Abstract
Introduction
Ductoscopy is a low invasive method enabling the diagnostics of intraductal proliferative lesions in breasts. Fiberoptic ductoscopy (FDS) is important in the diagnosis of patients with pathological nipple discharge. There are attempts to apply FDS in patients with breast cancer without the presence of nipple discharge.
Aim
To assess fiberoptic ductoscopy in the diagnostics of breast neoplasms.
Material and methods
The material was composed of a group of 164 patients treated for intraductal proliferative lesions in breasts. In the analyzed group of patients, FDS was conducted in 128 patients with pathological nipple discharge and 36 patients with the presence of breast cancer. The analyzed period was divided into three sub-periods. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FDS examination verified by post-operative histopathological examination were analyzed. The safety of the method was also assessed, taking into consideration the complications.
Results
An increasing number of successful ductoscopies together with the number of performed FDS examinations was noted. There were statistically significant differences in the percentage of successful cannulations in relation to the number of performed FDS examinations in the three subsequent stages of the project (p = 0.011). The duration of FDS examination in the third period was reduced in comparison with the first and second period (p < 0.001). Sensitivity of fiberoptic ductoscopy is 68.1%, specificity 77.3% and PPV 90.4%, but NPV is 44.1%.
Conclusions
The introduction of fiberoptic ductoscopy in our clinic has contributed to the widening of the diagnostic possibilities of small intraductal lesions of the mammary gland.
Keywords: fiberoptic ductoscopy, pathologic nipple discharge, diagnostic method of the breast
Introduction
Ductoscopy (fiberoptic ductoscopy – FDS) is a low invasive method enabling the diagnostics of intraductal proliferative lesions in breasts [1]. Fiberoptic ductoscopy is important in the diagnosis of patients with pathological nipple discharge [2–7]. Unilateral, spontaneous discharge as well as discharge from a single mammary duct belong to the group of discharges from the mammary gland defined as pathological [8–12]. The reasons for pathological nipple discharge (PND) may be as follows: duct ectasia, intraductal proliferative lesions such as usual ductal hyperplasia, ductal intraepithelial neoplasia, intraductal papillary neoplasms, intraductal lobular carcinoma. Among patients with breast cancer, PND occurs in 5–10% of patients [2–7]. In the light of current data, mammary ductoscopy is the best method to examine patients with nipple discharge. There have been attempts to use FDS in patients with breast cancer without nipple discharge, that is in forced or provoked discharge [13–16]. It is also a clinically important problem due to the fact that 36% of patients with breast cancer have breast lumps. However, in this case FDS is significant in the assessment of the extension of cancer in mammary ducts, and therefore it has an influence on the scope of the operation [2–7].
Aim
The aim of the article is to assess FDS in the diagnostics of intraductal breast neoplasms.
Material and methods
The material was composed of a group of 164 patients treated for intraductal proliferative lesions in breasts in the Department of Surgical Oncology, Medical University of Gdansk, Poland during the period from December 2004 to January 2010. In the analyzed group of patients, FDS was conducted in 128 patients with pathological nipple discharge and 36 patients with the presence of breast cancer but without a confirmed nipple discharge (Photo 1). In the first group, in 123 patients successful cannulation of mammary ducts was carried out with the possibility of assessment of the lesions in mammary ducts as well as qualification for surgical treatment. In the second group, successful cannulation was observed in 25 examined patients with the assessment of mammary ducts in the area of a lump with regard to number and character of lesions in mammary ducts.
