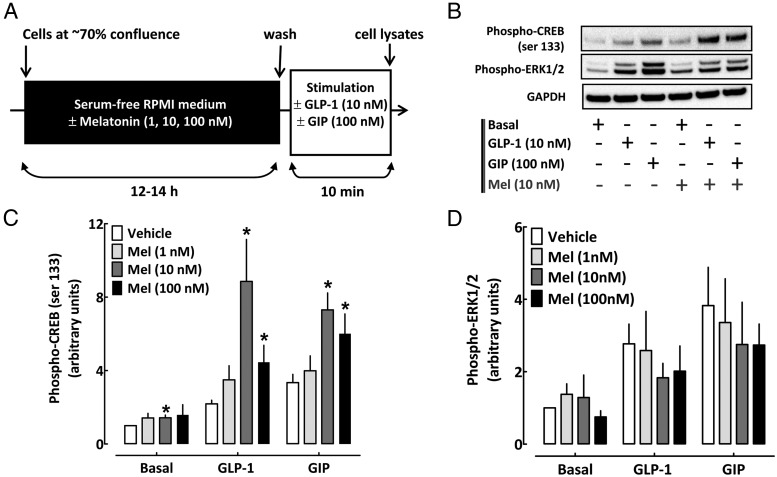
For acute stimulation experiments (see Figure 2A), INS 832/13 cells plated in six-well dishes (70% confluence) were cultured overnight (12–14 hours) in serum-free RPMI 1640 medium containing 2.8 mM glucose, 10 mM HEPES, 1 mM pyruvate, 200 U/mL penicillin, 100 μg/mL streptomycin, and 0.1% BSA (fatty acid free, low endotoxin [Sigma; A8806]) in the presence of MT (Bachem; 1, 10, or 100 nM) diluted in dimethylsulfoxide (DMSO; Sigma), MT receptor agonist ramelteon (LGM Pharma; 1 nM) diluted in DMSO (Sigma), or vehicle solution (DMSO; Sigma). DMSO vehicle treatment was administered to all (non-MT/ramelteon) conditions to correct for potential confounding effects of DMSO, which was used to prepare the MT/ramelteon solutions. Cells were subsequently exposed for 10 minutes to serum-free RPMI 1640 medium containing 2.8 mM glucose (basal conditions), 10 nM glucagon-like peptide 1 (GLP-1; Sigma), or 100 nM gastric inhibitory polypeptide (GIP; Tocris Bioscience) at 16.7 mM glucose.
Figure 2.
Persistent activation of β-cell MT receptor signaling enhances cAMP-PKA-CREB signaling pathway. A, Graphical illustration of the experimental work flow. B–D, Phospho-CREB (serine 133), phospho-ERK1/2, and GAPDH (loading control) protein levels and quantification in INS 832/13 cells exposed for 10 minutes to basal (2.8 mM glucose), GLP-1 (10 nM) at 16.7 mM glucose, or GIP (100 nM) at 16.7 mM glucose stimulation after overnight culture (12–14 hours) in media containing either melatonin (1–100 nM) or vehicle (DMSO). DMSO vehicle treatment was administered to all non-MT conditions to correct for potential confounding effects of DMSO, which was used to prepare MT solutions. Data are expressed as mean ± SEM and an average of four to six independent experiments. *, P < .05 vs vehicle.
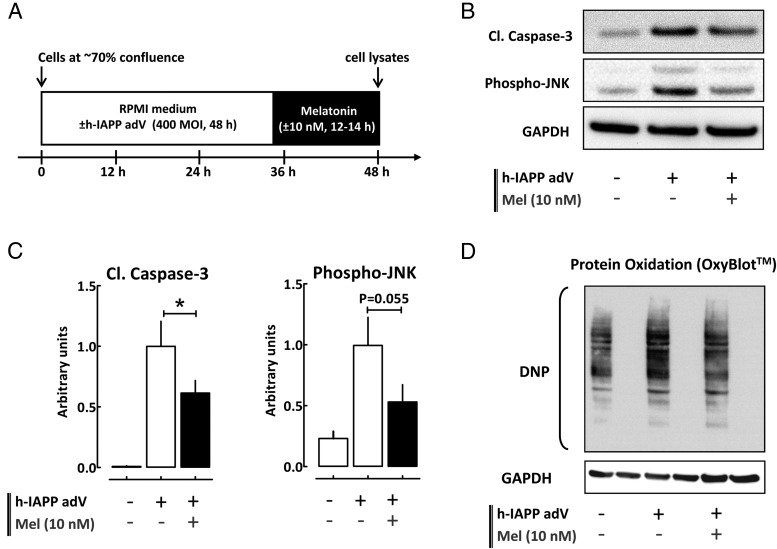
For adenovirus transduction experiments (see Figure 3A), INS 832/13 cells were plated on six-well plates at the density of 106 cells/well and cultured for 24 hours. Cells were transduced with human islet amyloid polypeptide (h-IAPP) adenovirus at 400 multiplicity of infection (MOI) in complete RPMI 1640 medium for 48 hours. MT (10 nM) was added during the last 12–14 hours. At the end of the experiment, cells were washed with cold PBS and lysed for 10 minutes at 4°C in Nonidet P-40 lysis buffer [0.5% Nonidet P-40; 20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 2 mM MgCl2; 1 mM dithiothreitol; 5 mM NaF; 1 mM Na3VO4; and protease inhibitors (Sigma)] and centrifuged at 10 000 rpm for 10 minutes to remove insoluble materials. Supernatant was stored at −20°C until use for the subsequent protein determination by bicinchoninic assay (Bio-Rad Laboratories) and Western blotting.
