Abstract
Objective:
The aim of the present case–control study was to evaluate the association between maternal periodontitis and preeclampsia. Association studies between maternal periodontitis and elevated risk for preeclampsia have shown conflicting results. Periodontal maintenance is necessary to reduce the risk of adverse pregnancy outcomes like preeclampsia.
Materials and Methods:
Periodontal parameters [bleeding on probing, probing depth (PD), and clinical attachment level (CAL)] of 1320 women were assessed, followed by retrieval of their demographic and medical data from the medical records. Based on the medical records, 80 women were excluded from the study, leaving 1240 females as the eligible sample for the study. The women were divided into control group (1120 non-preeclamptic women who gave birth to infants with adequate gestational age) and case group (120 preeclamptic women). Logistic regression analysis revealed that primiparity and maternal periodontitis were the two significant variables causing preeclampsia. Further analysis was carried out by matching the two groups for primiparity to find the significance of maternal periodontitis. Maternal periodontitis was defined as PD ≥4 mm and CAL ≥3 mm at the same site in at least four teeth.
Results:
The results showed that maternal periodontitis (odds ratio 19.8) was associated with preeclampsia. Maternal periodontitis also remained associated with preeclampsia after matching for primiparity, which was another significant confounding factor in the study (odds ratio 9.33).
Conclusion:
Maternal periodontitis is a risk factor associated with preeclampsia, emphasizing the importance of periodontal care in prenatal programs.
Keywords: Periodontitis, preeclampsia, primiparity
INTRODUCTION
“Mouth is the mirror of the whole body” is an age-old concept with its practical relevance even today. According to focal infection therapy, micro-organisms and their waste products enter the body through mouth. Numerous epidemiological, microbiological, and immunological studies have reinforced the concept that periodontal disease may be considered as a risk factor for various systemic diseases like cardiovascular disease, cerebrovascular disease, respiratory disease and for adverse pregnancy outcomes such as preterm delivery of low-birth-weight infants and preeclampsia.[1]
Periodontal disease is a multifactorial, chronic, gram-negative infection associated with atherosclerotic thromboembolic events.[2] It may act as a chronic reservoir of endotoxins and inflammatory cytokines that initiate and exaggerate atherogenesis and thrombogenesis.[3] Pregnancy-induced gingivitis and periodontitis are two oral conditions with prevalence rates ranging from 30 to 100% and from 5 to 20%, respectively.[4]
The second most leading cause for maternal mortality is hypertensive disorder of pregnancy, which accounts for 15% of total deaths and still births.[5] Preeclampsia is a complex disorder affecting about 5–10% of the obstetric population, resulting from deficient placental implantation during the first half of pregnancy and hypertensive disorders after 20th week of gestation in a previously normotensive woman.[2,6]
Preeclampsia and periodontitis have been found to be associated with high circulating levels of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-10, and IL-6 resulting in inflammatory vascular damage leading to placental endothelial alterations.[7] Periodontal microbiota plays a significant role in systemic diseases directly through a pro-inflammatory effect or indirectly through the host-mediated destruction. The similarities in their pathophysiologies have led to the hypothesis of periodontal disease being a risk factor for preeclampsia.[8]
The purpose of the present study was to assess the risk association between maternal periodontitis and preeclampsia before and after matching for known risk factors of preeclampsia like primiparity.
MATERIALS AND METHODS
The present study was conducted at a medical college in Gujarat and was approved by the Gujarat University Ethical Committee with the number KSD-281. Participants were informed about the aims of the study and a written informed consent was obtained from them.
An eligible sample was selected based on the following criteria.
Inclusion criteria
Women of age 18–35 years who gave birth to live infants in the hospital unit.
Exclusion criteria
Women who gave birth to more than one infant in a single delivery; those who had undergone in vitro fertilization; those who were suffering from any systemic diseases before pregnancy, or had placental, cervical, and/or uterine abnormalities; those who had human immunodeficiency virus infection; those who were on antibiotic prophylaxis for dental treatment; and smokers/alcoholics.