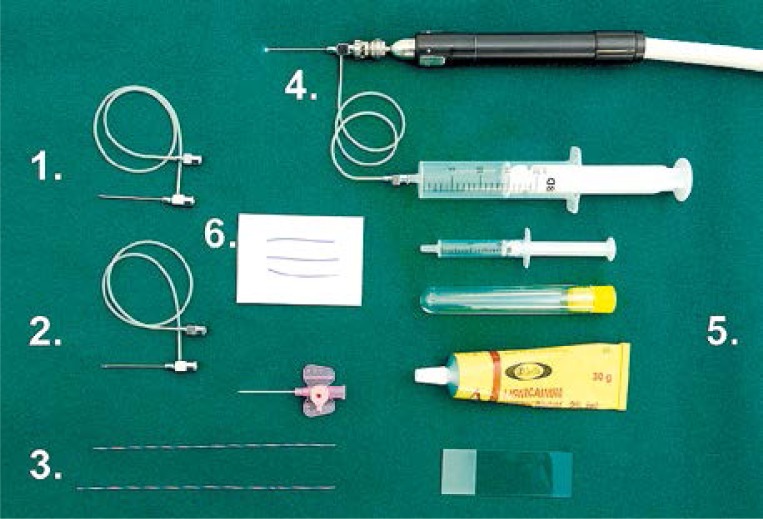
Photo 1.
Fiberoptic ductoscopy diagnostic package (Volpi AG, Switzerland): 1 – diagnostic cannula, 2 – biopsy cannula, 3 – dilatator, 4 – fiberoptic ductoscopy with diagnostic cannula, 5 – cytology set, 6 – sutures for marking mammary duct after microductectomy operation
Patients below the age of 20 in the period of lactation were excluded with the exception of cases of bleeding from the nipple and discharge from several ducts as a side effect of some medicines such as psychotics, antihypertensive drugs, anti-emetics as well as hormonal drugs, being the reason for pathological nipple discharge [4, 17].
Follow-ups in patients operated on due to pathological nipple discharge or disqualified from surgical treatment were conducted after 6 and 12 months, during which post-operative complications such as wound infection, pain, cosmetic effect and the presence of recurrence of nipple discharge were assessed.
In order to have a full assessment of the introduction of fiberoptic ductoscopy, the analyzed period was divided into 3 sub-periods (stage 1: 12.2004–08.2005; stage 2: 09.2005–05.2008; stage 3: 06.2008–02.2010). During FDS examination the mean time of the examination in three periods and the percentage of successful cannulations of mammary ducts were assessed. Moreover, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FDS examination verified by post-operative histopathological examination were analyzed. During FDS examination, the safety of the method was assessed, taking into consideration the complications and the pain level during the examination. The examination was conducted subject to the approval of the commission NKBBN (466/2004).
In patients with PND the mammary ducts were assessed by means of FDS, taking into consideration the character and number of intraductal proliferative lesions. During FDS, the intraductal proliferative lesion classification from 2002 was used; the classification was suggested by the Japanese Association of Mammary Ductoscopy (JAMD) according to their own modification in which the following were distinguished: normal duct, single papilloma, multiple papilloma, amputation of a duct, circular narrowing or hyperplasia, ductectasia, ambiguous results (reddening, red spots), microcalcifications [5, 18–20].
All examined patients with PND received local anesthesia in the form of lidocaine in aerosol (Lidocaini, Egis Pharmaceuticals Ltd., Hungary) – it was applied to 128 (79.9%) patients. General anesthesia with endotracheal intubation was applied to the patients with confirmed breast cancer in 36 (20.1%) patients.
Statistical analysis
Data concerning normal distribution were compared by means of Student's t-test after previous check of the normality of distribution by means of the Kolmogorov-Smirnov test. Data which did not have a normal distribution were analyzed by means of non-parametric tests (Mann-Whitney U test, Kruskal-Wallis test). For continuous variables, the mean and range were given. Categorical variable values were described by means of their number and percentage in sub-groups, rounding to the nearest value. The comparison of categorical valuables was done by means of a χ2 test according to Pearson. In case of a low number in sub-groups, Yates’ correction and Fisher's exact test were applied. Correlations between chosen variables were assessed on the basis of the Pearson product-moment correlation coefficient. Statistical programs SPSS v. 13.0 (SPSS Inc, USA) and Statistica v. 8.0 (Stat Soft Inc, USA) were used for statistical calculations.