Figure 3.
Persistent activation of β-cell MT receptor signaling protects against β-cell proteotoxicity. A, Graphical illustration of the experimental work flow. B and C, Cleaved caspase-3, phospho-JNK, and GAPDH (loading control) protein levels in control INS 832/13 cells and in cells transduced at 400 MOI with h-IAPP adenovirus for 48 hours and exposed for the final 12–14 hours to media containing either MT (10 nM) or vehicle (DMSO). The graph represents the quantification of the cleaved form of caspase-3 and JNK phosphorylation at threonine 183 and tyrosine 185 (n = 3–5 independent experiments). Data are expressed as mean ± SEM. *, P < .05. D, Immunoblot detection of carbonyl groups introduced into proteins due to oxidative stress and derivatized to DNP in INS 832/13 cells transduced at 400 MOI with h-IAPP adenovirus for 48 hours and exposed for the final 12–14 hours to media containing either MT (10 nM) or vehicle (DMSO) (a representative example of five independent experiments is shown). DMSO vehicle treatment was administered to all non-MT conditions to correct for potential confounding effects of DMSO, which was used to prepare melatonin solutions.
Immunofluorescent staining
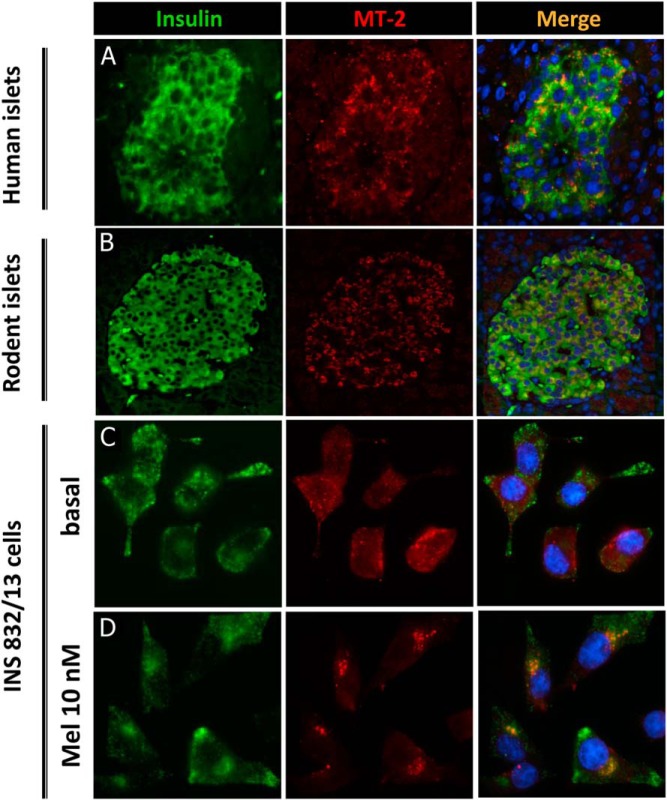
Cells were plated on coverslips in 12-well dishes and allowed to reach approximately 40% confluence. Cells were incubated for 48 hours in standard RPMI 1640 culture medium as described above. Cells were then treated with 10 nM MT for 1 hour at 37°C. After rinsing with PBS, cells were fixed with 4% PFA for 30 minutes at room temperature (RT), soaked in soaking buffer (Tris buffered saline and 2% Triton X-100) for 20–30 minutes at RT. Blocking was performed using blocking buffer (3% BSA and 1% Triton X-100) for 2 hours at RT. Cells on coverslips or pancreatic sections were stained with a goat antiMT-2 antibody (1:50; Santa Cruz Biotechnology) and guinea pig anti-insulin antibody (1:100; Invitrogen) and then mounted with Vectashield with 4′,6′-diamino-2-phenylindole (Vector Laboratories) and viewed using Leica DM6000 microscope (Leica Microsystem). Images were acquired using OpenLab software (Improvision).
Western blotting
Proteins (25–40 μg/lane) were separated on a 4%–12% Bis-Tris NuPAGE gel and blotted onto a polyvinylidene fluoride membrane (FluoroTrans; VWR). Membranes were probed overnight at 4°C with primary antibodies. Antiphospho-CREB antibody (which detects CREB when phosphorylated at serine 133), antiphospho-ERK1/2 (which selectively recognizes the doubly phosphorylated active forms of these kinases, Thr202/Tyr204), anticleaved caspase-3, antiphospho-stress-activated protein kinase/Jun-amino-terminal kinase (JNK) (Thr183/Tyr185), and antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Cell Signaling. Horseradish peroxidase-conjugated secondary antibodies were from Invitrogen. Proteins were visualized by enhanced chemiluminescence (Millipore), and protein expression levels were quantified using the Labworks software (UVP).
Detection of protein modification by Oxyblot
For the OxyBlot preparation, 5 μL of cell or islet lysates (∼15 μg of protein) obtained as previously described were mixed with 5 μL of 12% sodium dodecyl sulfate. Samples were then treated with 10 μL of 2,4-dinitrophenylhydrazine (DNP) solution or 10 μL of control derivatization solution and incubated at room temperature for 15 minutes, after which 7.5 μL of neutralization solution was added. Proteins were then separated on a 4%–12% Bis-Tris NuPAGE gel and transferred onto a polyvinylidene fluoride membrane (FluoroTrans; VWR), and the membrane was blocked in 1% BSA for 1 hour. The membrane was incubated overnight with a primary antibody (rabbit anti-DNP; Millipore) and then for 1 hour at room temperature with horseradish peroxidase-conjugated secondary antibody (goat antirabbit; Millipore). Proteins were visualized by enhanced chemiluminescence (Millipore), and the protein expression levels were quantified using the Labworks software (UVP) using GAPDH as loading control.
