Abstract
Aim:
The aim of the present study was to evaluate the bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400 (PEG 400), and polyethylene glycol 1000 (PEG 1000) against selected microorganisms in vitro.
Materials and Methods:
Five vehicles, namely propylene glycol, glycerine, PEG 400, PEG 1000, and combination of propylene glycol with PEG 400, were tested for their bactericidal activity. The minimum bactericidal concentration was noted against four standard strains of organisms, i.e. Streptococcus mutans American Type Culture Collection (ATCC) 25175, Streptococcus mutans ATCC 12598, Enterococcus faecalis ATCC 35550, and Escherichia coli ATCC 25922, using broth dilution assay. Successful endodontic therapy depends upon thorough disinfection of root canals. In some refractory cases, routine endodontic therapy is not sufficient, so intracanal medicaments are used for proper disinfection of canals. Intracanal medicaments are dispensed with vehicles which aid in increased diffusion through the dentinal tubules and improve their efficacy. Among the various vehicles used, glycerine is easily available, whereas others like propylene glycol and polyethylene glycol have to be procured from appropriate sources. Also, these vehicles, being viscous, aid in sustained release of the medicaments and improve their handling properties. The most commonly used intracanal medicaments like calcium hydroxide are ineffective on many microorganisms, while most of the other medicaments like MTAD (Mixture of Tetracycline, an Acid, and a Detergent) and Triple Antibiotic Paste (TAP) consist of antibiotics which can lead to development of antibiotic resistance among microorganisms. Thus, in order to use safer and equally effective intracanal medicaments, newer alternatives like chlorhexidine gluconate, ozonized water, etc., are being explored. Similarly, the five vehicles mentioned above are being tested for their antimicrobial activity in this study.
Results:
All vehicles exhibited bactericidal activity at 100% concentration.
Conclusion:
Propylene glycol was effective against three organisms namely S. mutans E. faecalis and E. coli and its bactericidal activity was at 50%, 25% and 50% respectively. PEG 1000 was effective against S. mutans and E. coli at 25%. Hence propylene glycol was effective on more number of organisms of which E. faecalis is a known resistant species. PEG 1000 was bactericidal at a lower concentration but was effective on two organisms only.
Keywords: Bactericidal activity, broth dilution, minimum bactericidal concentration, minimum inhibitory concentration, polyethylene glycol 400 and polyethylene glycol 1000, propylene glycol
INTRODUCTION
Last three decades have been very challenging, especially due to the global menace of developing antibiotic resistance.[1] Newer drugs are being discovered with millions and billions being invested in drug research.[2] Instead, the need for developing newer alternatives or unexplored properties of existing agents which can enhance the activity of pre-existing drugs or antibiotics is definitely a viable option.[3] For instance, in the field of Endodontics, both pediatric and adult, intracanal medicaments are commonly used for proper disinfection of the root canals, especially in refractory cases. Proper disinfection is the mainstay for successful endodontic therapy and it is achieved through the collective effects of biomechanical preparation, irrigation, and intracanal medication.[4,5,6]
Furthermore, previous studies have shown that bacteria in infected root canals and also periapical tissues (especially furcation area in primary molars) reside deep within dentine, cementum, and periapical tissues too.[7,8] In order to reach these areas effectively, enhanced penetration is attained by means of intracanal medicaments used along with carriers or vehicles, for example, propylene glycol.[9]
One of the properties which have been under-investigated is whether these vehicles or the so-called “excipients” in Pharmaceutics have antimicrobial property or activity on their own. If they really possess antimicrobial activity, they can be used as an effective alternative for disinfection of root canals, with reduced probability of development of antibiotic resistance. In view of the above-mentioned facts, we planned to study the antimicrobial activity of the vehicles propylene glycol, glycerin, polyethylene glycol 400 (PEG 400), polyethylene glycol 1000 (PEG 1000), and a combination of propylene glycol and PEG 400, as this might shed some light on the ideal vehicle for intracanal medicaments. Polyethylene glycol is also referred to as “Macrogol” and the number denotes its molecular weight. Higher the number, higher is its viscosity.[10] The combination of propylene glycol with Macrogol was first used in dentistry in vivo by Takushige et al., whereas PEG 1000 was used in vitro recently by Carreira et al.[11,12] To the best of our knowledge and literature search, no study of the bactericidal activity of these vehicles and their comparison has been conducted till date.