Study design
During the 1 year study period, 1320 women were admitted in the obstetric department. Periodontal examination was performed 48 h after delivery, but before assessment of medical records to avoid the occurrence of bias. Intraoral examination was done with the help of artificial light source, mouth mirror, William's periodontal probe, and cotton pliers. The following clinical parameters were used to evaluate the clinical signs of inflammation and periodontal tissue destruction:
Gingival bleeding index (Ainamo and Bay)
The gingival bleeding was determined dichotomously by gentle probing of the gingival crevice with the William's periodontal probe. The appearance of bleeding within 10 s indicated a positive score, which was expressed in percentage.[9]
Probing pocket depth
The distance between the base of the pocket and the gingival margin was measured at six sites of each tooth and the deepest/highest penetration was recorded for each individual tooth.
Clinical attachment level
It was determined by measuring the distance from the cemento-enamel junction to the probable base of the sulcus. Teeth were excluded if their cemento-enamel junction was not identifiable, with large undefined restorations, large carious lesions or fractures, or if they were erupting.
For the purpose of analysis, maternal periodontitis was defined as probing depth ≥4 mm and clinical attachment level (CAL) loss ≥3 mm at the same site in at least four teeth.
Medical data
Demographic data, medical history, and detailed information on events during pregnancy and delivery were obtained from patients’ medical records. Medical data were reviewed by an obstetrician to confirm the inclusion and exclusion criteria. Eighty women were removed from the study based on the exclusion criteria. Thus, a total of 1240 patients were selected for this study.
Women were diagnosed with preeclampsia if they had the following:
Blood pressure ≥140/90 mm Hg on two separate occasions after week 20 of gestation. Blood pressure was assessed by a trained medical group from the hospital unit
Proteinuria defined as protein concentration ≥0.30g/dl in 24 hours urine collection.
After assessment of the medical records, the women were first divided as follows:
Group A: Case group consisting of 120 preeclamptic women
Group B: Control group consisting of 1120 non-preeclamptic women.
Logistic regression analysis was then carried out to check the confounding factors for preeclampsia. According to this analysis, primiparity was found to be a significant confounding factor along with maternal periodontitis. So, the groups were subdivided (group C and group D) by matching for primiparous women in the ratio of 1:1.
Group C: 96 primiparous preeclamptic women from group A
Group D: 96 randomly selected primiparous non-preeclamptic women from group B.
Statistical analysis was carried out to check the significance of maternal periodontitis in these two groups. After obtaining the data, mean and standard deviation were estimated for the samples of each study group. Mean values were compared between different study groups by using one-way analysis of variance (ANOVA) or non-parametric Kruskal–Wallis test followed by Tukey's honestly significant difference (HSD) procedure by SPSS software ver. 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
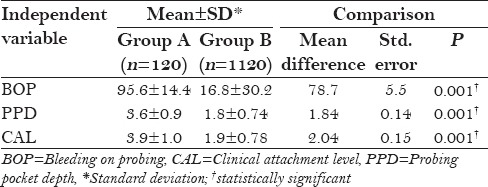
The periodontal condition of all the three groups is shown in Table 1. In group A, the mean percentage of sites with bleeding on probing (BOP) was found to be 95.6 ± 14.4% and in group B, it was found to be 16.8 ± 30.2%. In group A, the mean probing pocket depth (PPD) was found to be 3.6 ± 0.9 mm and in group B, it was found to be 1.8 ± 0.74 mm. In group A, the mean CAL was found to be 3.9 ± 1.0 mm and in group B, it was found to be 1.9 ± 0.78 mm. On comparing the periodontal status, there was statistically significant difference (P < 0.001) between group A and group B as shown in Table 2.
Table 1.
Mean and standard deviation of percentage of BOP sites, PPD, and CAL in the study groups
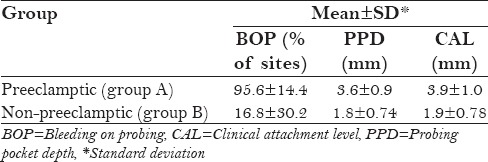
Table 2.
