Abstract
Extracellular proteolysis is an important regulatory nexus for coordinating synaptic functional and structural plasticity, but the identity of such proteases is incompletely understood. Matrix metalloproteinases (MMPs) have well-known, mostly deleterious roles in remodeling after injury or stroke, but their role in nonpathological synaptic plasticity and function in intact adult brains has not been extensively investigated. Here we address the role of MMP-9 in hippocampal synaptic plasticity using both gain- and loss-of-function approaches in urethane-anesthetized adult rats. Acute blockade of MMP-9 proteolytic activity with inhibitors or neutralizing antibodies impairs maintenance, but not induction, of long-term potentiation (LTP) at synapses formed between Schaffer-collaterals and area CA1 dendrites. LTP is associated with significant increases in levels of MMP-9 and proteolytic activity within the potentiated neuropil. By introducing a novel application of gelatin-substrate zymography in vivo, we find that LTP is associated with significantly elevated numbers of gelatinolytic puncta in the potentiated neuropil that codistribute with immunolabeling for MMP-9 and for markers of synapses and dendrites. Such increases in proteolytic activity require NMDA receptor activation. Exposing intact area CA1 neurons to recombinant-active MMP-9 induces a slow synaptic potentiation that mutually occludes, and is occluded by, tetanically evoked potentiation. Taken together, our data reveal novel roles for MMP-mediated proteolysis in regulating nonpathological synaptic function and plasticity in mature hippocampus.
INTRODUCTION
Long-lasting changes in strength of synaptic neurotransmission reflect cellular mechanisms enabling acquisition of new skills and formation of memories (Bliss and Collingridge 1993; Jorntell and Hansel 2006; Pastalkova et al. 2006; Rioult-Pedotti et al. 2000; Rioult-Pedotti et al. 1998; Rogan et al. 1997; Whitlock et al. 2006). Long-term potentiation (LTP) is an enduring increase in synaptic strength (Bliss and Lømo 1973), and in hippocampus, LTP elicited at synapses formed between the Schaffer collaterals and the dendrites of area CA1 neurons exhibits at least two phases. A rapidly appearing but transient early phase (E-LTP) arises through posttranslational modifications of proteins that regulate neurotransmitter release and neurotransmitter receptor function (Barria and Malinow 2005; Castillo et al. 2002; Liao et al. 1992; Shi et al. 1999; Silva et al. 1992). A subsequently emerging but persistent late phase (L-LTP) requires, additionally, mRNA transcription and coordinated synthesis and degradation of certain proteins (Fonseca et al. 2006; Frey et al. 1988; Impey et al. 1996; Karpova et al. 2006; Nguyen et al. 1994). The enduring nature of LTP has been attributed, in part, to long-term structural remodeling of synaptic contacts. For example, growth of new dendritic spines (Engert and Bonhoeffer 1999; Hosokawa et al. 1995), increased numbers of presynaptic boutons, or formation of multi-synapse boutons (Antonova et al. 2001; Bozdagi et al. 2000; Nägerl et al. 2004; Toni 1999) and enlargement of spine heads (Kopec et al. 2006; Matsuzaki et al. 2004) have all been associated with enduring LTP. Consistent with such morphological remodeling, it is well-recognized that the structural molecules of the synapse, including adhesion proteins and proteins of the extracellular matrix, are modified by plasticity-inducing stimuli and are required for long-lasting synaptic plasticity (reviewed in: Benson et al. 2000; Dityatev and Schachner 2003). These data suggest that there must be mechanisms for coordinating functional and structural remodeling of synaptic connectivity. Although such mechanisms remain incompletely understood, recent studies indicate that regulated extracellular proteolysis may be important for coupling functional and structural changes in synaptic architecture (Baranes et al. 1998; Huang et al. 1996; Komai et al. 2000; Madani et al. 1999; Mataga et al. 2004; Matsumoto-Miyai et al. 2003; Oray et al. 2004; Pang et al. 2004; Tamura et al. 2006).
Matrix metalloproteinases (MMPs) are a family of mostly secreted, very potent proteolytic enzymes that collectively can degrade the entire extracellular matrix as well as cleave certain cell-surface and other proteins (Sternlicht and Werb 2001). MMPs are generally secreted in an inactive (pro-) form, becoming proteolytically active by removal of the pro-peptide sequence. MMPs function canonically throughout most tissues of the body both in physical remodeling of the pericellular microenvironment and in cell-cell or cell-matrix signaling via activation or liberation of bioactive fragments (Nagase and Woessner 1999). In mature brain, their activity—and in particular, that of MMP-9 — has customarily been associated with protracted remodeling that occurs with injury, degeneration, inflammation, and other pathophysiological contexts (Lo et al. 2002; Reeves et al. 2003; Szklarczyk et al. 2002; Zhang et al. 1998). However, recent evidence suggests that MMP function in hippocampus can be regulated on much faster time scales by synaptic activity of the kind associated with normal, nonpathological brain function. In acute hippocampal slices, levels and proteolytic activity of MMP-9 are rapidly and selectively enhanced by stimuli that induce L-LTP, whereas pharmacological or genetic disruption of MMP-9 activity impairs LTP selectively (Nagy et al. 2006). These data suggest new mechanistic roles for MMPs in synaptic plasticity and brain function. Nevertheless, these data derive mostly from experiments conducted on acutely prepared hippocampal slices taken from young (3– 6 wk-old) rats and mice, thus it remains an open question as to whether MMP-9 activity is regulated by, and contributes to, LTP induced in intact adult brains in vivo.
Here we address this question directly using both gain- and loss-of-function approaches in urethane-anesthetized adult rats. Our data show that blocking MMP-9 proteolytic activity in area CA1 impairs maintenance of LTP selectively. By immunoblotting and through a novel application of gelatin-substrate zymography in vivo, we show elevated levels of MMP-9 protein and proteolytic activity within the potentiated neuropil. Such “hot spots” of gelatinolytic activity codistribute with markers of synapses and dendrites. Exposing intact area CA1 neurons to recombinant-active MMP-9 induces a slow synaptic potentiation that mutually occludes, and is occluded by, tetanically evoked potentiation. Taken together, our data suggest novel roles for MMP-mediated proteolysis in regulating non-pathological synaptic function and plasticity in mature hippocampus.
METHODS
Electrophysiology
A total of 90 young adult male Sprague Dawley rats (300 g, >3 mo old) were used. The care and treatment of all animals conformed strictly to guidelines approved by the National Institutes of Health and Mount Sinai’s Institutional Animal Care and Use Committee. Rats were anesthetized with urethane (1.5 g/kg ip) and placed in a stereotaxic frame. Rectal temperature was maintained at 37°C. A monopolar tungsten electrode was used to stimulate the Schaffer collateral-commissural projection (from bregma, in mm: AP: 3.5, ML: 3.0, DV: 2.3). Field excitatory postsynaptic potentials (fEPSPs) were recorded in area CA1 stratum radiatum (in mm: AP: 4, ML: 2.8, DV: 2.5) with a glass micropipette filled with 3 M NaCl. Test pulses (100-μs duration) were collected every 30 s; their intensity was adjusted to evoke fEPSP amplitudes that were ~50% of the maximal response. The slope of the fEPSPs was used to generate an input-output (I/O) relationship ranging from subthreshold to maximal response. All responses were expressed as percent change from the average responses recorded during the 20 –30 min immediately before drug application or LTP-inducing tetanic stimuli. LTP was induced with four trains of 100-Hz, 1-s stimulation separated by 5 min. Control (non-LTP-inducing) stimuli consisted of a total of 400 pulses delivered over a duration of 75 min. Paired-pulse facilitation (PPF) was induced by delivering two stimuli with a 20- to 100-ms interstimulus interval. At the termination of the experiments, rats were killed by intracardiac perfusion (4% paraformaldehyde, 10 min). Brain sections (50 μm) were sliced on a freezing sliding microtome, mounted, and Nissl-stained with cresyl-violet. These sections were used to verify placement of stimulating/recording electrodes histologically.