MATERIALS AND METHODS
This study was carried out in Dr Prabhakar Kore Basic Science Research Centre, KLE University, Belgaum. It was approved by the Institutional Review Board of KLE University (Ref No. KLEU/Ethic/14-15/D-73) and it is a part of an ongoing ex vivo study. The susceptibility of the test organisms to propylene glycol, glycerine, PEG 400, PEG 1000, and propylene glycol with PEG 400 was assessed using broth dilution assay, as minimum inhibitory concentration (MIC) can be readily converted to the minimum bactericidal concentration (MBC). Triplicates were performed for each of the standard strains.
Culture media: Brain Heart Infusion (BHI) broth
Test organisms: Four micro-organisms were selected for the study: Streptococcus mutans American Type Culture Collection (ATCC) 25175, Staphylococcus aureus ATCC 12598, Enterococcus faecalis ATCC 35550, and Escherichia coli ATCC 25922 [Figure 1]. All microorganisms were previously subcultured in appropriate media and under gaseous conditions to confirm their purity at 35°C for 48 h prior to testing of the vehicles
Inoculum preparation: The growth method or the log phase method was performed as follows. At least three to five well-isolated colonies of the same morphological type were selected from an agar culture plate. The top of each colony was touched with a loop, and the growth was transferred into a tube containing 4–5 ml of BHI broth. The broth culture was incubated at 35°C for 2–6 h until it achieved the turbidity of the 0.5 McFarland standard. The turbidity of actively growing broth culture was adjusted with broth to obtain a final turbidity optically comparable to that of the 0.5 McFarland standard, done visually by comparing the inoculum tube and the standard against a white card with contrasting black lines
Broth dilution method: A total of 10 tubes were taken and nine dilutions of the vehicle were done with BHI for MIC and MBC. In the initial tube, only 200 μl of vehicle was added. For further dilutions, 200 μl of BHI broth was added to the next nine tubes separately. In the second tube, 200 μl of vehicle was added which already contained 200 μl of BHI broth. This was considered as 10−1 dilution. From the 10−1 diluted tube, 200 μl was transferred to the second tube to make 10−2 dilution. The serial dilution was repeated up to 10−8 dilution for each vehicle. From the maintained stock cultures of the required organisms, 5 μl was taken and added to 2 ml of BHI broth. In each serially diluted tube, 200 μl of the above culture suspension was added. The last tube contained only the media and the culture suspension, i.e. the growth control. The tubes were kept for incubation for 24 h at 37°C in bacteriological incubator and observed for turbidity [Figure 2]
MBC: After recording the lowest concentration inhibiting the growth of organisms as MIC, all the tubes not showing visible growth were subcultured on BHI agar along with the control tube, i.e., growth control, and incubated overnight. The amount of growth was noted; no growth indicated the whole inoculum was killed and this highest dilution showing 99.99% inhibition was recorded as MBC [Figure 3][13]
Triplicates were performed for each of the standard strains. The experimental data were collected and statistically analyzed using Fisher's exact test.
Figure 1.
Standard strains against which bactericidal activity of the five vehicles was checked: (a) Streptococcus mutans ATCC 25175; (b) Staphylococcus aureus ATCC 12598; (c) Enterococcus faecalis ATCC 35550; (d) Escherichia coli ATCC 25922
Figure 2.
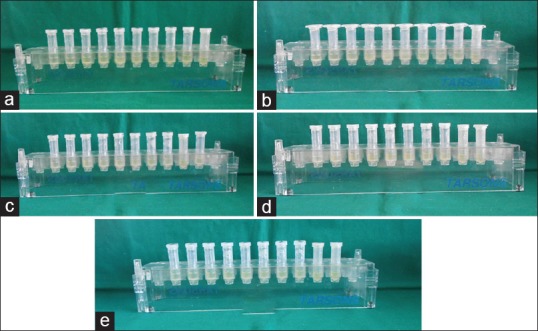
Susceptibility of Eschericia coli ATCC 25922 to (a) Propylene glycol, (b) Glycerine, (c) PEG 400, (d) PEG 1000 and (e) Propylene glycol with PEG 400 assessed using Broth dilution assay
Figure 3.