Targeted PCR arrays
INS 832/13 cells were transduced with h-IAPP adenovirus at 400 MOI in complete RPMI 1640 medium for 48 hours as previously described with MT (10 nM) or vehicle (DMSO) added during the last 12–14 hours. Subsequently, cells were homogenized using QIAshredder microcentrifuge spin-columns (79654; QIAGEN), and total RNA isolation was performed using the RNeasy mini kit (74104; QIAGEN) according to manufacturer's instructions. RNA abundance was quantified using the NanoDrop 1000 spectrophotometer (NanoDrop Technologies), and cDNA was synthesized from 0.5 μg total RNA using the RT2 first-strand kit (330520; QIAGEN) according to the manufacturer's instructions. The following targeted PCR arrays were performed in 96-well format using the RT2 SYBR Green ROX quantitative PCR master mix (330520; QIAGEN) and the QuantStudio 6 Flex real-time PCR system (Applied Biosystems) according to the manufacturer's instructions: rat apoptosis RT2 Profiler PCR array (PARN-012ZC-2; QIAGEN) and rat oxidative stress RT2 profiler PCR array (PARN-065ZC-2; QIAGEN). Free web-based RT2 Profiler PCR Array Data Analysis Software (version 3.5; SABiosciences) was used to determine δδcycle threshold based fold-changes in expression of target genes using standardized settings (ie, baseline, threshold) and β-actin as the endogenous control gene across all plates.
Human islets
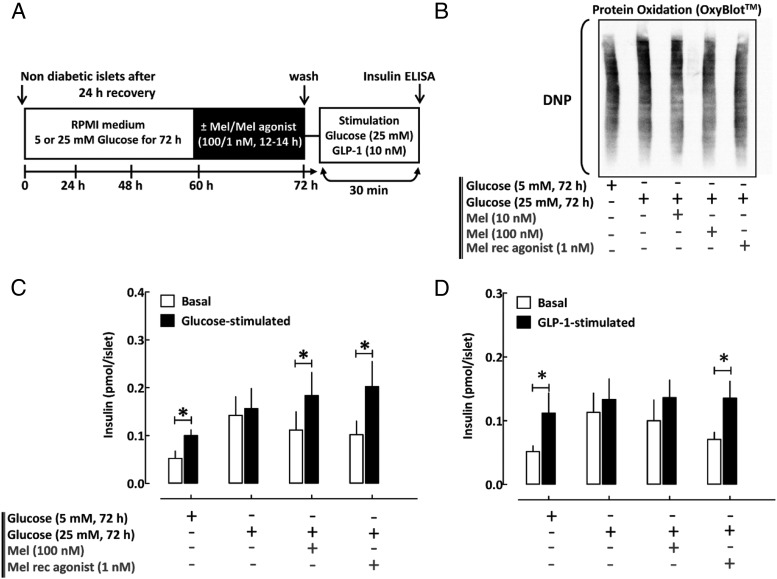
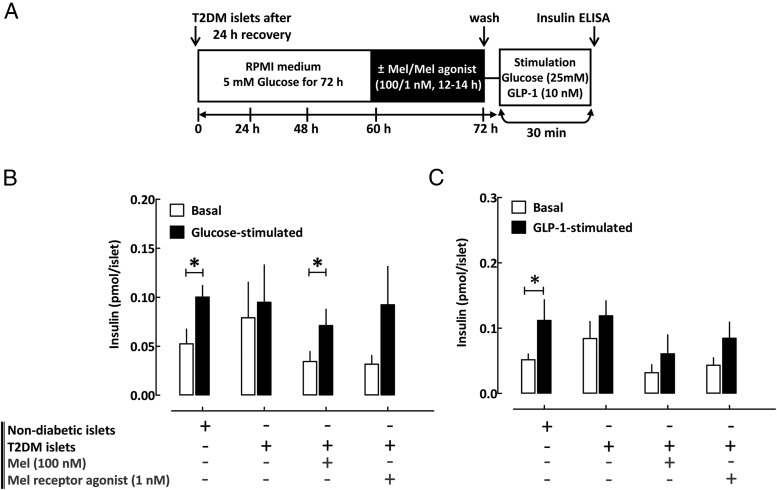
Nondiabetic (n = 5 shipments) and T2DM human islets (n = 4 shipments) were obtained through the Integrated Islet Distribution Program (Supplemental Table 1) and maintained in RPMI 1640 medium with 5 mM glucose and 10% fetal bovine serum at 37°C. Insulin secretion was assessed by static incubation at basal (5 mM glucose per 30 minutes) and hyperglycemic conditions (25 mM glucose per 30 minutes) or GLP-1 stimulation (10 nM, 30 minutes) with insulin measured by ELISA (ALPCO). GSIS and Oxyblot analysis in nondiabetic islets were assessed after an exposure to four conditions: 1) 72 hours' incubation in RPMI 1640 with 5 mM glucose (control), 2) 72 hours in 25 mM glucose (glucotoxicity model), 3) 72 hours in 25 mM glucose with 100 nM MT (for the final 12–14 hours), and 4) 72 hours in 25 mM glucose with 1 nM ramelteon (for the final 12–14 hours) (see Figure 5A). GSIS in T2DM islets was assessed after an overnight incubation (12–14 hours) in the presence of MT (100 nM), MT receptor agonist ramelteon (1 nM), or vehicle solution (DMSO) (see Figure 6A).