Reagents
MMP inhibitors or recombinant-active MMPs were delivered into area CA1 using either controlled pressure pulses of nitrogen (20 psi, every 2 min over a 20-min period) applied via a Picospritzer to one barrel of a double-barrel glass micropipette in which the other barrel was used for recording fEPSPs or via an infusion cannula connected to a Hamilton syringe driven by a syringe pump (200 nl/min; 2 μl total volume). The onset and duration of drug administration are indicated in individual figures. Concentrations of the inhibitors and active enzymes are: MMP-2/9 Inhibitor II, a gelatinase (MMP-2 and 9) inhibitor [250 μM, catalogue No. 444249, Calbiochem, La Jolla, CA, dissolved in 0.6% dimethylsulfoxide (DMSO)]; an MMP-9 function-blocking mouse monoclonal antibody (clone 6-6B) or nonimmune mouse IgG (10 μg/ml each, Calbiochem); recombinant active-MMP-9 (rMMP-9), recombinant active-MMP-2 (rMMP-2), or recombinant pro- (inactive) MMP-9 (each 0.1 μg/μl, Calbiochem). The selectivity and potency of Inhibitor II over a range of concentrations has been established previously using a fluorometric enzymatic assay (Nagy et al. 2006). The MMP-9 neutralizing antibody recognizes pro- and active forms of MMP-9, but neither form of MMP-2, as shown by enzyme-linked immunosorbent assay [ELISA (Ramos-DeSimone et al. 1993]. Functionally, the MMP-9 neutralizing antibody inhibits MMP-9 enzymatic activation, but has no effect on MMP-2 activation, in a radiolabeled gelatin assay (Ramos-DeSimone et al. 1993).
In vivo gelatin-substrate zymography and quantitative analysis
Loci of proteolysis by endogenous MMPs activated in response to tetanic or control stimuli were identified anatomically by in vivo gelatin zymography (Oh et al. 1999). In brief, gelatin zymography is an assay for gelatinolytic activity in which cells or tissues are exposed to a specific substrate (gelatin) that is recognized and cleaved by gelatinases (which are MMP-2 and -9). The gelatin substrate has been coupled to an intramolecularly quenched fluorophore (FITC). On proteolytic cleavage by MMP-2 and/or -9, the FITC-gelatin fragments fluoresce, and can be localized after fixation and visualization by microscopy. In situ zymography is customarily applied to tissues ex vivo; for the present in vivo studies, we modified a previously described protocol applied to hippocampal slices (Nagy et al. 2006). Two groups of rats (LTP group, n = 7 and control stimulation group, n = 6) were anesthetized and prepared for electrical stimulation and recording as described in the preceding text. The NMDA receptor antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP; 10 mg/kg in normal saline, Sigma) was injected intraperitoneally in one additional group (CPP group, n = 3) 30 min prior to preparation for stimulation and recording. In all groups, after establishing a baseline recording of synaptic responses for 30 min, FITC-DQ gelatin (1 μg/μl, Molecular Probes, Eugene, OR) was pressure-injected over ~10 min into area CA1 via a Picospritzer, delivering a total volume of 5 μl. The LTP and CPP groups then received tetanic stimulation while the control group received control stimuli as described above. Synaptic responses were monitored for 75 min, at which time the animals were transcardially perfused with 4% paraformaldehyde. Sections through the entire hippocampus were cut on a vibratome at a setting of 50 μm and mounted onto glass slides in serial order. Sections were then examined for gelatinolytic fluorescence using a Zeiss LSM 410 confocal microscope (Thornwood, NY). Single optical sections were acquired with a ×40, 1.3 numerical aperture oil-immersion objective, a 488 nm laser-line, and λ515–535 band-pass filter. Qualitative inspection of each series of hippocampal sections showed that the DQ-gelatin spread ~1.5 mm in the rostrocaudal and mediolateral dimensions, although no attempt was made to quantify precisely how far the DQ-gelatin spread in each animal. For quantitative analysis, we chose four to six consecutive tissue sections (for a total of 200 –300 μm of hippocampal tissue) passing through, and centered around, the injection/recording site. From each section, four fields were captured from area CA1 st. radiatum using identical image-capture parameters across conditions. Two fields were taken medial to the injection track, the other two fields were taken lateral to the injection track. The two fields flanking the injection track were ≥250 μm away from the track. The digital images were then imported to a PC computer employing Metamorph software (Universal Imaging), where a thresholding function was used to capture and quantify numbers of gelatinolytic puncta within each field.
In vivo gelatin-substrate zymography and immunolocalization
A group of rats (n = 3) were subjected to LTP-inducing tetanic stimulation followed by injection of DQ-gelatin as described in the antecedent section. Electrophysiological recording verified that LTP was induced in all animals. After perfusion with 4% paraformaldehyde, serial 50-μm-thick sections through the hippocampus were cut frozen on a microtome then incubated in one of the following primary antibodies or sera: rabbit anti-MMP-9 (1:5,000; Torrey Pines); mouse anti-microtubule-associated protein-2 (MAP-2, 1:5,000; Abcam); mouse anti-glial fibrillary acidic protein (GFAP, 1:200, Chemicon); or a cocktail of guinea-pig anti-vesicular glutamate transporter 1 and 2 antibodies (1:20,000 and 1:1,000, respectively; Chemicon). The specificity of the anti-MMP-9 antisera has been characterized by us previously (Nagy et al. 2006). Briefly, this antisera recognizes recombinant pro- and active-forms of MMP-9 in Western blots of recombinant proteins and hippocampal lysates but does not cross-react with either form (pro- or active-) of the structurally and functionally related MMP-2. Primary antibody binding was visualized using species-appropriate, direct fluorophore-conjugated secondary antibodies (Alexa-594,1:400, Jackson Labs). Sections were analyzed by confocal microscopy. Digital images were imported into Adobe Photoshop where minor adjustments in contrast and brightness were made. Control experiments consisted of omitting the primary antibody.