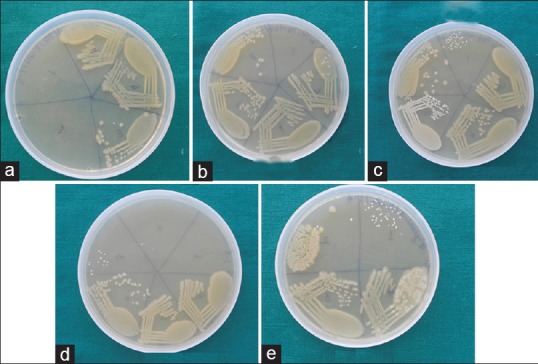
Minimum Bactericidal Concentration (MBC) of Eschericia coli ATCC 25922 to (a) Propylene glycol, (b)glycerine, (c) PEG 400, (d) PEG 1000 and (e) Propylene glycol with PEG 400 was assessed after being subcultured on BHI agar
RESULTS
The MBC results for the vehicles are shown in Table 1. The results showed that all vehicles did exhibit bactericidal activity on the selected microorganisms at different concentrations. Out of all the vehicles, PEG 1000 was the most effective antimicrobial vehicle while glycerine was the least effective on the basis of its MIC. Propylene glycol was the second most effective against all microorganisms except Sta. aureus.
Table 1.
Bactericidal activity of vehicles against ATCC strains of Str. mutans, Sta. aureus, En. faecalis and Es. coli

Among all the ATCC strains of microorganisms, Str. mutans and Es. coli were the most susceptible to the vehicles, En. faecalis exhibited intermediate susceptibility, and Sta. aureus was the most resistant to all the vehicles.
The combination of propylene glycol and PEG 400 did not show any synergistic antimicrobial activity and, in fact, its efficacy decreased against Str. mutans, En. faecalis, and Es. coli, in comparison to propylene glycol alone.
PEG 1000 was most effective bactericidal agent against Str. mutans and Es. coli among all the five vehicles and the difference was statistically significant (Fisher's exact test: P = 0.003 and P = 0.004, respectively). For Sta. aureus and En. faecalis, none of the five vehicles showed statistically significant difference in their bactericidal activity (Fisher's exact test: P = 1 and P = 0.326, respectively).
DISCUSSION
In endodontic therapy, few cases do not respond to the conventional therapy in the pediatric and adult Endodontics. This difficulty to treat such refractory cases may be due to many reasons such as anatomic variation, presence of biofilms, and development of antibiotic resistance.[9] To overcome the problem of the global menace of developing antibiotic resistance, the use of alternative medications and substances or a combination of pharmaceutical excipients is suggested to increase the spectrum of antimicrobial action.[3] The most difficult to tackle is facultative anaerobes, as they are the ones that develop resistance most of the time.[14]
The use of intracanal medicaments becomes mandatory in Endodontics as many non-vital and abscessed teeth lack blood circulation. As a result of this, systemic antibiotics fail to reach the site of infection and, hence, such infections cannot be treated.[15] Also, local drug delivery and sustained release along with better diffusion into the surrounding periradicular tissues may prove to be an added advantage.[9,16] The vehicles used to dispense the intracanal medicaments have a direct influence on the release, time of onset of action of the medicament, penetration of the intracanal medicaments into dentinal tubules, and also the dissociation of drugs. The in vitro bactericidal activity of a variety of glycols, especially monopropylene, dipropylene, and triethylene, has been investigated more extensively. The bactericidal activity of PEG 400 had been studied almost three decades ago by Vaamonde et al. in 1982 and Chirfe et al. in 1983,[17] whereas the antimicrobial effect of PEG 1000 has been studied only recently.[12] The antimicrobial activity of PEG 1000 might be related to the hydrophilic property of PEG. Removal of water possibly does not allow microbial growth, as a certain amount of water is essential for the multiplication and development of microbes.[12] It is interesting to note that PEG 1000, like propylene glycol, also allowed greater dentinal penetration when combined with intracanal medicaments.[9] After a thorough review of literature, we have not come across a single article which explores and compares the bactericidal activity of these pharmaceutical excipients.