Figure 5.
Persistent activation of β-cell MT receptor signaling improves β-cell dysfunction associated with glucotoxicity in human islets. A, Graphical illustration of the experimental work flow. B, Immunoblot detection of carbonyl groups introduced into proteins due to oxidative stress and derivatized to DNP in human islets previously incubated for 72 hours in standard RPMI 1640 media supplemented with either 5 mM glucose (control) or 25 mM glucose (glucotoxic conditions) and exposed for the final 12–14 hours to media containing MT (10 or 100 nM), MT receptor agonist (ramelteon, 1 nM), or vehicle (DMSO). C and D, Assessment of glucose and incretin (GLP-1, 10 nM)-stimulated insulin secretion in human islets previously incubated for 72 hours in standard RPMI 1640 media supplemented with either 5 mM glucose (control) or 25 mM glucose (glucotoxic conditions) and exposed for the final 12–14 hours to media containing MT (100 nM), MT receptor agonist (ramelteon, 1 nM), or vehicle (DMSO). DMSO vehicle treatment was administered to all non-MT/ramelteon conditions to correct for potential confounding effects of DMSO, which was used to prepare MT/ramelteon solutions. Data are expressed as mean ± SEM and an average of five independent islet shipments. *, P < .05 vs basal.
Figure 6.
Persistent activation of β-cell MT receptor signaling improves β-cell dysfunction in T2DM human islets. A, Graphical illustration of the experimental work flow. B and C, Assessment of glucose and incretin (GLP-1, 10 nM)-stimulated insulin secretion in T2DM human islets incubated in standard RPMI 1640 media and exposed to overnight incubation (12–14 hours) in media containing MT (100 nM), MT receptor agonist (ramelteon, 1 nM), or vehicle (DMSO). Data are expressed as mean ± SEM and an average of four independent islet shipments. *, P < .05 vs basal. DMSO vehicle treatment was administered to all non-MT/ramelteon conditions to correct for potential confounding effects of DMSO, which was used to prepare MT/ramelteon solutions.
Statistical analysis
Results are expressed as the means ± SEM for n independent experiments, as indicated in figure legends. Statistical analyses were carried out by a Student's t test. A value of P < .05 was taken as evidence of statistical significance.
Results
MT receptor activation potentiates cAMP/PKA/CREB pathway in β-cells
Consistent with previous reports, we confirmed robust MT receptor expression in human and rodent β-cells as well as in INS 832/13 cells used in the current study (Figure 1). Acute exposure of β-cells to MT (10 nM) led to MT-2 internalization (Figure 1), consistent with the kinetics of MT receptor activation.
Figure 1.
MT receptor expression in β-cells. Representative examples of nondiabetic human (A) and rat (B) islets stained for insulin (green), MT-2 (red) and nuclei (blue). C and D, INS 832/13 cells stained for insulin (green), MT-2 (red), and nuclei (blue) treated with either MT (10 nM) for 1 hour or vehicle solution.
We next investigated whether persistent overnight exposure to MT potentiates activation of CREB and ERK1/2 pathways (Figure 2 and Supplemental Figure 1), both known to play important roles in the regulation of β-cell function and survival (19–21). Exposure to MT (in concentrations ranges 1–100 nM for 12–14 hours) led to the enhancement of CREB activation (phosphorylation of serine 133), particularly evident at 10 nM MT concentration (Figure 2 and Supplemental Figure 1). Pretreatment with 10 nM MT enhanced phospho-CREB levels in the basal state (1.4 fold vs vehicle, P < .05, Figure 2, B and C, n = 6) as well as in response to incretin hormones such as GIP (1.7-fold vs vehicle, P < .05, Figure 2, B and C, n = 6) and GLP-1 (3.1-fold vs vehicle, P < .05, Figure 2, B and C, n = 6). In contrast, overnight exposure to MT (in concentrations ranges 1–100 nM for 12–14 hours) did not augment activation/phosphorylation of ERK1/2 (p44/p42 MAPK) (Figure 2, B and D, and Supplemental Figure 1, n = 4–6).
MT receptor activation promotes β-cell survival in a cell model of proteotoxicity
We next examined whether pretreatment of β-cells with MT promotes β-cell survival in T2DM. We transduced INS 832/13 cells with adenovirus expressing h-IAPP, a cell model of β-cell apoptosis that recapitulates molecular features of β-cell demise in patients with T2DM (ie, induction of endoplasmic reticulum and oxidative stress [22]). As expected, cells overexpressing h-IAPP exhibited robust β-cell apoptosis associated with induction of caspase-3 cleavage, activation/phosphorylation of JNK (stress activated protein kinase/Jun-amino-terminal kinase) at threonine 183 and tyrosine 185 (Figure 3, B and C), and induction of oxidative stress assessed by detection of oxidatively modified proteins (Figure 3D). MT exposure (10 nM, 12–14 hours) blunted h-IAPP-induced β-cell apoptosis evidenced by reduced caspase-3 cleavage (∼40% vs vehicle, P < .05, Figure 3, B and C, n = 5) and reduced phosphorylation of JNK (∼50% vs vehicle, P = .055, Figure 3, B and C, n = 3). Furthermore, MT treatment also decreased proteotoxicity-associated oxidative stress (∼50% vs vehicle, P < .05, Figure 3D and Supplemental Figure 2, n = 5). The experiments conducted with INS 832/13 cells overexpressing the nonamyloidogenic rodent IAPP ascertained that MT receptor activation decreases the toxicity associated with the amyloidogenic properties of h-IAPP rather than just protein overload (Supplemental Figure 3).