In situ hybridization histochemistry and semi-quantitative analysis
Complementary RNA (cRNA) probes were generated from a mouse-specific cDNA (a gift from Dr. Dylan Edwards) corresponding to a 220-bp fragment of MMP-9 (Tanaka et al. 1993). Specificity of this probe has been confirmed previously by Northern blot (Das et al. 1997). Sense and antisense cRNA probes were transcribed from 1 to 2 μg of purified DNA plasmid template in the presence of 35S-UTP with either T7 or T3 polymerase using an RNA transcription kit (Stratagene, Cedar Creek, TX). One group of rats (n = 6) received LTP-inducing stimulation as described in the preceding text, a second group (n = 4) received control stimuli. Animals were transcardially perfused with cold 4% paraformaldehyde 75 min poststimulation and prepared for in situ hybridization histochemistry. Free-floating tissue sections through hippocampus were processed for hybridization of radioactive cRNA probes using protocols described in detail previously (Barlow et al. 2002; Gil et al. 2002). After overnight probe hybridization, RNase treatment, and the final stringency wash (0.1× SSC, 65°C, 30 min), slides were then dried and exposed to autoradiographic film (β-max, Amersham, Arlington Heights, IL) for 3– 6 days. Quantification of relative intensity of probe hybridization was determined from film autoradiograms using densitometry according to previously published methods of analysis (Gil et al. 2002; Golshani et al. 1997). Sections through hippocampus bilaterally from each group of rats were exposed together on the same sheets of film along with 14C plastic standards (Amersham). Film autoradiograms were scanned at high resolution on a flat-bed scanner, maintaining constant settings across conditions. All images were then imported into ImageJ. Sampling boxes of constant dimensions were placed over st. pyramidale (CA1 and CA3), st. radiatum (CA1), and the dentate gyrus granule cell layer, from which optical density readings were taken and converted to absolute values of radioactivity (nCi/g) by reference to optical density readings of the radioactive standards, used to generate a standard curve of known amounts of radioactivity. Mean values (± SD) were determined across regions analyzed (4 –5 sections/condition, per rat) to compare relative overall levels of probe hybridization across conditions and hippocampal layers.
Immunoblotting
Detailed procedures for Western blot analysis of MMP-9 protein levels in hippocampal lysates have been described in detail previously (Nagy et al. 2006). Briefly, whole hippocampus (n = 3 rats) was removed bilaterally 1-h after LTP induction by tetanic stimulation on one side (ipsilateral) as described in the preceding text. Hippocampi were snap-frozen, followed by dissection of both ipsilateral (LTP) and contralateral area CA1. For these experiments only, the contralateral area CA1, which receives commissural afferents from the stimulated side (Gottlieb and Cowan 1973), was used as the control tissue to take into account interanimal variability in basal levels of MMP-9. Tissue was homogenized in 50 μl of radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 8; 150 mM NaCl; 0.1% SDS; 1% NP-40; 0.5% sodium deoxycholate; and 1× Complete Protease Inhibitor Cocktail, Roche Diagnostics, Mannheim, Germany); protein concentrations were determined using Bio-Rad Protein Assay (Hercules, CA), and 50 μg tissue were loaded onto a 10% SDS-polyacrylamide gel and electrophoresed. Gels were then transferred onto membranes which were probed with antisera to MMP-9 (1:500; Torrey Pines Biolabs, Houston, TX). After incubation in HRP-conjugated secondary antibodies, antibody binding was visualized using SuperSignal West Pico Lumino/Enhancer Solution (Pierce, Rockford, IL) and developed on X-Omat LS Imaging Film (Eastman Kodak, Rochester, NY). Blots were then stripped and reprobed with antisera to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:5,000; Trevigen, Gaithersburg, MD), which was used as a loading control. Blots were scanned and analyzed by Metamorph software using densitometry. Within each lane, pro- and active-MMP-9 band intensities were normalized against the appropriate GAPDH band intensities; data were expressed as a ratio of MMP-9 level in the ipsilateral LTP side to those in the corresponding contralateral control side.
Statistics
Data are expressed as means ± SD and compared using Student’s t-test or one-way ANOVA and a Scheffe’s post hoc test as appropriate where P < 0.05 is considered significant.
RESULTS
Neutralizing MMP-9 proteolytic activity pharmacologically or with blocking antibodies impairs maintenance of LTP in vivo
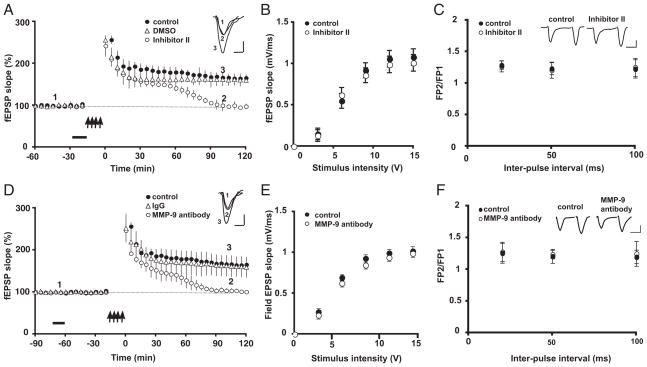
We first established that our tetanic stimulation protocol produces robust, long-lasting potentiation in area CA1 of urethane-anesthetized adult rats. A control LTP group of rats (n = 8) received tetanic stimulation of the Schaffer collateral-commissural pathway. This resulted in a significant increase over baseline in fEPSP slope recorded in area CA1 that was sustained for ≥120 min (165.0 ± 7.9% of baseline at 120 min posttetanus; Fig. 1A, ●), the typical duration of these experiments. To test the role of MMP activity in LTP in vivo, a different set of rats was used to induce LTP in the presence of a pharmacological blocker of MMP activity to determine the effects of this blocker on induction and maintenance of LTP. Inhibitor II is a potent gelatinase (MMP-2 and -9) inhibitor that has been used previously to impair L-LTP in hippocampal slices from young rats (Nagy et al. 2006). After a baseline of synaptic responses was established, Inhibitor II was delivered intrahippocampally either by pressure injection (n = 10) or by infusion pump (n = 8), followed by tetanic stimulation. In both groups, such stimulation produced a strong potentiation that was slightly but not significantly lower in magnitude in comparison with control LTP animals over the first 30 min (P > 0.3 at 30 min posttetanus), but thereafter, potentiation decayed rapidly to baseline by 90 –120 min (Fig. 1A, ○). There were no differences in the effects on LTP between the two methods of Inhibitor II delivery (e.g., at 120 min posttetanus, fEPSP slope was 104.8 ± 6.8% of baseline in the pressure-injected group vs. 97.6 ± 6.1% of baseline in the infusion pump group), therefore the two groups were combined. To rule out nonspecific effects on LTP from the diluent in which Inhibitor II was dissolved, a separate set of animals (n = 8) received an equivalent amount of 0.6% DMSO intrahippocampally via pressure injection. Here we found that potentiation was no different from the control LTP group at any of the time points tested (160.3 ± 7.8% of baseline, 120 min posttetanus, Fig. 1A, ▵, P > 0.4). We also determined that Inhibitor II had no effects on basal synaptic transmission by examining parameters that reflect normal synaptic function. We found that Inhibitor II had no effect on the relationship between stimulus strength and the size of the postsynaptic response (input-output relationship, Fig. 1B) or PPF (Fig. 1C).
FIG. 1.