Results
The average time of follow-up of FDS patients was 12 months (range of observation: 5–36 months). The examined group consisted of 128 patients (78%) with pathological nipple discharge and 36 patients (22%) with breast cancer without discharge. Median age in the whole group was 53 years (range of age: 21–84 years). Other demographic data are presented in Table I.
Table I.
Demographic characteristics of patients undergoing fiberoptic ductoscopy (n = 164)
| Parameter | Median | Range |
|---|---|---|
| Age [years] | 53.0 | 21–84 |
| Weight [kg] | 67.5 | 49–112 |
| Height [cm] | 164 | 149–178 |
| Body mass index [kg/m2] | 24.3 | 20–41 |
Cannulation
The analysis of the percentage of successful cannulations assessed in sub-periods of introduction of the method showed an increase in the percentage of successful cannulations along with the number of performed FDS examinations. The comparison of unsuccessful cannulations in the three subsequent stages of introducing FDS is presented in detail in Table II. In the first period of introduction of the project (stage 1: 12.2004–08.2005) among 85 performed FDS examinations, there were 71 (83.5%) successful cannulations and 14 (14.7%) unsuccessful cannulations. During stage 2 (stage 2: 09.2005–05.2008) 39 FDS examinations were performed with 38 (97.4%) successful cannulations and 1 (2.6%) unsuccessful cannulation. During stage 3 (06.2008–02.2010) among 40 FDS there were 39 (97.5%) successful cannulations and 1 (2.5%) unsuccessful cannulation. Statistically significant differences in the percentage of cannulations in relation to the number of performed FDS examinations in the three successive stages of the project (p = 0.011) occurred. Unsuccessful cannulations in 16 patients examined by FDS were caused by: narrow mammary ducts in 8 (4.9%), lack of visible outlet of mammary duct on the nipple in 6 (3.7%) patients and direct location of intraductal proliferative lesions behind the duct on the nipple in 2 (1.2%) patients.
Table II.
Comparison of the number of unsuccessful vs. successful cannulations at consecutive sub-periods of implementation of fiberoptic ductoscopy procedure in Department of Oncological Surgery in Gdansk (p = 0.011) (n = 164)
| Sub-periods of the project* | Successful cannulations | Unsuccessful cannulations | Value of p |
|---|---|---|---|
| 1 stage | 71 (83.5%) | 14 (16.4%) | 0.011 |
| 2 stage | 38 (97.4%) | 1 (2.6%) | |
| 3 stage | 39 (97.5%) | 1 (2.5%) |
Stage 1: 12.2004–08.2005; stage 2: 09.2005–05.2008; stage 3: 06.2008–02.2010
Fiberoptic ductoscopy duration
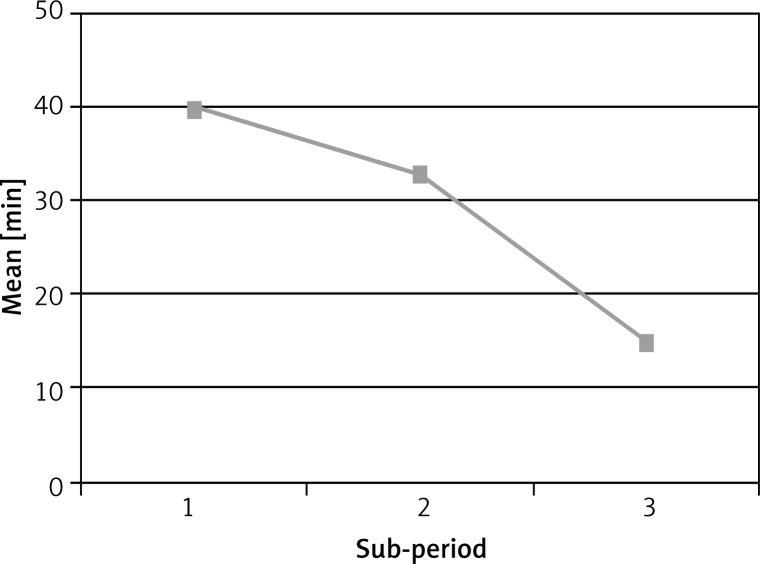
Mean duration of FDS among 164 qualified patients was 26.5 min and median duration of the examination was 25 min (range of FDS duration: 10–65 min). In the first period of introducing the method FDS duration median was 40 min, but in the second and third it was 33 min and 15 min. Figure 1 presents a gradual reduction of time medians for FDS examination along with gaining experience. Duration of FDS examination differed statistically between the above periods of introducing the examination (p < 0.001).