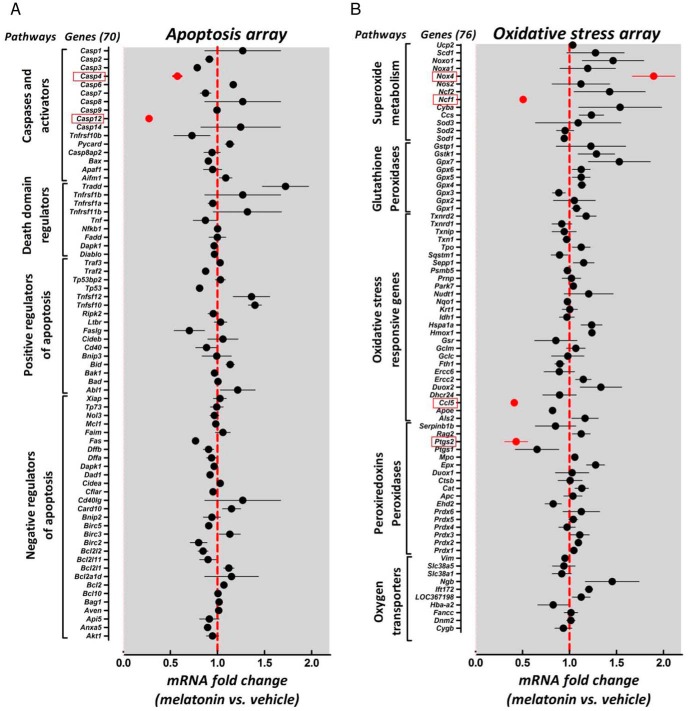
To delineate putative transcriptional targets of MT signaling in β-cells, we used INS 832/13 cells with adenovirus-expressing cytotoxic h-IAPP exposed (12–14 hours) to either vehicle (DMSO) or MT (10 nM) treatment and subsequently assessed approximately 150 genes known to mediate cellular apoptosis or oxidative stress pathways (Figure 4). Most notably, the activation of MT signaling resulted in a significant reduction in Caspase 12 (0.27 ± 0.02-fold vs vehicle, P < .05, n = 3) and Caspase 4 (0.57 ± 0.04-fold vs vehicle, P < .05, n = 3) mRNA expression, purported mediators of β-cell loss in diabetes (22) (Figure 4A). Also, activation of MT signaling reduced mRNA expression of cellular mediators of reactive oxygen species production and oxidative stress response such as Ncf1 (p47phox) (0.50 ± 0.04-fold vs vehicle, P < .05, Figure 4B, n = 3) and Ptgs2 (Cox-2) (0.42 ± 0.12-fold vs vehicle, P < .05, n = 3), genes known to promote β-cell failure (23, 24). In addition, MT treatment also increased mRNA expression of Nox4 (1.9 ± 0.23-fold vs vehicle, P = .058, Figure 4B, n = 3), a gene shown to enhance β-cell functionality (25).
Figure 4.
Persistent activation of β-cell MT receptor signaling modulates apoptotic and oxidative stress gene expression in response to β-cell proteotoxicity. Apoptosis RT2 Profiler PCR (A) and oxidative stress RT2 Profiler PCR (B) arrays demonstrating relative mRNA expression of 146 genes purported to mediate cellular apoptotic and oxidative stress pathways in INS 832/13 cells transduced with h-IAPP adenovirus for 48 hours and exposed to either MT (10 nM) or vehicle (DMSO). The graph represents mRNA levels expressed as fold change for MT (10 nM) vs vehicle (DMSO)-treated cells (n = 3 independent experiments for each array). Data are expressed as mean ± SEM. Dashed red line represents mRNA expression in vehicle (DMSO)-treated cells. Genes demonstrating notably significant changes vs vehicle are highlighted in red. DMSO vehicle treatment was administered to all non-MT conditions to correct for potential confounding effects of DMSO, which was used to prepare MT solutions.
MT receptor activation promotes β-cell function in human islets
We next tested the potential of MT receptor activation to mitigate β-cell dysfunction in human islets (see Supplemental Table 1 for islet donor information). To induce β-cell dysfunction characteristic of T2DM, we first exposed isolated human islets to chronic hyperglycemia (72 hours at 25 mM glucose), a condition associated with decreased CREB expression (26), induction of oxidative stress (27), and loss of GSIS (28). Chronic hyperglycemia led to the induction of oxidative stress and loss of glucose and incretin-stimulated insulin secretion (Figure 5, B–D). MT as well as MT receptor agonist pretreatment (12–14 hours) resulted in a significant improvement in oxidative stress as well as partial restoration of glucose-stimulated (P < .05, basal vs glucose-stimulated for MT and MT receptor agonist, Figure 5C, n = 5) and incretin-stimulated (P < .05, basal vs GLP-1 stimulated for MT receptor agonist, Figure 5D, n = 5) insulin response. Finally, we studied β-cell function in cadaveric islets obtained from patients with T2DM, which demonstrated a decline in glucose- (P = .2 for basal vs glucose stimulated, Figure 6B, n = 4) and incretin-stimulated insulin responsiveness (P = .1 for basal vs GLP-1-stimulated, Figure 6C, n = 4). Activation of MT receptor signaling was beneficial in restoring GSIS in islets from patients with T2DM (P < .05, basal vs glucose stimulated for MT pretreatment, Figure 6B, n = 4), but failed to show significant improvement for incretin-mediated insulin release (Figure 6C, n = 4).