Matrix metalloproteinase 9 (MMP-9) inhibition impairs long-term potentiation (LTP) maintenance in adult rat area CA1 in vivo. A: tetanic stimulation (↑, 4 trains of 100 Hz, 1-s stimulation separated by 5 min) of the Schaffer collaterals in a control group produces robust potentiation of field excitatory postsynaptic potentials (fEPSPs; ●). In contrast, in rats subjected to intrahippocampal injection of the MMP-9 blocker Inhibitor II (250 μM; bar), potentiation returns to baseline by 90–120 min posttetanic stimulation (○) with no significant effects on LTP induction through the 1st 30 min (P > 0.3 at 30 min compared with control values). There were no effects on LTP after injection of the diluent alone (0.6% DMSO; ▵). Inset: representative EPSP traces were recorded before tetanus (1), 120 min after tetanus in the presence of Inhibitor II (2) and 120 min after tetanus in the presence of the DMSO diluent (3). Calibration: 10 ms, 0.3 mV. B: input–output curves, representing the relationship between stimulus intensity and the size of the fEPSP slope, did not differ significantly between animals injected with Inhibitor II (250 μM, ○) and control animals (●). C: there were no differences in the paired-pulse facilitation (PPF) ratios between animals injected with Inhibitor II (250 μM) and control animals. The PPF ratio represents the slope of the second fEPSP (FP2) divided by the slope of the first fEPSP (FP1) for the interpulse intervals (IPI) shown. Inset: representative EPSP traces from control and inhibitor-treated animals (IPI: 50 ms). Calibration: 25 ms, 0.2 mV. D: rats receiving an intrahippocampal injection of an MMP-9 function-blocking antibody (15 min, bar) show a deficit in LTP maintenance (○) in comparison with control animals (●). Such deficits were similar to those observed with Inhibitor II shown in A. There were no effects on LTP in animals receiving an intrahippocampal injection of nonimmune mouse IgG (▵). Inset: representative EPSP traces were recorded before tetanus (1), 120 min after tetanus in the presence of MMP-9 function-blocking antibody (2) and 120 min after tetanus in the presence of IgG control antibody (3). Calibration as in A. E: there were no differences between control animals and those receiving MMP-9 blocking antibody in input–output curves. F: there were no differences in the PPF ratios between animals injected with MMP-9 blocking antibody and control animals. Inset: representative EPSP traces from control and MMP-9 antibody-treated animals. Other conventions as in C.
To distinguish whether the impairment in LTP maintenance observed with the pharmacological gelatinase (MMP-2/9) inhibitor could be attributed to MMP-2 or -9, we next repeated these experiments on rats receiving intrahippocampal infusions of an MMP-9-specific proteolytic function-blocking antibody (n = 4). Here we found effects on LTP that were very similar to those observed with Inhibitor II (Fig. 1D). In rats receiving blocking-antibody (Fig. 1D, ○), the magnitude of potentiation was slightly but not significantly lower through the first 15 min in comparison with control LTP rats (Fig. 1D, ●, P > 0.25 at 15 min posttetanus) or with rats receiving nonimmune IgG (Fig. 1D, ▵, n = 4), but then potentiation decayed rapidly to baseline values by 90 –120 min. There were no effects of the blocking antibody on input-output relationships (Fig. 1E) or PPF (Fig. 1F), suggesting that the blocking-antibody does not change properties of normal synaptic neurotransmission. Taken together, these observations suggest that blocking MMP-9 activity pharmacologically or with specific neutralizing antibodies in vivo affects maintenance, but not induction, of LTP.
Regulation of MMP-9 levels and proteolytic activity in association with LTP
We next investigated if induction of LTP is associated with regulation of MMP-9 levels and/or MMP proteolytic activity as a basis for the functional role of MMP-9 in LTP maintenance. Immunoblot analysis of area CA1 lysates prepared from rats 60 min after receiving tetanic stimuli (n = 3) shows a significant elevation in levels of both pro- and active forms of MMP-9 in the potentiated area CA1 in comparison with levels in the contralateral control sides (Fig. 2A, P < 0.05). To verify that increased levels of the active form of MMP-9 during LTP represents enhanced proteolytic activity, in vivo zymography was performed in which the MMP-9 substrate DQ-gelatin was injected into hippocampal area CA1 prior to induction of LTP or administration of control stimuli (Fig. 2B). We first determined that DQ-gelatin had no significant effects on synaptic potentiation through 75 min post-LTP induction, when the animals were killed (Fig. 2C; P > 0.05), and no effects on basic synaptic properties such as input-output relationships or PPF (data not shown). At 75 min postinjection, animals were perfused, and hippocampal sections through the injection/recording site were cut and analyzed by confocal microscopy. In rats receiving control stimuli, only occasional fluorescent puncta of gelatinolytic activity were evident in area CA1 st. radiatum (Fig. 2, D and G). In contrast, in rats in which LTP was elicited, significantly greater numbers of fluorescent gelatinolytic puncta were present in area CA1 st. radiatum (Fig. 2, E and G). To rule out that such increased MMP proteolytic activity is a nonspecific reaction to the tetanic stimulation per se, rather than the LTP which results from it, we repeated the experiment on rats that received tetanic stimulation but failed to develop LTP due to prior intraperitoneal administration of the NMDA receptor antagonist CPP (Fig. 2C). In the CPP animals, the numbers of gelatinolytic puncta in area CA1 st. radiatum were similar to those in rats receiving control stimuli (Fig. 2, F and G), indicating that MMP proteolytic activity is enhanced in association with LTP specifically.
FIG. 2.
Regulation of MMP-9 levels and proteolytic activity during LTP. A: representative immunoblots (left) of rat area CA1 homogenates (n = 3 rats) frozen 60 min post-LTP induction. For each animal, homogenates were prepared from ipsilateral (LTP) and contralateral (contra) sides. Membranes were probed with antibodies that recognize both the pro- and active-form of MMP-9 (pro-9, act-9 respectively) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH), used as a loading control. Quantification of band intensity (right) showed significant increases in levels of both pro- and active-forms of MMP-9 in sides undergoing LTP in comparison with those in contralateral sides (*P < 0.05). B: low-magnification confocal image of rat hippocampus showing representative injection of the MMP-9 substrate DQ gelatin into area CA1. SO, stratum oriens; P, s. pyramidale; SR, s. radiatum; S L-M, s. lacunosummoleculare. Bar, 500 μm. C: intrahippocampal injection of DQ-gelatin (bar) has no effects on induction or maintenance of LTP (○, n = 7) in comparison with control animals (●; n = 6, P > 0.05 at 60 min). LTP is abolished in animals receiving an intraperitoneal injection of the N-methyl-D-aspartate (NMDA) receptor antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) prior to tetanic stimulation (triangles, n = 3). Inset: representative EPSP traces were recorded before and 60 min after LTP induction in control, DQ-gelatin (gel) injected and CPP-administered animals. Calibration: 10 ms, 0.5 mV. D—F: high-magnification confocal images through area CA1 s. radiatum taken from animals 75 min after receiving control stimulation (D), LTP-inducing tetanic stimulation (E), or LTP-inducing tetanic stimulation after CPP administration (F). Numerous “hot spots” of gelatinolytic puncta are evident within the potentiated neuropil in the LTP animals in comparison with very few puncta present in animals receiving control stimuli or those in which LTP was blocked by CPP. Bars, 10 μm. G: quantitative analysis of numbers of gelatinolytic puncta within area CA1 s. radiatum. There was significantly greater numbers of puncta in LTP animals in comparison with the other 2 groups (*P < 0.01).
