Dear Sirs
Dietary vitamin K influences warfarin response, but its effect on anticoagulation control during the initiation phase of therapy is still poorly quantified. Studies of patients already on stable warfarin therapy have shown that lower mean daily vitamin K intake is associated with unstable and non-therapeutic International Normalised Ratios (INRs) (1, 2), and higher vitamin K intake may lead to sub-therapeutic INRs (3). The risk of adverse bleeding and thromboembolic events, however, is greatest during the initiation phase of warfarin therapy when INRs tend to be non-therapeutic (4). In this prospective cohort study, we evaluated dietary vitamin K intake as a risk factor for poor therapeutic INR control during the initiation phase of warfarin therapy.
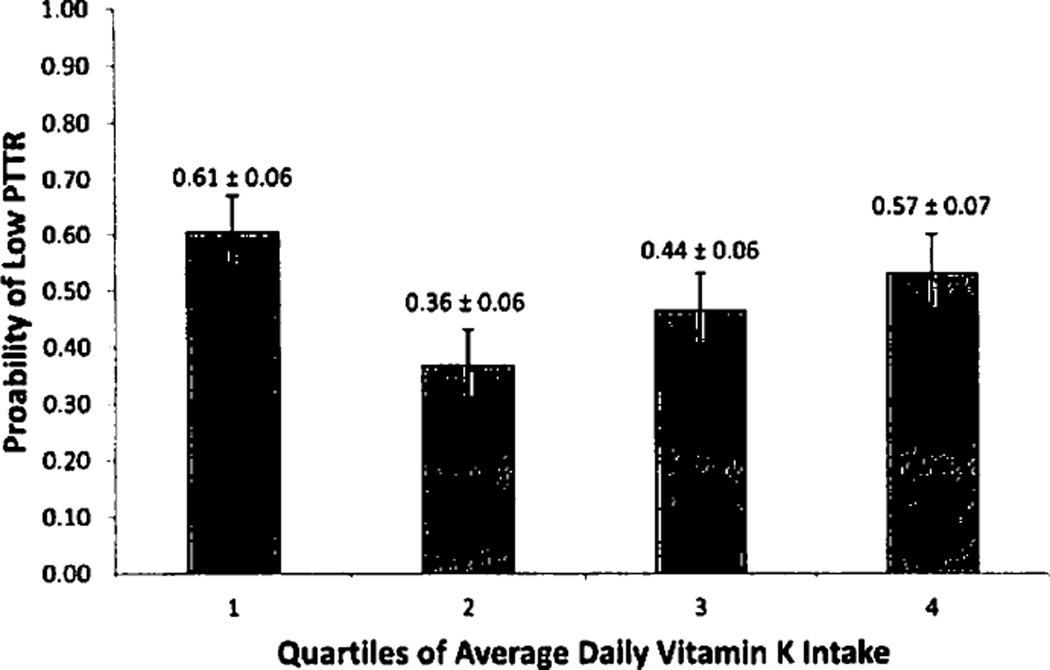
Data were obtained from a U.S. prospective cohort study designed to determine the associations between genetics and adherence with warfarin response (5). The 368 patients were recruited from three outpatient anticoagulation clinics from 2002–2005. All patients who completed a dietary vitamin K food diary were included in this study. Patients were considered to reach maintenance dose after having three consecutive INRs in therapeutic range without a dose change. Vitamin K intake was assessed using a validated prospective food diary given to patients at enrollment and collected at the first follow-up visit (6). The exposure, average daily vitamin K intake (ADVK), was calculated as the mean self-reported daily vitamin K intake over a 7-day period. The primary outcome was having a low percent time in therapeutic range (PTTR), defined as a PTTR below the median (7). Only INRs collected between the time of vitamin K assessment and attainment of maintenance dose were used for analysis. The proportion of patients with low PTTR for each ADVK quartile was compared using the Chi-square test, and the ADVK quartile containing the lowest proportion of patients with low PTTR was designated as the reference quartile for all analyses. Age, interacting medications, and VKORC1 and CYP2C9 polymorphisms were included a priori in the model due to previously described associations with INR values (8). Because patients were enrolled at different times during therapy, time from therapy initiation, defined as the first anticoagulation clinic visit to study enrollment, was also included a priori as a covariate in the final model. All other covariates that were associated with the primary outcome with a p<0.2 cut-off were identified as potential confounders and added to the final model. Predicted probabilities of low PTTR for each ADVK quartile were calculated for the fully adjusted model, holding all other covariates at their sample means. Of the 368 patients in the total cohort, 282 (77%) returned the vitamin K diet diary by the first follow-up visit. Median PTTR was 82%. With the exception of time from therapy initiation to enrollment, none of the baseline patient, clinical, and genetic variables differed