Figure 1.
Mean duration of fiberoptic ductoscopy procedure in consecutive sub-periods of the project (n = 164)
Changes in mammary ducts
Among 148 successful FDS, intraductal proliferative lesions were found in 130 (87.8%) patients and multiple intraductal proliferative lesions in 18 (12.2%) patients. No intraductal proliferative lesions in mammary ducts were found in 66 (43.9%) patients. Among intraductal proliferative lesions, single papillomas were found in 13 (8.9%) and multiple papillomas in 11 (7.4%). In 23 (15.5%) there was amputation of a duct, and circular narrowing or hyperplasia in 11 (7.4%) patients. Ambiguous results (reddening, red spots) were found in 20 (13.5%) patients, and in 4 (2.7%) microcalcifications were noticed (Table III).
Table III.
Results of fiberoptic ductoscopy of patients with PND and forced discharge (n = 164)
| Parameter | Patients (%) |
|---|---|
| Anesthesia during FDS: | |
| Local | 128 (78) |
| General | 36 (22) |
| Number of lesions found in the duct (n = 148): | |
| Single | 130 (87.8) |
| Multiple | 18 (12.2) |
| * Results: FDS (n = 148) | |
| Normal | 65 (43.9) |
| Single papilloma | 13 (8.9) |
| Multiple papilloma | 11 (7.4) |
| Amputation of a duct | 23 (15.5) |
| Circular narrowing or hyperplasia | 11 (7.4) |
| Ductectasia | 1 (0.7) |
| Ambiguous results (reddening, red spots) | 20 (13.5) |
| Microcalcifications | 4 (2.7) |
Modified classification of intraductal proliferative lesions proposed by JAMD in 2002 [5].
On the basis of the results of FDS it was possible to make a decision concerning the performance of a surgical procedure or recommend further observation of the patient. In the group of 123 patients with PND after a successful FDS, 72 (58.5%) patients were qualified for a surgical procedure and 51 (41.5%) were recommended further clinical observation without a surgical procedure. Patients with forced discharge due to breast cancer diagnosis were treated surgically according to the pre-operative decision of the interdisciplinary team.
Sensitivity, specificity, positive predictive value, negative predictive value of fiberoptic ductoscopy in patients with pathological nipple discharge
Comparison of the results of FDS examination with post-operative histopathological examination was possible in 72 patients qualified for a surgical procedure. Among 47 (65.3%) patients whose lesions were confirmed by FDS examination, in 42 (58.3%) lesion presence was confirmed by post-operative histopathological examination (PPV). However, in the remaining 5 (7%) patients, lesions in mammary ducts seen during FDS examination were not confirmed during post-operative histopathological examination (NPV). Lack of lesions in mammary ducts in FDS examination was confirmed in post-operative histopathological examination in 17 (23.6%) patients (PPV). On the basis of obtained data concerning the results of FDS verified by post-operative histopathological examination it was calculated that sensitivity of FDS is 68.1%, specificity 77.3%, PPV 90.4%, and NPV 44.1% (Table IV).
Table IV.
Comparison of fiberoptic ductoscopy against postoperative histopathological evaluation
| Results of histopathology | Total | |||
|---|---|---|---|---|
| + | – | |||
| Result of fiberoptic ductoscopy | + | 42 (58.3%) | 5 (7%) | 47 (65.3%) |
| – | 8 (11.1%) | 17 (23.6%) | 25 (34.7%) | |
| Total | 50 (69.4%) | 22 (30.6%) | 72 (100%) | |
(+) Pathological data, (–) normal data; sensitivity – 68.1%; specificity – 77.3%; PPV – 90.4%; NPV – 44%.