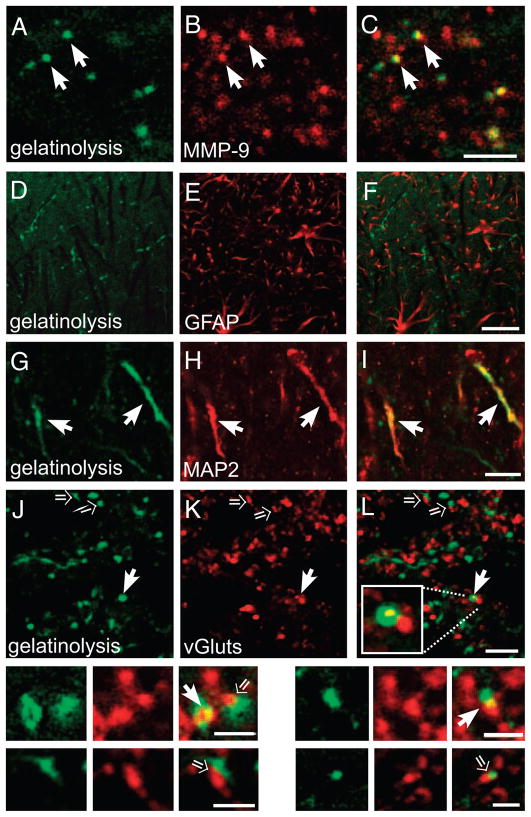
To identify with which elements of the neuropil the gelatinolytic puncta were associated, we immunolabeled tissue sections containing the LTP-associated gelatinolytic puncta for several neuronal and glial markers. We first confirmed that the vast majority of gelatinolytic puncta codistributed with immunolabeling for MMP-9 (Fig. 3, A–C), consistent with the idea that the proteolytically active form of MMP-9 was directly contributing to the gelatinolysis. In contrast, there were many more MMP-9 immunolabeled puncta that were not gelatinolytic, but this was expected because the MMP-9 antibody recognizes both pro- (inactive) and active forms of the enzyme (Nagy et al. 2006). Because both glia and neurons are capable of synthesizing MMP-9 (Arai et al. 2003; Nagy et al. 2006; Szklarczyk et al. 2002), we labeled sections for GFAP, a marker of most astrocytes, but found no evidence for codistribution with the gelatinolytic puncta (Fig. 3, D–F). In contrast, some gelatinolysis codistributed with immunolabeling for microtubule-associated protein-2 (MAP-2), a marker of dendrites (Fig. 3, G–I). Additionally, many gelatinolytic puncta showed small regions of overlapping codistribution with immunolabeling for vesicular glutamate transporters (vGluts), a marker of presynaptic terminals (solid arrows, Fig. 3, J—L, and top row of insets). In other cases, gelatinolytic and vGlut puncta were directly abutted or apposed, but did not overlap (open arrows, Fig. 3, J–L, and bottom row of insets).
FIG. 3.
Gelatinolytic puncta codistribute with dendritic and synaptic markers. A–L, insets: high-power confocal images through area CA1 s. radiatum taken 75 min after LTP induction in rats receiving an intrahippocampal injection of the MMP-9 substrate DQ-gelatin. Left: gelatinolytic puncta (green); middle: immunolabeling for indicated markers (red); and right: merged images; regions of codistributed overlap are indicated by yellow pixels. A–C: most gelatinolytic puncta codistribute with immunolabeling for MMP-9 (arrows). There are numerous MMP-9 immunolabeled puncta that are not gelatinolytic, which presumably represents a pool of inactive (pro) MMP-9, as the antibody recognizes both pro- and active-forms of MMP-9. D—F: gelatinolytic puncta did not codistribute with GFAP immunolabeling, a marker of astrocytes. G–I: many puncta or elongated regions of gelatinolysis codistributed with immunolabeling for MAP-2, a dendritic marker (arrows). J–L: many gelatinolytic puncta codistributed with immunolabeling for vGluts, a presynaptic terminal marker (solid arrows), whereas others appeared abutted or directly apposed to vGlut-immunolabeled puncta (open arrows). Insets: underneath show higher-power images of such labeling patterns. Bars, 3 μm A-L; 2 μm in insets.
Previous in situ hybridization studies have shown that hippocampal seizure activity elevates levels of MMP-9 mRNAs and leads to a redistribution of MMP-9 mRNA into s. radiatum (Szklarczyk et al. 2002). We therefore investigated whether LTP led to a similar enhancement and redistribution of MMP-9 mRNA into area CA1 s. radiatum, a region rich in dendrites and the location of the potentiated synapses. Hippocampal sections taken from rats 75 min post-LTP induction, when MMP-9 protein levels are elevated, or from those receiving control stimuli, were subjected to in situ hybridization of radioactive MMP-9 cRNA probes and exposed to film. Qualitative (Fig. 4, A and B) and quantitative (C) densitometric analyses of film autoradiograms showed no differences between stimulation conditions in the intensity of probe hybridization in st. pyramidale of areas CA1 and CA3 or in the granule cell layer of the dentate gyrus nor were there any differences between stimulation conditions in intensity of probe hybridization within neuropil of area CA1 st. radiatum. These data suggest that the LTP-associated elevation in MMP-9 protein level does not reflect major changes in levels or distribution of MMP-9 mRNAs.
FIG. 4.

There are no changes in MMP-9 mRNA levels or distribution with LTP. A and B: representative film autoradiograms of bilateral hipppocampal sections showing pattern and relative intensity of MMP-9 cRNA probe hybridization. Section in A was taken from an animal 75 min after receiving control stimuli on one side (control stim); section in B was taken from an animal 75 min after LTP was induced tetanically on 1 side (LTP). In both images, the contralateral sides (contra) are also shown for comparison. There are no apparent differences in levels or patterns of probe hybridization across sides or stimulation conditions. Bars, 2 mm. C: quantitative densitometry shows no differences in relative levels of probe hybridization between LTP (n = 6) animals and those receiving control stimuli (n = 4) in s. pyramidale of areas CA1 and CA3, the granule cell layer of the dentate gyrus (DG), or area CA1 st. radiatum (st. rad).
Proteolytically active MMP-9 induces LTP that is occluded by, and occludes, tetanically-evoked LTP
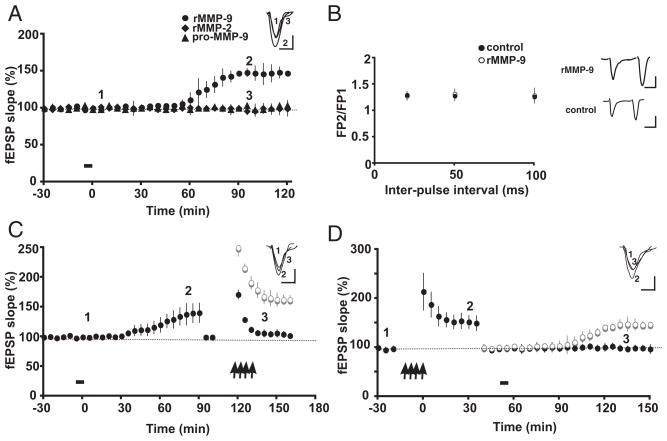
We next addressed how proteolytically active MMP-9 affects synaptic function by administering recombinant-active MMP-9 (rMMP-9; 0.1 μg/μl, 2 μl) by infusion cannula into area CA1 while recording synaptic responses. Here, we found that rMMP-9 produced a slowly developing, persistent potentiation of the extracellular fEPSP initial slope, reaching a maximum by 90 –120 min postadministration (147.5% ± 1.17% of baseline, n = 5; Fig. 5A, ●), which was sustained through ≥180 min when the experiments were terminated. Such potentiation was specific for proteolytically active MMP-9 in that administration of the inactive pro-form of MMP-9 (▲, Fig. 5A, n = 3) or active MMP-2 (◆, Fig. 5A, n = 3) had no effect on synaptic responses. In a separate series of experiments, we determined that the rMMP-9-mediated enhancement of synaptic responses was not associated with changes in PPF (Fig. 5B, P > 0.05), suggesting that the site of MMP-9 action is not presynaptic. Because rMMP-9 alone could elicit long-lasting synaptic potentiation, we next asked whether rMMP-9-mediated potentiation occludes tetanically evoked potentiation. After rMMP-9-induced synaptic enhancement reached a plateau and had stabilized by ~90 min, fEPSP initial slope was adjusted to yield synaptic responses that matched those of the original baseline (Fig. 5C), whereupon tetanic stimuli were applied. However, there was no further persistent potentiation of the fEPSP slope after tetanic stimulation (Fig. 5C; 40 min posttetanus: 102.2% ± 2.5% of baseline, n = 4). Conversely, in a different set of animals, stable LTP was first induced tetanically followed by a reduction in stimulus intensity to produce synaptic responses similar to those at baseline levels. Subsequently, rMMP-9 was infused as described in the preceding text. However, there was no further synaptic potentiation following rMMP-9 administration (Fig. 5D; 150 min posttetanus: rMMP-9, 99.6% ± 3.5% of baseline, n = 4). Taken together, the mutual occlusion of synaptic potentiation elicited tetanically or by rMMP-9 exposure suggests that these two types of plasticity share some common cellular mechanisms.