Discussion
A variant in MTNR1B (gene encoding MT-2 receptor) is associated with impaired MT receptor signaling and consequent increased susceptibility for T2DM (29), raising a postulate that MT receptor signaling is involved in the regulation of glucose control. Importantly, the association between the MTNR1B variant and T2DM is attributed to β-cell failure rather than alteration in insulin sensitivity or body size (6, 30, 31). In particular, in a recent study of 43 single-nucleotide polymorphisms associated with T2DM, the MTNR1B variant displayed the strongest effect on diminished insulin secretion and disposition index during an oral glucose tolerance test (7). Given that β-cell failure is essential to the onset of T2DM, in the current study, we tested whether activation of MT signaling in vitro is beneficial for the preservation of β-cell function and survival in the context of molecular stress associated with T2DM. We report that MT signaling has the potential to attenuate proteotoxicity-induced β-cell apoptosis and improve deleterious consequences of glucotoxicity and T2DM on insulin secretion and oxidative stress in human islets.
A role for MT in the regulation of glucose homeostasis and β-cell health was proposed decades ago (32), an observation supported by a number of recent studies (8, 11, 13, 33, 34). Pinealectomized rodents develop diurnal hyperglycemia, impaired glucose tolerance, and loss of GSIS (33, 34). These deleterious metabolic effects of pinealectomy are presumably mediated due to the loss of tissue MT signaling because a majority of these effects are reversed by nightly MT supplementation (33). Interestingly, MT levels are reduced in humans with T2DM (and rodent models of diabetes), whereas nondiabetic individuals exhibiting low nightly MT secretion appear to be at an increased risk of developing diabetes (8, 11). Whether a progressive loss of MT secretion and signaling in humans contributes to the induction of β-cell failure and predisposition to T2DM remains to be explored; however, our current data provide further support for the functional link between β-cell MT receptor signaling and regulation of β-cell survival and function in T2DM.
Activation of MT receptor signaling (with duration designed to mimic the period of darkness ie, > 8 hours) has been shown to sensitize cAMP-dependent signal transduction pathway, particularly by potentiating PKA and CREB activation, a phenomenon shown to be present in pancreatic β-cells (15, 18). The ability of MT to potentiate the cAMP-dependent signal transduction pathway is important for MT's ability to regulate circadian rhythms and clock gene expression (12, 35) and also appears to contribute to regulation of β-cell function and survival in human islets. Previous work has shown that adequate PKA activity is essential for the stimulation of acute insulin response to glucose (19, 21). Moreover, CREB expression is essential for maintaining proper β-cell mass, function, and protection against β-cell apoptosis (21). Mice deficient in CREB expression develop diabetes characterized by reduced β-cell mass and increased β-cell apoptosis (36). Furthermore, expression of a CREB dominant-negative mutant in isolated human islets leads to increased β-cell apoptosis, whereas overexpression of CREB reverses β-cell apoptosis by raising Bcl-2 levels (26). Interestingly, MTNR1B variants characterized by impaired cAMP-dependent signaling display increased association with T2DM, whereas a variant with a loss of ERK1/2 signaling (another pathway activated by MT signaling) did not demonstrate an association with T2DM risk (29). Further studies are needed to delineate the exact molecular transduction pathways responsible for the beneficial effects of MT signaling on β-cell function and survival.
To assess the potential transcriptional targets responsible for beneficial effects of MT signaling on the β-cell, we used a cell model of β-cell failure due to h-IAPP-induced proteotoxicity (37–39). Importantly, this cell model recapitulates key molecular features of β-cell failure in T2DM, particularly the induction of endoplasmic reticulum and oxidative stress (40). Because MT signaling has been previously shown to attenuate the cellular apoptosis program and reduce oxidative burden (41), we performed targeted PCR arrays to examine effects of MT signaling on expression of nearly 150 genes purported to mediate apoptotic and oxidative stress pathways (Figure 4). Apoptotic array data revealed significantly attenuated mRNA expression of proapoptotic caspases-3, -4, and -12 in response to MT treatment, consistent with the antiapoptotic effects of MT signaling on the β-cell (Figure 4A). Importantly, a nearly 70% reduction in Caspase-12 expression is particularly notable, given that an increase in β-cell caspase-12 expression is a feature of T2DM (22, 42).
Oxidative stress array data also revealed intriguing biological targets of β-cell MT signaling (Figure 4B). Activation of MT signaling resulted in a substantial reduction in Ncf1 (also known as p47phox) expression, which encodes an activating subunit of nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzymes. Ncf1-dependent NOX activation is an important determinant of cellular reactive oxygen species accumulation and consequent induction of oxidative stress (43). Interestingly, the deletion of Ncf1 results in the attenuation of hyperglycemia-induced oxidative stress and β-cell dysfunction in a mouse model of diabetes (23). Moreover, the activation of MT signaling also led to a significant decline in prostaglandin-endoperoxidase synthase 2 (Ptgs2, also known as Cox-2) mRNA. Induction of Ptgs2 expression, which occurs in response to a number of proinflammatory and prooxidative stimuli in β-cells, leads to increased prostaglandin E2 biosynthesis, which acts as a potent inhibitor of β-cell function (24). Importantly, the inhibition of Ptgs2 gene expression has been shown to enhance β-cell function and attenuate the deleterious effects of prooxidative stressors on the β-cell (44). Taken together, further studies are needed to delineate the exact signaling pathways linking MT receptor activation with downstream transcriptional targets. In that context, a number of described genes have been shown to contain cAMP responsive elements in their promoter regions (45, 46).