Comparison of BOP, PPD, and CAL between group A and group B
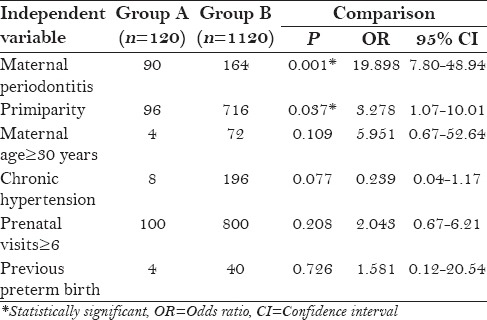
Logistic regression multivariate analysis of all the variables for preeclampsia showed that only maternal periodontitis [odds ratio (OR) 19.8] and primiparity (OR 3.27) were the two statistically significant variables when group A and group B were compared [Table 3].
Table 3.
Logistic regression multivariate analysis for preeclampsia: Unconditional (group A and group B)
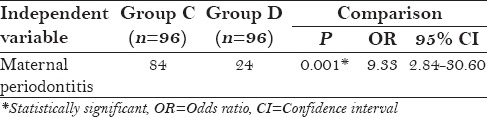
After matching for primiparity in group C and group D, maternal periodontitis was still found to be a statistically significant factor affecting preeclampsia with OR 9.33 [Table 4].
Table 4.
Logistic regression analysis for preeclampsia (after matching for primiparity between group C and group D)
DISCUSSION
Periodontal disease is a disease characterized by intermittent periods of activity and inactivity that initiates local inflammation leading to tissue destruction. Many authors like Offenbacher et al.,[10] Lin et al.,[11] and Boggess et al.[12] have emphasized the role of active periodontal disease causing translocation of periopathogenic bacteria to the fetal-placental unit, resulting in placental damage and manifestations of preeclampsia.
The mechanisms leading to preeclampsia have been speculated to be because of impaired placental perfusion leading to an insufficient placental implantation.[7] Endothelial cell dysfunction caused by cytokines like TNF-α and oxidative stress lead to changes in the structure of placental bed as well as uterine blood vessels, resulting in high levels of fibronectin, endothelin, tissue plasminogen activator, Von Willebrand factor, and thrombomodulin in the maternal blood.[4] Levels of free 8-isoprostane, which is a marker of lipid peroxidation and a strong vasoconstrictor, are found to be increased in preeclamptic women. The burden of endotoxins, inflammatory cytokines, and oxidative stressors at the level of maternal–fetal unit can also be increased by periodontitis.[7] Potent vascular stressors like soluble intercellular adhesion molecules (sICAMs) are found to be increased in periodontitis,[13] thus compounding preeclampsia. So, any maternal infection or inflammatory disease like periodontitis and atheroma formation can initiate and propagate acute uteroplacental atherosis and preeclampsia.[14]
Oral cavity and systemic diseases form a two-way street, i.e. both entities can affect each other. Golub et al.[15] proposed a “two-hit” model for chronic periodontitis and systemic diseases like arthritis and osteoporosis. They concluded that the periodontopathic bacteria provided one “hit,” whereas systemic inflammations elevating the levels of pro-inflammatory biomarkers like C-reactive protein (CRP), IL-6, and matrix metallopeptidase-9 (MMP-9) in serum or plasma act as a second “hit.” It is possible that preeclampsia could also lead to an aggravation of pre-existing periodontal problems or even co-induce periodontal destruction, but further studies regarding this co-relation have to be carried out.
The aim of the present study was to assess the risk association between maternal periodontitis and preeclampsia.