Safety and quality of life of the patients after fiberoptic ductoscopy
Among 164 patients qualified for FDS examination, complications were found only in 6 (3.6%) patients. Early complications (found during FDS examination), i.e. damage to a mammary duct, were confirmed in 2 (1.2%) patients. Both complications were noted during the first stage of introducing FDS in the clinic. Late complications were noted during follow-ups after performing FDS in the form of topical inflammation in the place of cannulation of mammary ducts in 4 (2.4%) patients. All patients who had late complications also experienced associated symptoms such as reddening near the nipple and pain. After introducing a wide spectrum of antibiotic therapy the complications receded. In the group of patients who had FDS examination and were disqualified from a surgical procedure, no changes in the quality of life were observed during follow-ups (6 months and 12 months). In only one patient, a continuous nipple discharge was noted 6 months after the first FDS examination. The patient was qualified for microductectomy. During a follow-up conducted after 6 months no relapse of nipple discharge was observed in this patient. In patients who had FDS and then microductectomy, follow-ups were performed after 6 and 12 months. In only 2 patients after microductectomy, after 3 months of observation a recurrence of nipple discharge was confirmed. After conducting another FDS examination a suspicious mammary duct was noted and the patient was qualified for microductectomy, after which the symptoms receded. In the remaining group of patients, neither recurrence of nipple discharge nor change in the quality of life was observed.
Discussion
Constant aspiration for the improvement of unsatisfying results of breast cancer treatment manifests itself in searching for a more and more perfect method of diagnosis of breast cancers. Its aim is to diagnose the disease in an early stage. The fact that approximately 80% of breast cancers are formed in the epithelium of mammary ducts, from which invasive ductal cancer develops through the stage of intraductal cancer, indicates the usefulness of searching for new methods of diagnosis at the level of mammary ducts. One of the diagnostic options is FDS [21, 22]. The creators of the method were probably motivated by the fact that at the level of the nipple in mammary ducts, papillomas near the nipple and Paget's disease develop. The lactiferous sinus lying just behind the nipple is a common place of development of ductectasia. Segmental ducts which lie just behind it are a common area of formation of single papillomas. Sub-segmental ducts are usually the origin for fibroadenomas and benign cysts, and they are a place of abnormal epithelial cell division and development of the majority of breast cancers.
Zagouri et al. tried to determine the number of examinations necessary to master the FDS method. In order to do that they performed FDS on nipples after performing mastectomy (ex vivo) to master the procedure. They estimated that performing at least 20 FDS examinations allows one to master the technique – mastering the technique was defined by 90% successful cannulations [23]. The differences in the results of the cited study and the results from our clinic are due to significant differences in methodology. The disadvantage of the study conducted by Zagouri et al. was the small group of patients examined. Moreover, those authors did not present the results of FDS examination of the examined mammary glands. In our material, a rate of 90% successful cannulations (counting from the first successful cannulation) was achieved after performing 160 examinations; however, in order to master the method within the learning curve it is necessary to perform 20–40 FDS examinations. In the case of other surgical procedures such as marking the sentinel lymph node in breast cancer or melanoma, it is thought that in order to master the method within the learning curve it is necessary to perform about 40 procedures. In the case of fine-needle biopsy, it is thought that this number is approximately 20 examinations [24]. On the basis of the data from literature and our own results, it was concluded that mastering the technique of FDS is not difficult. The gradual increase in the percentage of successful cannulations was also directly connected with the time of performing FDS. Since the moment of starting FDS, a gradual reduction of ductoscopy has been observed. The duration of FDS examination has not been analyzed in the literature so far, though it is very important due to the quality of performing the examination. Optimal reduction of the duration of the examination was achieved in the third stage of the introduction of the method in the clinic, as in other procedures, such as sialoendoscopy, video-assisted thyroidectomy and management of pancreatic pseudocysts [25–27].