Also, after a series of in vitro and in situ studies, the Cariology Research Unit of the Niigata University School of Dentistry developed the concept of 3M-MP, in which 3M stands for triple mix of antibiotics and MP stands for Macrogol, i.e. polyethylene glycol, and propylene glycol, respectively.[11,18] This group of investigators have carried out extensive research on the most ideal antibiotics to be used for disinfection of dentinal tubules, but there is only one such study regarding penetration of propylene glycol as vehicle for intracanal medicament.[9] Propylene glycol is a known antimicrobial and is an effective preservative.[19] A recent article by Carreira et al. published in 2007 has thrown some light on the phenomenon of synergism in bactericidal activity, which was observed in Ciprofloxacin–PEG 1000 association, Metronidazole–PEG 1000 association, and Ciprofloxacin–Metronidazole–PEG 1000 combination. Also, PEG 400 produced severe plasmolysis, cell wall collapse, and finger-like extrusions in Klebsiella pneumonia.[20] Promising results have been shown by PEG-coated nanoparticles which were found to be most effective in killing Es. coli, Sta. aureus, and multi-drug resistant clinical isolates of Shigella spp. and Vibrio cholera.[21] Of all the vehicles being used in Endodontics, camphorated monochlorophenol (CMCP) is effective but tissue toxic, whereas distilled water possesses no antimicrobial activity.[22]
In our study, all vehicles exhibited antimicrobial activity at 100% concentration. We chose broth dilution over agar well diffusion, as the diffusion of vehicles through agar would be difficult due to them being viscous. Of them, propylene glycol exhibited maximum activity against En. faecalis, which was otherwise the least susceptible to the remaining vehicles. In 1965, Olitzky reported that propylene glycol is a known antimicrobial with marked germicidal activity.[23] Glycerine exhibited activity only at 100% concentration and, therefore, was the least effective antimicrobial. This is in accordance with the results observed by Antony et al. in 1997.[24] Other studies demonstrated bactericidal activity at 30% and 25% concentration but at a longer incubation time, i.e. after 48 h and 7 days, which is contrary to the findings of the present study.[25]
PEG 400, glycerine, and propylene glycol combination with PEG 400 showed similar bactericidal activity at 100% concentration only, against all the selected organisms. PEG 1000 showed bactericidal activity at the lowest concentration, i.e. at 25% against Str. mutans and Es. coli, but it exhibited bactericidal activity against Sta. aureus and En. faecalis, the most resistant of the selected standard strains, at 100% concentration only.
Str. mutans and Es. coli were the most susceptible organisms. Susceptibility of Str. mutans and Es. coli to 25% of PEG 1000 is in accordance with the findings by Carreira et al., but the results of Sta. aureus and En. faecalis vary and are not in agreement, which might be due to the selection of different standard strains. The results of the antimicrobial activity of PEG 400 and glycerine are contrary to the findings of Gomes et al., which could be because they had used agar diffusion method to test the antimicrobial activity. The negative results may be due to their inability to diffuse through agar due to their viscous nature and not necessarily due to the absence of antimicrobial activity.[12,26] Both PEG and propylene glycol possess low toxicity and are well-recognized vehicles for drugs.[10,27] Also, they result in better handling properties of the resulting paste.[22] Combination of propylene glycol with PEG 400 exhibited a non-synergistic effect. PEG 400 has an inherent advantage that it does not interact with other components of the paste and exerts its antibacterial activity because it has a low water activity.[28]
CONCLUSION
The purpose of conducting this study was to test the bactericidal activity of the above-mentioned vehicles and to determine the vehicle with the maximum bactericidal activity. This will aid in enhancing the diffusion, antimicrobial activity, and release of the intracanal medicaments for a longer period of time.[9] Hence, the most effective antimicrobial vehicle has its applications in Endodontics as an intracanal medicament,[4] in Pedodontics for lesion sterilization and tissue repair,[11,18] in chronic periodontitis for local drug delivery,[16] and also in the emerging field of Regenerative Endodontics.[29] The use of vehicles with good bactericidal activity might reduce the usage of antibiotics and toxic substances like CMCP and encourage the use of innovative substances like chlorhexidine gluconate as intracanal medicaments which do not develop resistance and are more biocompatible.[30]
Within the limitations of this study, of all the vehicles, PEG 1000 followed by propylene glycol were found to have better antimicrobial and bactericidal activities. Also, it had better handling properties of the resulting paste and diffusion through dentinal tubules.[9,12] The use of combination of propylene glycol with PEG 400 is not justified. PEG 1000 has greater bactericidal activity on Str. mutans and Es. coli.