In our human islet studies, prolonged exposure to hyperglycemia resulted in elevated basal insulin secretion and the subsequent inability to further elevate insulin secretion in response to a glucose challenge, an observation consistent with previous reports (28). The mechanisms underlying this observation appear to be related to changes in genetic β-cell programming (dedifferentiation), likely implemented to defend against ensuing oxidative damage (47). Subsequently, a number of typically repressed genes (eg, MCT1, LDHA, and HK1) have been shown to be up-regulated, and β-cell identity genes (PDX-1, Mafa, and NKX6.1) be suppressed in β-cells exposed to prolonged hyperglycemia and/or oxidative stress (47, 48). This metabolic switch favors glycolytic over oxidative metabolism and results in the stimulation of ATP production and insulin secretion at basal glucose levels and the consequent failure to further enhance ATP production and insulin secretion after a glucose challenge (49). How does the activation of MT signaling reverse increased basal insulin secretion and partially restore glucose-stimulated insulin release in glucotoxic islets? One plausible hypothesis will be through the attenuation of oxidative stress, which will likely result in a diminution of β-cell dedifferentiation as recently described by Guo et al (48).
Another potential mechanism by which MT signaling may regulate β-cell function and survival is through the modulation of circadian clock gene expression. Accumulating evidence suggests that the functional β-cell circadian clock is essential for proper β-cell function and survival as well as maintenance of normal glucose homeostasis (50–52). Indeed, disrupting the β-cell circadian clock either via genetic manipulation or exposure to circadian misalignment in vivo leads to a loss of insulin secretion, diminished β-cell growth, and increased susceptibility to oxidative stress and β-cell apoptosis (50–53). Diurnal activation of MT receptor signaling has been shown to modulate clock gene expression (eg, Per-1) in the pituitary (54) and the pancreas (55) in vivo as well as potentiate clock gene expression in cultured β-cells (12). It is unknown whether the beneficial effects of MT on the β-cell observed in our study are attributed to improved β-cell circadian clock function; however, it is important to point out that islets isolated from T2DM patients appear to exhibit disrupted clock gene expression patterns (56). Further work is needed to understand the role of MT receptor signaling in the control of β-cell circadian clock function.
In conclusion, our data demonstrates that the persistent activation of MT signaling in vitro has the potential to attenuate β-cell loss and dysfunction associated with molecular stress present in T2DM. This work provides further support for the functional link between MT receptor signaling and regulation of β-cell survival and function in T2DM and suggests that chronic activation of MT signaling presents a potential preventative and treatment strategy to preserve β-cell mass and function in T2DM.
Acknowledgments
We are grateful to Kevin Hsu (University of California, Los Angeles) for excellent technical support. We also thank Dr Peter C. Butler and Dr Christopher S. Colwell (University of California, Los Angeles) for their helpful discussions and insightful comments.
This work was supported by Grant R01DK098468 from the National Institutes of Health and an investigator-initiated grant from Takeda Pharmaceuticals (to A.V.M.). S.C. was supported by Grant 2012-D-001-SUP from the Larry L. Hillblom Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- adenylate cyclase
- CREB
- cAMP responsive element binding protein
- DMSO
- dimethylsulfoxide
- DNP
- 2,4-dinitrophenylhydrazine
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide 1
- GSIS
- glucose-stimulated insulin secretion
- h-IAPP
- human islet amyloid polypeptide
- JNK
- Jun-amino-terminal kinase
- MOI
- multiplicity of infection
- MT
- melatonin
- MT-2
- MT receptor 2
- NOX
- nicotinamide adenine dinucleotide phosphate oxidase
- PKA
- protein kinase A
- RT
- room temperature
- T2DM
- type 2 diabetes mellitus.