FIG. 5.
Proteolytically active MMP-9 induces potentiation in rat area CA1 in vivo that occludes, and is occluded by, tetanic stimulation. A: recombinant active MMP-9 (rMMP-9; 0.1 μg/μl) delivered intrahippocampally (bar) induces a slowly emerging, persistent potentiation that begins to arise approximately 60 min postinjection (●, n = 5). Administration of inactive pro-MMP-9 (▲) or recombinant active MMP-2 (rMMP-2; ◆) did not elicit an increase in fEPSP slope (n = 3 each). Inset: representative EPSP traces were recorded before (1), 90 min after rMMP-9 application (2), and 90 min after rMMP-2 application (3). Calibration: 10 ms, 0.3 mV. B: intrahippocampal infusion of rMMP-9 has no effect on PPF. Plot shows PPF measured before (●) and 120 min after (○) rMMP-9 administration. Inset: representative EPSP traces (50 ms IPI) recorded before and after rMMP-9 injection. Calibration: 25 ms, 0.2 mV. C: rMMP-9 potentiation occludes tetanically-induced potentiation. After rMMP-9 potentiation reached a plateau (~90 min), fEPSP slope was reduced to yield synaptic responses that matched those of baseline levels, followed by tetanic stimulation (↑). There was no further potentation after tetanic stimulation (●). The ghostline (○) indicates the level of tetantically induced LTP in control animals (see Fig. 1A). Representative EPSP traces were recorded at time points indicated in the panel. D: tetanic stimulation occludes rMMP-9 potentiation. Tetanic stimulation (↑) produces LTP (●); at ~45 min postinduction, stimulus intensity was reduced to produce synaptic responses that matched those of the original baseline, followed by administration of rMMP-9 (bar). There was no further potentiation following delivery of rMMP-9 (●). The ghostline (○) indicates the level of rMMP-9-induced potentiation from animals shown in A. Representative EPSP traces were recorded at time points indicated in the panel. Calibration as in Fig. 1A.
DISCUSSION
The results of this study demonstrate that MMP-9, an extracellularly acting protease, functions critically in modulating hippocampal synaptic physiology and plasticity in adult animals in vivo. This is important because the prevailing view of MMP-9 function in adult brain is that it is activated under injurious or pathophysiological conditions, where, for example, it has protracted, deleterious roles in neurovascular remodeling and neuronal degeneration after stroke (Lee et al. 2004; Lo et al. 2002; Zhao et al. 2006). Here, in urethane-anesthetized adult rats, we show that protein levels of MMP-9 are elevated and numbers of MMP-9 immunopositive “hot spots” of gelatinolytic activity are significantly increased relatively rapidly in area CA1 by LTP-inducing tetanic stimuli. When MMP-9 activity is blocked acutely, either with pharmacological inhibitors or with MMP-9-specific neutralizing antibodies, LTP maintenance at Schaffer collateral-area CA1 synapses is eliminated, but LTP induction and properties of normal synaptic neurotransmission are unaffected. Once proteolytically active, MMP-9 induces a slowly emerging synaptic potentiation that occludes, and is occluded by, tetanically evoked LTP. Taken together, the data indicate that tightly regulated, highly localized MMP-mediated extracellular proteolysis contributes fundamentally to modulating normal, nonpathological synaptic physiology and function in mature brain.
In other tissues, MMPs can serve at least two important functions (Sternlicht and Werb 2001). One is in cell-signaling through release or processing of ligands that then bind cell-surface receptors and activate signal-transduction cascades. The second is in physical remodeling of the pericellular microenvironment via cleavage of cell-surface adhesion and/or matrix proteins that maintain cellular architecture. During LTP, it is likely that proteolytically active MMP-9 serves both signaling and remodeling functions. The MMP-9-triggered, slow potentiation described here in vivo is identical to that shown previously in acute hippocampal slices, where such potentiation is mediated by integrin receptors that recognize the conserved tripeptide arginine-glycine-aspartate (RGD) sequence (Nagy et al. 2006). This suggests that in vivo, MMP-9 mediated proteolysis furnishes an endogenous RGD-bearing ligand that then binds and activates integrin receptors. The identity of endogenous MMP-9 substrates that could trigger such integrin-activated cascades is unknown, although there are a number of putative RGD-bearing matrix and cell-surface proteins expressed in hippocampus that are potential targets (Dityatev and Schachner 2003), and the levels of some of these are upregulated by activity (Hoffman et al. 1998). The present and previous observations (Nagy et al. 2006) that MMP-9 is involved in maintenance, but not induction, of LTP is consistent with a large body of work showing a similar involvement of integrins in LTP maintenance but not induction (Bahr et al. 1997; Chan et al. 2003; Staubli et al. 1990; Xiao et al. 1991). The majority of integrins in hippocampus are present postsynaptically within dendritic spines (Bi et al. 2001; Chan et al. 2003; Einheber et al. 1996; Grooms et al. 1993; King et al. 2001); this would indicate that MMP-9 effects mediated through integrins would also be expressed postsynaptically. This is consistent with our present observations that there were no effects of proteolytically active MMP-9 on paired-pulse facilitation, a measure of presynaptic function. Integrin stimulation in hippocampus leads to activation of several kinase signaling cascades (Bernard-Trifilo et al. 2005); this in turn results in phosphorylation of certain glutamate receptor subunits and a slow enhancement of glutamate receptor currents (Kramar et al. 2003; Lin et al. 2003). Integrin stimulation also increases levels of filamentous actin within dendritic spines and changes spine shape and size (Kramar et al. 2006; Shi and Ethell 2006). Similarly, LTP elicited both in vivo and in slices also affects actin dynamics within spines and is associated with spine enlargement (Desmond and Levy 1983; Fukazawa et al. 2003; Kim and Lisman 1999; Kopec et al. 2006; Krucker et al. 2000; Lin et al. 2005). Thus MMP-9 may be facilitating structural remodeling of potentiated spines, either proactively through integrin receptor signaling or, alternatively, by simply creating a permissive environment for such remodeling by local degradation of matrix or cell-surface proteins that normally maintain neuronal membrane apposition. Cadherins, for example, are synaptic adhesion proteins that are cleaved by MMPs under certain conditions (Monea et al. 2006; Steinhusen et al. 2001). Although it remains to be determined definitively whether or not MMP-9 affects spine morphology, a recent study of hippocampal neurons grown in culture has shown that MMP-7 exposure leads to conversion of mature, mushroom-shaped spines to filopodia-like structures (Bilousova et al. 2006). This is a morphological conversion that would not be expected in LTP (Engert and Bonhoeffer 1999; Matsuzaki et al. 2004), which may mean that MMP-7 produces different effects on spine morphology than those expected for MMP-9 or young neurons in culture behave differently than mature neurons in vivo.