Further studies should be carried out to verify and compare the diffusion of all the vehicles through dentinal tubules and against clinical isolates in vitro. Due to the synergistic action or even nullifying effect of combination of these vehicles with various intracanal medicaments, for instance, commonly used antibiotics, in vitro studies should be carried out to find the best medicament and vehicle combination, followed by in vivo studies.
ACKNOWLEDGMENTS
We would like to thank Dr. Alka Kale, Dr. Shivayogi Hugar and Dr. Sunil Jalalpure for their timely help and invaluable support throughout the conduct of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dhawane BS, Konda SG, Shaikh BM, Chobe SS, Khandare NT, Kamble VT, Bhosale RB. Synthesis and in vitro antimicrobial activity of some new 1-thiazolyl-2-pyrazoline derivatives. Int J Pharm Sci Rev Res. 2010;1:44–8. [Google Scholar]
- 2.Light DW, Lexchin JR. Pharmaceutical research and development: What do we get for all that money? BMJ. 2012;345:e4348. doi: 10.1136/bmj.e4348. [DOI] [PubMed] [Google Scholar]
- 3.Siedenbiedel F, Tiller JC. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers. 2012;4:46–71. [Google Scholar]
- 4.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Peters OA, Peter CI. Cleaning and shaping of root canal. In: Hargreaves KM, Cohen S, editors. Chen's Pathways of Pulp. 10th ed. Haryana, India: Elsevier; 2010. pp. 283–348. [Google Scholar]
- 6.Young GR, Parashos P, Messer HH. The principles of techniques for cleaning root canals. Aust Dent J. 2007;52(Suppl):S52–63. doi: 10.1111/j.1834-7819.2007.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 7.Ando N, Hoshino E. Predominant obligate anaerobes invading the deep layers of root canal dentin. Int Endod J. 1990;23:20–7. doi: 10.1111/j.1365-2591.1990.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 8.Peters LB, Wesselink PR, Buijs JF, van Winkelhoff AJ. Viable bacteria in root dentinal tubules of teeth with apical periodontitis. J Endod. 2001;27:76–81. doi: 10.1097/00004770-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Cruz EV, Kota K, Huque J, Iwaku M, Hoshino E. Penetration of propylene glycol into dentine. Int Endod J. 2002;35:330–6. doi: 10.1046/j.1365-2591.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 10.Rowe RC, Sheskey PJ, Quinn ME. 6th ed. Italy: Pharmaceutical Press and American Pharmacists Association and RPS Publishing; 2009. Handbook of Pharmaceutical Excipients; pp. 519–22. [Google Scholar]
- 11.Takushige T, Cruz EV, Asgor Moral A, Hoshino E. Endodontic treatment of primary teeth using a combination of antibacterial drugs. Int Endod J. 2004;37:132–8. doi: 10.1111/j.0143-2885.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 12.Carreira Cde M, dos Santos SS, Jorge AO, Lage-Marques JL. Antimicrobial effect of intracanal substances. J Appl Oral Sci. 2007;15:453–8. doi: 10.1590/S1678-77572007000500015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cockerill FR, Wikler MA, Alder J, Dudley MN, Eliopoulos GM, Ferraro MJ, et al. Wayne PA: Clinical and Laboratory Standards Institute; 2012. CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. CLSI document M100-S22; pp. 136–8. [Google Scholar]
- 14.Gaetti-Jardim Júnior E, Landucci LF, Lins SA, Vieira EM, de Oliveira SR. Susceptibility of strict and facultative anaerobes Isolated from endodontic infections to metronidazole and beta-lactams. J Appl Oral Sci. 2007;15:539–45. doi: 10.1590/S1678-77572007000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peedikayil FC. Antibiotics: Use and misuse in pediatric dentistry. J Indian Soc Pedod Prev Dent. 2011;29:282–7. doi: 10.4103/0970-4388.86368. [DOI] [PubMed] [Google Scholar]