References
- 1. Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–229. [DOI] [PubMed] [Google Scholar]
- 2. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. [DOI] [PubMed] [Google Scholar]
- 3. Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest. 1967;46:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marchetti P, Del Guerra S, Marselli L, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89:5535–5541. [DOI] [PubMed] [Google Scholar]
- 5. Yoneda S, Uno S, Iwahashi H, et al. Predominance of β-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab. 2013;98:2053–2061. [DOI] [PubMed] [Google Scholar]
- 6. Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jonsson A, Ladenvall C, Ahluwalia TS, et al. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on α- and β-cell function and insulin action in humans. Diabetes. 2013;62:2978–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mantele S, Otway DT, Middleton B, et al. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS One. 2012;7:e37123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peschke E, Frese T, Chankiewitz E, Peschke D, et al. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006;40:135–143. [DOI] [PubMed] [Google Scholar]
- 10. Bach AG, Muhlbauer E, Peschke E. Adrenoceptor expression and diurnal rhythms of melatonin and its precursors in the pineal gland of type 2 diabetic Goto-Kakizaki rats. Endocrinology. 2010;151:2483–2493. [DOI] [PubMed] [Google Scholar]
- 11. McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishiyama K, Hirai K. The melatonin agonist ramelteon induces duration-dependent clock gene expression through cAMP signaling in pancreatic INS-1 β-cells. PLoS One. 2014;9:e102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44:26–40. [DOI] [PubMed] [Google Scholar]
- 14. Korkmaz A, Topal T, Tan DX, Reiter RJ. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 2009;10:261–270. [DOI] [PubMed] [Google Scholar]
- 15. von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. [DOI] [PubMed] [Google Scholar]
- 16. Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. [DOI] [PubMed] [Google Scholar]
- 17. Barrett P, Schuster C, Mercer J, Morgan PJ. Sensitization: a mechanism for melatonin action in the pars tuberalis. J Neuroendocrinol. 2003;15:415–421. [DOI] [PubMed] [Google Scholar]
- 18. Kemp DM, Ubeda M, Habener JF. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic β cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol Cell Endocrinol. 2002;191:157–166. [DOI] [PubMed] [Google Scholar]
- 19. Kaihara KA, Dickson LM, Jacobson DA, et al. β-Cell-specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes. 2013;62:1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costes S, Longuet C, Broca C, et al. Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann NY Acad Sci. 2004;1030:230–242. [DOI] [PubMed] [Google Scholar]
- 21. Dalle S, Quoyer J, Varin E, Costes S. Roles and regulation of the transcription factor CREB in pancreatic β-cells. Curr Mol Pharmacol. 2011;4:187–195. [DOI] [PubMed] [Google Scholar]
- 22. Huang CJ, Lin CY, Haataja L, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. [DOI] [PubMed] [Google Scholar]
- 23. Liu GC, Fang F, Zhou J, et al. Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse. Diabetologia. 2012;55:2522–2532. [DOI] [PubMed] [Google Scholar]
- 24. Robertson RP, Chen M. A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J Clin Invest. 1977;60:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uchizono Y, Takeya R, Iwase M, et al. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80:133–139. [DOI] [PubMed] [Google Scholar]
- 26. Costes S, Vandewalle B, Tourrel-Cuzin C, et al. Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in β-cells and human pancreatic islets. Diabetes. 2009;58:1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J Biol Chem. 2004;279:42351–42354. [DOI] [PubMed] [Google Scholar]
- 28. Eizirik DL, Korbutt GS, Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the β-cell function. J Clin Invest. 1992;90:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonnefond A, Clement N, Fawcett K, et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langenberg C, Pascoe L, Mari A, et al. Common genetic variation in the melatonin receptor 1B gene (MTNR1B) is associated with decreased early-phase insulin response. Diabetologia. 2009;52:1537–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staiger H, Machicao F, Schafer SA, et al. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine β-cell function. PLoS One. 2008;3:e3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milcou SM, Vrejoin G, Marcean R, Nanu L. [Effect of a hypoglycemic pineal hormone on the endocrine pancreas in alloxanized animals; morphological study.]. Ann Endocrinol (Paris). 1957;18:621–627. [PubMed] [Google Scholar]
- 33. la Fleur SE, Kalsbeek A, Wortel J, van der Vliet J, Buijs RM. Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night-time glucose concentrations. J Neuroendocrinol. 2001;13:1025–1032. [DOI] [PubMed] [Google Scholar]
- 34. Lima FB, Machado UF, Bartol I, et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol. 1998;275:E934–E941. [DOI] [PubMed] [Google Scholar]
- 35. von Gall C, Garabette ML, Kell CA, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5:234–238. [DOI] [PubMed] [Google Scholar]
- 36. Jhala US, Canettieri G, Screaton RA, et al. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Costes S, Huang CJ, Gurlo T, et al. β-Cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes. 2011;60:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124:3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rivera JF, Gurlo T, Daval M, et al. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic β-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ. 2011;18:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pappolla MA, Sos M, Omar RA, et al. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J Neurosci. 1997;17:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang CJ, Haataja L, Gurlo T, et al. Induction of endoplasmic reticulum stress-induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293:E1656–E1662. [DOI] [PubMed] [Google Scholar]
- 43. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 44. Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1β-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet β-cell function. Diabetes. 2002;51:1772–1778. [DOI] [PubMed] [Google Scholar]
- 45. Zhang X, Zhang J, Yang X, Han X. Several transcription factors regulate COX-2 gene expression in pancreatic β-cells. Mol Biol Rep. 2007;34:199–206. [DOI] [PubMed] [Google Scholar]
- 46. Peshavariya HM, Liu GS, Chang CW, Jiang F, Chan EC, Dusting GJ. Prostacyclin signaling boosts NADPH oxidase 4 in the endothelium promoting cytoprotection and angiogenesis. Antioxid Redox Signal. 2014;20:2710–2725. [DOI] [PubMed] [Google Scholar]
- 47. Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. β-Cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(suppl 1):S154–S159. [DOI] [PubMed] [Google Scholar]
- 48. Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123(8):3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J Biol Chem. 2002;277:10912–10921. [DOI] [PubMed] [Google Scholar]
- 50. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee J, Moulik M, Fang Z, et al. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol Cell Biol. 2013;33:2327–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic β-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann NY Acad Sci. 2005;1040:508–511. [DOI] [PubMed] [Google Scholar]
- 55. Muhlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. [DOI] [PubMed] [Google Scholar]
- 56. Stamenkovic JA, Olsson AH, Nagorny CL, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985. [DOI] [PubMed] [Google Scholar]