The contribution of FDS examination to the diagnosis lies in the recognition of PND, especially in the case of minor lesions in mammary ducts not visible during conventional examination such as galactography, ultrasonography and mammography [7]. The FDS is a method enabling the performance of direct examination of mammary ducts. In the case of patients who had papillomas, FDS turned out to be very useful when making therapeutic decisions [28]. In patients with PND who underwent FDS and no lesions in mammary ducts were confirmed, it seems that the surgical procedure may be abandoned. It has been reported that owing to FDS examination it is possible to reduce the number of procedures by approximately 40% [28]. In our material consisting of 128 patients with PND who were qualified for the examination, owing to FDS examination the surgical procedure was abandoned in 51 (41.5%) patients. In the group of patients disqualified from surgical treatment in the first period of introducing the examination, further FDS observation was conducted.
The FDS may be significant in planning the procedure in breast surgery. It should be emphasized that FDS may be useful during procedures of removal of milk ducts. Due to the fact that FDS allows for selective marking of a mammary duct with a visible papilloma, it also enables the reduction of the scope of removal of the mammary gland [29].
In our material the assessment of compliance of described images in FDS with post-operative histopathological examination was carried out. The aim was to determine the best method to assess the quality of diagnostic examinations. It is in compliance with the opinions of other authors [19, 29–32]. Among 47 (65.3%) patients examined by FDS, the presence of lesions in mammary ducts in post-operative histopathological examination was confirmed in 42 (58.3%) patients. False positive results were noted in 5 (7%) patients. In post-operative histopathological examination, lack of lesions in mammary ducts was confirmed in 17 (23.6%) patients. On the basis of data concerning the results of FDS verified by the results of post-operative histopathological examination, the following parameters were calculated: sensitivity, specificity, PPV and NPV. Their values were: 68.1%, 77.3%, 90.4% and 44% respectively. High FDS sensitivity, specificity and PPV results are conclusive evidence for an important and decisive role in the diagnosis of intraductal lesions in breasts. However, the low NPV of FDS (44%) indicates that in case of a negative result of examination in some patients without lesions in mammary ducts there is a possibility of overlooking existing intraductal lesions. It seems that the reason for this could be an insufficient number of FDS examinations performed in the Department of Surgical Oncology of the Medical University of Gdansk.
Despite many publications concerning FDS in the last two decades, little attention was paid to complications and limitations connected with performing this procedure. Kamali et al. assessed FDS examination taking into consideration its usefulness in the diagnostics of patients with PND, and they also analyzed the complications connected with performing this examination. In 43 patients FDS was not completed due to: damage to a mammary duct in 9 (20.9%) patients, deformation of the mammary gland in 5 (11.6%) patients, presence of nipple ulceration in one patient (2.3%), severe pain which made the examination impossible in 2 (4.6%) patients, relocated (ectopic) mammary duct in 1 (2.3%) and a narrow milk duct on a nipple in 25 (5.8%) patients [19].
Among 164 patients qualified for FDS examination, complications were observed in 6 (3.7%) patients. In the group of patients with PND during performing FDS in 4 (2.45%) patients topical inflammation was observed. However, the inflammation receded after several days of antibiotic therapy. Damage of the mammary duct during FDS examination was observed in 2 (1.2%) patients. The appearance of this complication was connected with the presence of narrow mammary ducts. It seems that in cases of narrow mammary ducts together with the presence of bloody discharge from a nipple, it is advisable to abandon FDS examination and it is recommended to perform a surgical procedure.
Conclusions
The introduction of FDS in our clinic has contributed to the development of the diagnostic possibilities in slight intratubular changes of the breast and has become a valuable supplement of radiological methods applied so far. Moreover, due to this examination in uncertain cases such as PND with duct ectasia it was possible to abandon the operation in view of lack of intratubular hyperplastic lesions.