Levels of MMP-9 protein and numbers of MMP-9 immunopositive hot spots of gelatinolytic activity were both significantly elevated by LTP-inducing tetanic stimuli. Previous studies in acute slices have shown that the increase in MMP-9 protein level after LTP induction was dependent on protein synthesis and NMDA receptor activation (Nagy et al. 2006). The present study demonstrates that in vivo the increase in proteolytic activity also required NMDA receptor activation as there was no increase in numbers of gelatinolytic puncta in animals receiving an injection of the NMDA receptor antagonist CPP prior to tetanic stimulation. What is unclear at present are the mechanistic and temporal relationships between synthesis and activation of MMP-9 during LTP. One possibility is that there is an initial and rapid conversion of the preexisting pool of pro-MMP-9, already present, prior to stimulation, within the neuropil (Lee et al. 2004; Nagy et al. 2006; Szklarczyk et al. 2002), into its proteolytically active form, followed in time by synthesis and secretion of new pro-MMP-9. The soluble gas nitric oxide is released rapidly during LTP in an NMDA receptor-dependent manner (Zhuo et al. 1999) and can activate MMP-9 (Gu et al. 2002). Other candidates include the extracellular tissue-plasminogen activator (tPA)-plasmin cascade, which can convert pro- to active-MMP-9 via activation of other MMPs (Cuzner and Opdenakker 1999). tPA is an immediate early gene the rapid, activity-dependent mRNA upregulation of which in hippocampus requires NMDA receptors (Qian et al. 1993), although its release may be gated through metabotropic glutamate receptors (Shin et al. 2004). In some other cell types, MMP-9 activity levels are regulated largely through transcriptional control of MMP-9 mRNA levels (Sternlicht and Werb 2001), and in hippocampus, kainic acid-induced seizures increases MMP-9 mRNA levels within 6 –12 h concomitantly with increases in proteolytic activity (Szklarczyk et al. 2002). In contrast, during LTP, our in situ hybridization data suggest that there are not major changes in levels or distribution of MMP-9 mRNAs, at least within the first hour. It is possible that at longer times after LTP induction than those examined here, increases in MMP-9 mRNA levels may occur. It also remains unknown what mechanisms are responsible for catabolizing active MMP-9. Studies of LTP in rat prefrontal cortex or seizure activity in hippocampus suggest that increased expression of the endogenous MMP inhibitor TIMP-1 (tissue inhibitor of metalloproteinases-1) may be involved (Jaworski et al. 1999; Okulski et al. 2007; Rivera et al. 1997).
The anatomical localization of MMP-9 activity in brain has customarily been conducted by in situ zymography on tissue sections ex vivo. This process typically requires long incubation times in DQ gelatin and brain slicing to produce tissue sections, all of which can complicate interpretation of any proteolytic activity as truly representative of the state and position of MMP proteolysis at the time of relevant interest (e.g., the height of plasticity). To circumvent these potential limitations, we introduced a novel modification of the basic zymographic technique by injecting DQ gelatin, a MMP-9 substrate, directly into the hippocampus in vivo. The numerous gelatinolytic puncta that we observed in the LTP-expressing hippocampi are similar to those described earlier in acute slices subjected to chemically induced L-LTP and visualized by conventional in situ zymography (Nagy et al. 2006). In most tissues generally, secreted MMPs are anchored to the plasma-lemma via association with other molecules rather than floating freely in the extracellular space (Sternlicht and Werb 2001). This is consistent with the punctate immunolabeling of MMP-9 that we have observed both in vivo and in vitro (Nagy et al. 2006) and likely underlies the discrete foci of gelatinolytic activity that also appears discretely localized to or around dendritic spines. Although we confirmed that most of these gelatinolytic puncta codistributed with immunolabeling for MMP-9, we cannot rule out that other proteases may have also contributed to the gelatinolytic activity. Nevertheless, levels and activity of MMP-2, which is the other major gelatinase, are not affected by tetanic stimulation of the Schaffer collateral–CA1 pathway (Nagy et al. 2006). The numerous hot spots of MMP-9 immunopositive gelatinolytic puncta distributed throughout the potentiated neuropil codistributed with synaptic and dendritic markers or were firmly abutted against the synaptic puncta, consistent with the idea of highly local proteolysis ideally positioned anatomically to modify synaptic structure and function as discussed in the preceding text. We did not find, however, association with the astrocytic marker GFAP. This was a surprise because glia contain MMP-9 mRNA (Arai et al. 2003) and in acute hippocampal slices or sections from perfused brains, GFAP positive astrocytes and processes coimmunolabel for MMP-9 using antibodies that recognize both pro- and active forms (Nagy et al. 2006; Szklarczyk et al. 2002). It is possible that in our material, glial-associated MMP-9 represents only the pro-form and that this pool of MMP-9 is not activated during LTP in vivo. However, under pathological conditions—for example, as a consequence of stroke or inflammation—glial-associated MMP-9 does become active (Duchossoy et al. 2001; Goussev et al. 2003; Jourquin et al. 2003; Magnoni et al. 2004). Alternatively, fine, distal astrocytic processes often lack immunocytochemically detectable levels of GFAP (Ong et al. 1995), thus it is possible that such fine GFAP-negative astrocytic processes do associate with active MMP-9 but were simply undetectable in our material. In any event, the zymographic visualization of gelatinolysis by direct injection of the substrate into brain parenchyma has potentially widespread benefits for visualizing endogenous MMP activity under a variety of pathophysiological or normal conditions.
In conclusion, we have shown that MMP-9 becomes proteolytically active in discrete hot spots within the neuropil in association with LTP elicited in vivo. Because LTP in area CA1 accompanies forms of hippocampal-dependent learning (Whitlock et al. 2006), the data suggest that MMP-9-mediated local proteolysis is required for enabling synaptic modifications necessary for long-term memory. Although such modifications are likely to include both a signaling component—via integrins, for example—as well as synaptic structural remodeling— of dendritic spines, for example—the precise identity of the synaptic target molecules with which MMP-9 interacts awaits future studies.
Acknowledgments
We gratefully acknowledge the help of Bharti Patil in analyzing the film autoradiograms and Dr. Nazan Dolu for help with some of the initial electro-physiology experiments. We also thank Dr. Dylan Edwards for kindly providing us with cDNAs and Dr. Greg Phillips for many helpful discussions.
GRANTS
This work was supported by The Mount Sinai School of Medicine and National Institutes of Health Grants (NIMH) MH-075783-01 and NS-041687-04.