The present study was conducted on 1240 patients who were selected based upon their demographic, obstetric, and medical history from the medical records under a standardized protocol to eliminate any possibility of a bias. Clinical signs of inflammation and periodontal tissue destruction were assessed using BOP, PPD, and CAL based upon the criteria proposed by Lopez et al.[16] The statistical analysis showed that the percentage of sites with BOP, PPD, and CAL was more in group A as compared to group B [Table 1]. On investigating BOP, PPD, and CAL in both the groups, there were statistically significant difference (P < 0.001) between the preeclamptic group and the normotensive group as shown in Table 2. Findings from the present study showed a significant risk association between maternal periodontitis and preeclampsia after analysing other variables like maternal age, chronic hypertension, previous preterm birth and number of prenatal visits. The adjusted OR for preeclampsia was 19.8 (95% CI 7.80–48.94; P < 0.001). The result of the present study is in agreement with the studies done by Kunnen et al. (OR 7.9),[17] Shetty et al. (20.15),[18] and Moura da Silva et al. (OR 8.60).[19]
In the present study, there was also a significant association between primiparity (OR 3.27) and preeclampsia when the preeclamptic and normotensive groups were compared by logistic regression analysis as shown in Table 3. The result of the present study is in agreement with the findings of Cota et al. (OR 2.4)[3] and Siqueira et al. (OR 2.3).[20]
After matching for primiparity, maternal periodontitis still remained a significant factor in the preeclamptic group with an OR of 9.33 as shown in Table 4. Studies done by Siqueira et al. (OR 1.52)[20] and Canakci et al. (OR 3.47)[21] also showed similar significant association.
Comparison of preeclamptic group and normotensive group showed no significant risk association between maternal age, chronic hypertension, previous preterm birth, number of prenatal visits, and preeclampsia. The result of the present study is in accordance with the study result of Canakci et al.[21]
In the present study, maternal periodontitis is associated with an elevated risk for preeclampsia. This finding is consistent with the findings of Boggess et al.,[2] Canakci et al.,[21] Contreras et al.,[7] Cota et al.,[3] and Siqueira et al.,[20] and is contrary to the study findings of Khader et al.[22] and Rai,[23] who found no relation between maternal periodontitis and preeclampsia. The slight variation in the present study could be related to biases introduced by sample size and heterogeneity of the criteria to define periodontitis. The frequency of preeclampsia in the present study is 9.67%, which is higher compared to the values reported by Riche et al.[24] and Boggess et al.,[2] and is lower compared to the values reported by Castaldi et al.,[25] Siqueira et al.,[20] and Cota et al.[3]
The association between periodontitis and preeclampsia has been supported by the hypothesis that chronic periodontitis infection increases the risk of developing preeclampsia in pregnant women. The pathogenesis of periodontitis and preeclampsia is multifactorial and similar in many ways. So, periodontal treatment should be considered for reducing the risk of preeclampsia.[16] Furthermore, longitudinal and interventional studies are necessary to better address the association between periodontitis and preeclampsia, as well as the benefits of periodontal treatment in the prevention of adverse pregnancy outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Srinivas SK, Sammel MD, Stamilio DM, Clothier B, Jeffcoat MK, Parry S, et al. Periodontal disease and adverse pregnancy outcomes: Is there an association? Am J Obstet Gynecol. 2009;200:497.e1–8. doi: 10.1016/j.ajog.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227–31. doi: 10.1016/s0029-7844(02)02314-1. [DOI] [PubMed] [Google Scholar]
- 3.Cota LO, Guimarães AN, Costa JE, Lorentz TC, Costa FO. Association between maternal periodontitis and an increased risk of preeclampsia. J Periodontol. 2006;77:2063–9. doi: 10.1902/jop.2006.060061. [DOI] [PubMed] [Google Scholar]