The assessment of the results of FDS examination in patients with PND and with breast cancer without discharge allowed for the following conclusions:
The development of FDS technique in the Department of Surgical Oncology of the Medical University of Gdansk has allowed for a systematic increase in the number of successful cannulations and a reduction of the duration of FDS.
The FDS applied to patients with pathological nipple discharge is characterized by high sensitivity and high specificity in assessment of location and character of intratubular hyperplastic lesions.
The FDS is a safe method both in diagnostic tests and when used as an auxiliary tool in surgery of patients with breast cancers. It is associated with few early and late complications, which enables the application of this method in ambulatory environments.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ferlay JF, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Dietz JR, Crowe JP, Grundfest S, et al. Directed duct excision by using mammary ductoscopy in patients with pathologic nipple discharge. Surgery. 2002;132:582–8. doi: 10.1067/msy.2002.127672. [DOI] [PubMed] [Google Scholar]
- 3.Grunwald S, Heyer H, Paepke S, et al. Diagnostic value of ductoscopy in the diagnosis of nipple discharge and intraductal proliferations in comparison to standard methods. Onkologie. 2007;30:243–8. doi: 10.1159/000100848. [DOI] [PubMed] [Google Scholar]
- 4.Hünerbein M, Raubach M, Gebauer B, et al. Ductoscopy and intraductal vacuum assisted biopsy in women with pathologic nipple discharge. Breast Cancer Res Treat. 2006;99:301–7. doi: 10.1007/s10549-006-9209-9. [DOI] [PubMed] [Google Scholar]
- 5.Makita M, Akiyama F, Kimura K, et al. Mammary ductoscopic diagnosis of intraductal spread of breast cancer. Jap J Breast Cancer. 2001;16:274–8. [Google Scholar]
- 6.Mokbel K, Escobar PF, Matsunaga T. Mammary ductoscopy: current status and future prospects. Eur J Surg Oncol. 2005;31:3–8. doi: 10.1016/j.ejso.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer MH, Perloff LJ, Kelley RI, et al. The significance of age in patients with nipple discharge. Surg Gynecol Obstet. 1970;131:519–22. [PubMed] [Google Scholar]
- 8.Goldstein NS, Decker D, Severson D, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007;110:1687–96. doi: 10.1002/cncr.22981. [DOI] [PubMed] [Google Scholar]
- 9.Bjerring P, Arendt-Nielsen L. Depth and duration of skin analgesia to needle insertion after topical application of EMLA cream. Br J Anaesth. 1990;64:173–7. doi: 10.1093/bja/64.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Escobar PF, Baynes D, Crowe JP. Ductoscopy-assisted microdochectomy. Int J Fertil Womens Med. 2004;49:222–4. [PubMed] [Google Scholar]
- 11.King TA, Carter KM, Bolton JS. A simple approach to nipple discharge. Am Surg. 2000;66:960–6. [PubMed] [Google Scholar]
- 12.King EB, Chew KL, Petrakis NL, et al. Nipple aspirate cytology for the study of breast cancer precursors. J Natl Cancer Inst. 1983;71:1115–21. [PubMed] [Google Scholar]
- 13.Aleksandrowicz A. Anatomical nomenclature [Polish]; Warsaw: PZWL; 1989. [Google Scholar]
- 14.Brogi E, Robson M, Panageas KS, et al. Ductal lavage in patients undergoing mastectomy for mammary carcinoma: a correlative study. Cancer. 2003;98:2170–6. doi: 10.1002/cncr.11758. [DOI] [PubMed] [Google Scholar]
- 15.Jassem J. Breast cancer: manual for students and doctors [Polish]; Warsaw: PWN; 1998. [Google Scholar]