significantly across ADVK quartiles (see Suppl. Table 1, available online at www.thrombosis-online.com). The proportion of patients with low PTTR differed significantly across ADVK quartiles (p=0.017), with ADVK Q2 having the best anticoagulation control. Univariable logistic regression demonstrated that the odds of low PTTR were higher in ADVK Q1 vs ADVK Q2 (odds ratio [OR] = 2.76, 95% confidence interval [CI]: 1.31–5.81) as well as in ADVK Q4 vs ADVK Q2 (OR = 2.25, 95% CI: 1.03–4.91). Odds of low PTTR were higher in ADVK Q3 versus ADVK Q2, but this difference was not statistically significant (OR = 1.44, 95% CI: 0.68–3.03). In the fully adjusted model controlling for age, race, interacting medications, marital status, smoking status, VKORC1 and CYP2C9 polymorphisms, and time to enrollment, the increase in odds of low PTTR persisted for ADVK Ql (OR = 2.80; 95% CI: 1.32–5.91) and ADVK Q4 (OR = 2.28; 95% CI: 1.06–4.93). ADVK Q2 had the lowest adjusted predicted probability for low PTTR (0.36; 95% CI: 0.24–0.48) (► Figure 1). For a list of vitamin K content of foods and corresponding vitamin K content used in the vitamin K prospective food diary (in ascending order of vitamin K content) see Suppl. Table 2 (available online at www.thrombosis-online.com).
Figure 1. Predicted probabilities of low PTTR stratified by ADVK quartile.
Calculated at the sample means for age, gender, race, CYP2C9 and VKORC1 polymorphisms, marital status, smoking status, interacting medications, time to enrollment. Error bars signify standard error.
Maintaining therapeutic INRs during the initiation phase of warfarin therapy is critical for minimising adverse events and maximising therapeutic effectiveness, yet difficult given the increased INR variability and rate of adverse events early in therapy (4). Vitamin K intake is among several factors thought to influence INRs during warfarin therapy, but has not been well studied among patients in the initiation phase of therapy. Clinical guidelines stress maintaining a stable level of dietary vitamin K intake during warfarin therapy but are inconsistent about absolute levels of intake and do not distinguish between the initiation and maintenance phases of therapy (9–11). Our prospective study shows that a moderate level of dietary vitamin K intake is associated with the lowest risk of non-therapeutic INRs during the initiation phase of warfarin therapy, suggesting that careful modulation of vitamin K intake when starting warfarin may be important for anticoagulation control. Such dietary control may be challenging since dietary vitamin K intake varies widely in the general population, particularly among the elderly (12), who derive significant benefit from anticoagulation but are at increased risk for adverse events (8). In conjunction with dietary counseling, low-dose vitamin K supplements, which help stabilise INRs among unstable warfarin patients during maintenance phase (13), may improve warfarins therapeutic effectiveness during the initiation phase.