References
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji Z-X, Esteban ET, Lynch G. Arg-gly-asp-ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. J Neurosci. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Barlow JZ, Kelley KA, Bozdagi O, Huntley GW. Testing the role of the cell-surface molecule Thy-1 in regeneration and plasticity of connectivity in the CNS. Neuroscience. 2002;111:837–852. doi: 10.1016/s0306-4522(02)00023-4. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Benson DL, Schnapp L, Shapiro L, Huntley GW. Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol. 2000;10:473–482. doi: 10.1016/s0962-8924(00)01838-9. [DOI] [PubMed] [Google Scholar]
- Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93:834–849. doi: 10.1111/j.1471-4159.2005.03062.x. [DOI] [PubMed] [Google Scholar]
- Bi X, Lynch G, Zhou J, Gall CM. Polarized distribution of alpha5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol. 2001;435:184–193. doi: 10.1002/cne.1201. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloprotein-ase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet. 1997;21:44–54. doi: 10.1002/(SICI)1520-6408(1997)21:1<44::AID-DVG5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Synaptic correlates of associative potentiation/depression: an ultrastructural study in the hippocampus. Brain Res. 1983;265:21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Duchossoy Y, Horvat JC, Stettler O. MMP-related gelatinase activity is strongly induced in scar tissue of injured adult spinal cord and forms pathways for ingrowing neurites. Mol Cell Neurosci. 2001;17:945–956. doi: 10.1006/mcne.2001.0986. [DOI] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gil OD, Needleman L, Huntley GW. Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. J Comp Neurol. 2002;453:372–388. doi: 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Golshani P, Truong H, Jones EG. Developmental expression of GABA(A) receptor subunit and GAD genes in mouse somatosensory barrel cortex. J Comp Neurol. 1997;383:199–219. doi: 10.1002/(sici)1096-9861(19970630)383:2<199::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gottlieb DI, Cowan WM. Autoradiographic studies of the commissural and ipsilateral association connection of the hippocampus and detentate gyrus of the rat. I. The commissural connections. J Comp Neurol. 1973;149:393–422. doi: 10.1002/cne.901490402. [DOI] [PubMed] [Google Scholar]
- Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z, Noble-Haeusslein LJ. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg. 2003;99:188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms SY, Terracio L, Jones LS. Anatomical localization of beta 1 integrin-like immunoreactivity in rat brain. Exp Neurol. 1993;122:253–259. doi: 10.1006/exnr.1993.1125. [DOI] [PubMed] [Google Scholar]
- Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- Hoffman KB, Pinkstaff JK, Gall CM, Lynch G. Seizure induced synthesis of fibronectin is rapid and age dependent: implications for long-term potentiation and sprouting. Brain Res. 1998;812:209–215. doi: 10.1016/s0006-8993(98)00727-6. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Rusakov DA, Bliss TV, Fine A. Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: evidence for changes in length and orientation associated with chemically induced LTP. J Neurosci. 1995;15:5560–5573. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long- lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Biedermann IW, Lapinska J, Szklarczyk A, Figiel I, Konopka D, Nowicka D, Filipkowski RK, Hetman M, Kowalczyk A, Kaczmarek L. Neuronal excitation-driven and AP-1-dependent activation of tissue inhibitor of metalloproteinases-1 gene expression in rodent hippocampus. J Biol Chem. 1999;274:28106–28112. doi: 10.1074/jbc.274.40.28106. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, Le Diguardher T, Khrestchatisky M, Rivera S. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. Eur J Neurosci. 2003;18:1507–1517. doi: 10.1046/j.1460-9568.2003.02876.x. [DOI] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Thomas U, Knopfel T, Behnisch T. Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J Neurosci. 2006;26:4949–4955. doi: 10.1523/JNEUROSCI.4573-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VR, McBride A, Priestley JV. Immunohistochemical expression of the alpha5 integrin subunit in the normal adult rat central nervous system. J Neurocytol. 2001;30:243–252. doi: 10.1023/a:1012753808599. [DOI] [PubMed] [Google Scholar]
- Komai S, Matsuyama T, Matsumoto K, Kato K, Kobayashi M, Imamura K, Yoshida S, Ugawa S, Shiosaka S. Neuropsin regulates an early phase of schaffer-collateral long-term potentiation in the murine hippocampus. Eur J Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Bernard JA, Gall CM, Lynch G. Integrins modulate fast excitatory transmission at hippocampal synapses. J Biol Chem. 2003;278:10722–10730. doi: 10.1074/jbc.M210225200. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci USA. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci USA. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Tsuji K, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24:671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Jones A, Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992;9:1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Lin B, Arai AC, Lynch G, Gall CM. Integrins regulate NMDA receptor-mediated synaptic currents. J Neurophysiol. 2003;89:2874–2878. doi: 10.1152/jn.00783.2002. [DOI] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli JD. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. Embo J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni S, Baker A, George SJ, Duncan WC, Kerr LE, McCulloch J, Horsburgh K. Differential alterations in the expression and activity of matrix metalloproteinases 2 and 9 after transient cerebral ischemia in mice. Neurobiol Dis. 2004;17:188–197. doi: 10.1016/j.nbd.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Ninomiya A, Yamasaki H, Tamura H, Nakamura Y, Shiosaka S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–7736. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monea S, Jordan BA, Srivastava S, DeSouza S, Ziff EB. Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J Neurosci. 2006;26:2300–2312. doi: 10.1523/JNEUROSCI.3521-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okulski P, Jay TM, Jaworski J, Duniec K, Dzwonek J, Konopacki FA, Wilczynski GM, Sanchez-Capelo A, Mallet J, Kaczmarek L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Ong WY, Garey LJ, Leong SK, Reynolds R. Localization of glial fibrillary acidic protein and glutamine synthetase in the human cerebral cortex and subcortical white matter—a double immunolabelling and electron microscopic study. J Neurocytol. 1995;24:602–610. doi: 10.1007/BF01257375. [DOI] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Ramos-DeSimone N, Moll UM, Quigley JP, French DL. Inhibition of matrix metalloproteinase 9 activation by a specific monoclonal antibody. Hybridoma. 1993;12:349–363. doi: 10.1089/hyb.1993.12.349. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Prins ML, Zhu J, Povlishock JT, Phillips LL. Matrix metal-loproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J Neurosci. 2003;23:10182–10189. doi: 10.1523/JNEUROSCI.23-32-10182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CY, Kundel M, Wells DG. Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J Neurosci. 2004;24:9425–9433. doi: 10.1523/JNEUROSCI.2457-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long term potentiation in a-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–205. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Staubli U, Vanderklish P, Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behav Neural Biol. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Ishikawa Y, Hino N, Maeda M, Yoshida S, Kaku S, Shiosaka S. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J Physiol. 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Hojo K, Yoshida H, Yoshioka T, Sugita K. Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun. 1993;190:732–740. doi: 10.1006/bbrc.1993.1110. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Xiao P, Bahr BA, Staubli U, Vanderklish PW, Lynch G. Evidence that matrix recognition contributes to stabilization but not induction of LTP. Neuroreport. 1991;2:461–464. doi: 10.1097/00001756-199108000-00013. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Deb S, Gottschall PE. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur J Neurosci. 1998;10:3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Laitinen JT, Li XC, Hawkins RD. On the respective roles of nitric oxide and carbon monoxide in long-term potentiation in the hippocampus. Learn Mem. 1999;6:63–76. [PMC free article] [PubMed] [Google Scholar]