- 16.Montroni I, Santini D, Zucchini G, et al. Nipple discharge: is its significance as a risk factor for breast cancer fully understood? Observational study including 915 consecutive patients who underwent selective duct excision. Breast Cancer Res Treat. 2010;123:895–900. doi: 10.1007/s10549-010-0815-1. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki A, Okazaki M, Asaishi K, et al. Fiberoptic ductoscopy of the breast: a new diagnostic procedure for nipple discharge. Jpn J Clin Oncol. 1991;21:188–93. [PubMed] [Google Scholar]
- 18.Hou MF, Huang TJ, Huang YS, et al. A simple method of duct cannulation and localization for galactography before excision in patients with nipple discharge. Radiology. 1995;195:568–9. doi: 10.1148/radiology.195.2.7724785. [DOI] [PubMed] [Google Scholar]
- 19.Kamali S, Bender O, Aydin MT, et al. Ductoscopy in the evaluation and management of nipple discharge. Ann Surg Oncol. 2010;17:778–83. doi: 10.1245/s10434-009-0820-y. [DOI] [PubMed] [Google Scholar]
- 20.Zieliński J, Jaworski R, Świerblewski M, et al. The evaluation of ductoscopy as a new diagnostic tool in diseases of the breast: our experiences and data from the literature [Polish] Nowotwory J Oncol. 2010;60:116–21. [Google Scholar]
- 21.Dooley WC. Breast ductoscopy. In: Bland KI, Copeland EM, editors. The breast: comprehensive management of benign and malignant disorders. 4th ed. Philadelphia: Sauders; 2009. pp. 731–5. [Google Scholar]
- 22.Mokbel K. Nipple endoscopy: focus on a new diagnostic tool. Int J Fertil Womens Med. 2003;48:197–9. [PubMed] [Google Scholar]
- 23.Zagouri F, Sergentanis TN, Giannakopoulou G, et al. Breast ductal endoscopy: how many procedures qualify? BMC Research Notes [on-line] 2009;2:115. doi: 10.1186/1756-0500-2-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamaki Y, Miyoshi Y, Noguchi S. Application of endoscopic surgery for breast cancer treatment. Nippon Geka Gakkai Zasshi. 2002;103:835–8. [PubMed] [Google Scholar]
- 25.Kopec T, Wierzbicka M, Piskadło K, et al. Sialoendoscopy – a diagnostics and therapeutic approach subjectively rated by patients. Videosurgery Miniinv. 2014;9:505–10. doi: 10.5114/wiitm.2014.44176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barczynski M, Konturek A, Stopa M, et al. Minimally invasive video-assisted thyreidectomy: seven-year experience with 240 cases. Videosurgery Miniinv. 2012;7:175–80. doi: 10.5114/wiitm.2011.28871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sileikis A, Beisa A, Jurevicius S, et al. Minimally invasive management of pancreatic pseudocysts. Videosurgery Miniinv. 2013;8:211–5. doi: 10.5114/wiitm.2011.33809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling H, Liu GY, Lu JS, et al. Fiberoptic ductoscopy-guided intraductal biopsy improve the diagnosis of nipple discharge. Breast J. 2009;15:168–75. doi: 10.1111/j.1524-4741.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 29.Dietz JR, Kim JA, Malycky JL, et al. Feasibility and technical considerations of mammary ductoscopy in human mastectomy specimens. Breast J. 2000;6:161–5. doi: 10.1046/j.1524-4741.2000.99098.x. [DOI] [PubMed] [Google Scholar]
- 30.Hünerbein M, Raubach M, Gebauer B, et al. Intraoperative ductoscopy in women undergoing surgery for breast cancer. Surgery. 2006;139:833–8. doi: 10.1016/j.surg.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Makita M, Sakamoto G, Akiyama F, et al. Duct endoscopy and endoscopic biopsy in the evaluation of nipple discharge. Breast Cancer Res Treat. 1991;18:179–88. doi: 10.1007/BF01990034. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki A, Hirata K, Okazaki M, et al. Nipple discharge disorders: current diagnostic management and the role of fiber-ductoscopy. Eur Radiol. 1999;9:583–90. doi: 10.1007/s003300050715. [DOI] [PubMed] [Google Scholar]