- 4.Ruma M, Boggess K, Moss K, Jared H, Murtha A, Beck J, Offenbacher S. Maternal periodontal disease, systemic inflammation and risk for preeclampsia. Am J Obstet Gynecol. 2008;198:389.e1–5. doi: 10.1016/j.ajog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. 2007;78:670–6. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- 6.Rustveld LO, Kelsey SF, Sharma R. Association between maternal infections and preeclampsia: A systematic review of epidemiologic studies. Matern Child Health J. 2008;12:223–42. doi: 10.1007/s10995-007-0224-1. [DOI] [PubMed] [Google Scholar]
- 7.Contreras A, Herrera JA, Soto JE, Arce RM, Jaramillo A, Botero JE. Periodontitis is associated with preeclampsia in pregnant women. J Periodontol. 2006;77:182–8. doi: 10.1902/jop.2006.050020. [DOI] [PubMed] [Google Scholar]
- 8.Keelan JA, Wong PM, Bird PS, Mitchell MD. Innate inflammatory responses of human decidual cells to periodontopathic bacteria. Am J Obstet Gynecol. 2010:202.e1–471. doi: 10.1016/j.ajog.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 10.Offenbacher S, Jared HL, O’Reilly PG, Wells SR, Salvi GE, Lawrence HP, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3:233–50. doi: 10.1902/annals.1998.3.1.233. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Smith MA, Champagne C, Elter J, Beck J, Offenbacher S. Porphyromonas gingivalis infection during pregnancy increases maternal tumor necrosis factor alpha, suppresses maternal interleukin-10, and enhances fetal growth restriction and resorption in mice. Infect Immun. 2003;71:5156–62. doi: 10.1128/IAI.71.9.5156-5162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boggess KA, Edelstein BL. Oral health in women during preconception and pregnancy: Implications for birth outcomes and infant oral health. Matern Child Health J. 2006;10(Suppl):S169–74. doi: 10.1007/s10995-006-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: A state-of-the-science review. Ann Periodontol. 2001;6:9–15. doi: 10.1902/annals.2001.6.1.9. [DOI] [PubMed] [Google Scholar]
- 14.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;81:642–8. [PubMed] [Google Scholar]
- 15.Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85:102–5. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 16.López NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J Dent Res. 2002;81:58–63. doi: 10.1177/002203450208100113. [DOI] [PubMed] [Google Scholar]
- 17.Kunnen A, Blaauw J, van Doormaal JJ, van Pampus MG, van der Schans CP, Aarnoudse JG, et al. Women with a recent history of early-onset pre-eclampsia have a worse periodontal condition. J Clin Periodontol. 2007;34:202–7. doi: 10.1111/j.1600-051X.2006.01036.x. [DOI] [PubMed] [Google Scholar]
- 18.Shetty M, Shetty PK, Ramesh A, Thomas B, Prabhu S, Rao A. Periodontal disease in pregnancy is a risk factor for preeclampsia. Acta Obstet Gynecol Scand. 2010;89:718–21. doi: 10.3109/00016341003623738. [DOI] [PubMed] [Google Scholar]
- 19.Moura da Silva G, Coutinho SB, Piscoya MD, Ximenes RA, Jamelli SR. Periodontitis as a risk factor for preeclampsia. J Periodontol. 2012;83:1388–96. doi: 10.1902/jop.2012.110256. [DOI] [PubMed] [Google Scholar]
- 20.Siqueira FM, Cota LO, Costa JE, Haddad JP, Lana AM, Costa FO. Maternal periodontitis as a potential risk variable for preeclampsia: A case-control study. J Periodontol. 2008;79:207–15. doi: 10.1902/jop.2008.070174. [DOI] [PubMed] [Google Scholar]
- 21.Canakci V, Canakci CF, Canakci H, Canakci E, Cicek Y, Ingec M, et al. Periodontal disease as a risk factor for pre-eclampsia: A case-control study. Aust N Z J Obstet Gynaecol. 2004;44:568–73. doi: 10.1111/j.1479-828X.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- 22.Khader YS, Jibreal M, Al-Omiri M, Amarin Z. Lack of association between periodontal parameters and preeclampsia. J Periodontol. 2006;77:1681–7. doi: 10.1902/jop.2006.050463. [DOI] [PubMed] [Google Scholar]
- 23.Rai B. Periodontal disease risk factor for preeclampsia: Clinical periodontal profile. Advance Med Dent Sci. 2008;2:88–9. [Google Scholar]
- 24.Riché EL, Boggess KA, Lieff S, Murtha AP, Auten RL, Beck JD, et al. Periodontal disease increases the risk of preterm delivery among preeclamptic women. Ann Periodontol. 2002;7:95–101. doi: 10.1902/annals.2002.7.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Castaldi JL, Bertin MS, Giménez F, Lede R. Periodontal disease: Is it a risk factor for premature labor, low birth weight or preeclampsia? Rev Panam Salud Publica. 2006;19:253–8. doi: 10.1590/s1020-49892006000400005. [DOI] [PubMed] [Google Scholar]