- 16.Kalsi R, Vandana KL, Prakash S. Effect of local drug delivery in chronic periodontitis patients: A meta-analysis. J Indian Soc Periodontol. 2011;15:304–9. doi: 10.4103/0972-124X.92559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirfe J, Herszage L, Joseph A, Bozzini JP, Leardini N, Kohn ES. In vitro antibacterial activity of concentrated polyethylene glycol 400 solutions. Antimicrob Agents Chemother. 1983;24:409–12. doi: 10.1128/aac.24.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhakar AR, Sridevi E, Raju OS, Satish V. Endodontic treatment of primary teeth using combination of antibacterial drugs: An in vivo study. J Indian Soc Pedod Prev Dent. 2008;26(Suppl 1):S5–10. [PubMed] [Google Scholar]
- 19.Kinnunen T, Koskela M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1,3-butylene glycol in vitro. Acta Derm Venereol. 1991;71:148–50. [PubMed] [Google Scholar]
- 20.Bozzini JP, Kohn ES, Joseph A, Herszage L, Chirife J. Submicroscopical changes in Klebsiella pneumoniae cells treated with concentrated sucrose and polyethylene glycol 400 solutions. J Appl Bacteriol. 1986;60:375–9. doi: 10.1111/j.1365-2672.1986.tb05081.x. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya D, Samanta S, Mukherjee A, Santra CR, Ghosh AN, Niyogi SK, et al. Antibacterial activities of polyethylene glycol, tween 80 and sodium dodecyl sulphate coated silver nanoparticles in normal and multi-drug resistant bacteria. J Nanosci Nanotechnol. 2012;12:2513–21. doi: 10.1166/jnn.2012.6148. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh MR, Chaurasia VR, Masamatti VK, Mujeeb A, Jhamb A, Agarwal JH. In vitro evaluation of antibacterial efficacy of calcium hydroxide in different vehicles. J Int Soc Prev Community Dent. 2014;4:56–60. doi: 10.4103/2231-0762.131268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olitzky I. Antimicrobial properties of a propylene glycol based topical therapeutic agent. J Pharm Sci. 1965;54:787–8. doi: 10.1002/jps.2600540528. [DOI] [PubMed] [Google Scholar]
- 24.Anthony DR, Gordon TM, del Rio CE. The effect of three vehicles on the pH of calcium hydroxide. Oral Surg Oral Med Oral Pathol. 1982;54:560–5. doi: 10.1016/0030-4220(82)90195-5. [DOI] [PubMed] [Google Scholar]
- 25.Barr M, Tice LF. A study of the inhibitory concentration of various sugars and polyols on the growth of microorganisms. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1971;46:219–21. doi: 10.1002/jps.3030460405. [DOI] [PubMed] [Google Scholar]
- 26.Gomes BP, Ferraz CC, Vianna ME, Rosalen PL, Zaia AA, Teixeira FB, et al. In vitro antimicrobial activity of calcium hydroxide pastes and their vehicles against selected microorganisms. Braz Dent J. 2002;13:155–61. doi: 10.1590/s0103-64402002000300002. [DOI] [PubMed] [Google Scholar]
- 27.Li BQ, Dong X, Fang SH, Gao JY, Yang GQ, Zhao H. Systemic toxicity and toxicokinetics of a high dose of polyethylene glycol 400 in dogs following intravenous injection. Drug Chem Toxicol. 2011;34:208–12. doi: 10.3109/01480545.2010.500292. [DOI] [PubMed] [Google Scholar]
- 28.Ambrose U, Middleton K, Seal D. In vitro studies of water activity and bacterial growth inhibition of sucrose-polyethylene glycol 400-hydrogen peroxide and xylose-polyethylene glycol 400-hydrogen peroxide pastes used to treat infected wounds. Antimicrob Agents Chemother. 1991;35:1799–803. doi: 10.1128/aac.35.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayaraghavan R, Mathian VM, Sundaram AM, Karunakaran R, Vinodh S. Triple antibiotic paste in root canal therapy. J Pharm Bioallied Sci. 2012;4(Suppl 2):S230–3. doi: 10.4103/0975-7406.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komorowski R, Grad H, Wu XY, Friedman S. Antimicrobial substantivity of chlorhexidine- treated bovine root dentin. J Endod. 2000;26:315–7. doi: 10.1097/00004770-200006000-00001. [DOI] [PubMed] [Google Scholar]