Strengths of this study include a well-characterised, racially diverse cohort of patients in the initiation phase of warfarin therapy and use of a validated, prospective 7-day food diary for accurate dietary vitamin K assessment. Nevertheless, our vitamin K estimates are self-reported and semi-quantitative, so they are not sufficient to generate specific intake guidelines. Further, our patients were not all enrolled at the time of therapy initiation, although adjustment for time to enrollment in our primary analysis and a sensitivity analysis performed using all INRs from the initiation of warfarin therapy yielded similar estimates (data not shown). Our results suggest that moderate vitamin K intake may be optimal when initiating warfarin. Future prospective studies, including those with quantitative vitamin K measures at the beginning of therapy initiation, are warranted.
Acknowledgments
Financial support:
This work was supported by NIH grant R01HLO66176. a grant from the Doris Duke Charitable Foundation, and the USDA, Agricultural Research Service under Cooperative Agreement No. 58–1950–7–707. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors, and do not necessarily reflect the view of the USDA or the National Institutes of Health.
Dr. Kimmel has received research funding from, and served as a consultant to, several pharmaceutical companies, all unrelated to warfarin or vitamin K.
Footnotes
Conflicts of interest
All other authors have no conflicts of interest to disclose.
References
- 1.Rombouts EK, Rosendaal FR, Van der Meer FJM. Influence of dietary vitamin K intake on subtherapeutic oral anticoagulant therapy. Br J Haematol. 2010;149:598–605. doi: 10.1111/j.1365-2141.2010.08108.x. [DOI] [PubMed] [Google Scholar]
- 2.Sconce E, Khan T, Mason J, et al. Patients with un-stable control have a poorer dietary intake of vitamin K compared to patients with stable control of anticoagulation. Thromb Haemost. 2005;93:872–875. doi: 10.1160/TH04-12-0773. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen F, Hamberg O, Hess K, et al. The effect of dietary vitamin K on warfarin-induced anticoagulation. J Intern Med. 1991;229:517–520. doi: 10.1111/j.1365-2796.1991.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 4.Ansell J, Hirsh J, Hylek K, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 5.Platt AB, Localio AR, Brensinger CM, et al. Can we predict daily adherence to warfarin?: Results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Chest. 2009;137:883–889. doi: 10.1378/chest.09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courts RR, Tataronis GR, Booth SL, et al. Development of a self-assessment instrument to determine daily intake and variability of dietary vitamin K. J Am Coll Nutr. 2000;19:801–807. doi: 10.1080/07315724.2000.10718081. [DOI] [PubMed] [Google Scholar]
- 7.Rosendaal F, Cannegieter S, Van der Meer F, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 8.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemosl. 2009;102:268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 9.Booth SI, Centurelli M. Vitamin K: a practical guide to the dietary management of patients on warfarin. Nutrition Rev. 1999;57:288–296. doi: 10.1111/j.1753-4887.1999.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson M. Influence of Vitamin K on Anticoagulant Therapy Depends on Vitamin K Status and the Source and Chemical Forms of Vitamin K. Nutrition Rev. 2005;63:91–100. doi: 10.1111/j.1753-4887.2005.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 11.Warren Grant Magnuson Clinical Center. National Institutes of Health Drug Interaction Task Force. Important information to know when you are taking: Coumadin' and vitamin K. 2003 Available at: http://ods.od.nih.gov/pubs/factsheels/coumad.inl.pdf.
- 12.Thane CW, Paul aa, Bates CJ, Bolton-Smith C, Prentice A, Shearer MJ. Intake and sources of phyl-loquinone (vitamin KI): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr. 2007;87:605. doi: 10.1079/BJNBJN2002583. [DOI] [PubMed] [Google Scholar]
- 13.Sconce E, Avery P, Wynne H, et al. Vitamin K supplementation can improve stability of anticoagulation for patients with unexplained variability in response to warfarin. Blood. 2007;109:2419–2423. doi: 10.1182/blood-2006-09-049262. [DOI] [PubMed] [Google Scholar]