Abstract
Objective
Lower-extremity peripheral artery disease (LE-PAD), is strongly related to traditional risk factors (smoking, hypertension, dyslipidemia, diabetes). We hypothesized that the prevalence of LE-PAD in the absence of traditional CVD risk factors is not negligible, and that this condition would remain associated with subclinical atherosclerosis in other territories.
Methods
In the Multi-Ethnic Study of Atherosclerosis, we classified participants without any traditional risk factor according to their ankle-brachial index (ABI) into 3 groups: low (<1.00), normal (1.00–1.30) and high (>1.30) ABI. Coronary or carotid artery diseases were defined by the presence of any coronary artery calcification (CAC score > 0) or carotid plaque, respectively.
Results
Among the 6814 participants, 1932 had no traditional risk factors. A low- and high ABI were found in 176 (9%) and 149 (7.8%) cases, respectively. Lower glomerular filtration rate (OR: 0.88/10 units, p = 0.04) and higher Interleukin-6 levels (OR: 1.42/natural-log unit, p = 0.02) were associated with low ABI. Past smoking (cessation > 10 years) and pulse pressure had borderline association with low ABI. In adjusted models, low-ABI was significantly associated with CAC prevalence (OR: 1.22, p < 0.03). No significant association was found with carotid plaque.
Conclusion
In the absence of traditional CVD risk factors, LE-PAD is still common and associated with coronary artery disease.
Keywords: Atherosclerosis, Peripheral artery disease, Risk, Subclinical
The epidemiological studies during the last 50 years provided major findings on the risk factors for atherosclerosis and occurrence of cardiovascular events [1]. Besides age and gender, four other “modifiable” conditions, namely smoking, dyslipidemia, hypertension and diabetes, appeared as major contributors to the dramatic cardiovascular disease epidemic in western countries [2,3]. Clinical series and epidemiological studies have highlighted the role of these 4 traditional risk factors in the development of clinical and subclinical lower extremity peripheral artery disease (LE-PAD) [4,5]. These studies also demonstrate that PAD is frequently associated with atherosclerotic lesions in other vascular beds, especially the coronary and cerebrovascular territories [6,7]. However, a particular risk factor (i.e., smoking) does not affect all arteries to the same extent [8].
In several longitudinal studies, LE-PAD defined by a low ankle-brachial index (ABI) was associated with higher rates of fatal and non-fatal coronary and cerebrovascular events [9]. Recently, a meta-analysis showed that the association between LE-PAD and increased coronary events is present even in the presence of a low Framingham risk score [2], obtained by the summation of traditional risk factors [9].
Several potential risk factors including African-American ethnicity, other lipid disorders, psychosocial factors, inflammation, thrombotic and fibrinolytic factors are now considered as “novel” risk factors of LE-PAD [1,10–12]. However, the contribution of these risk factors in the development of LE-PAD in the absence of traditional risk factors is unknown. Additionally, the relationship between subclinical LE-PAD and subclinical CVD in other territories in the absence of traditional risk factors has not previously been assessed.
The Multi-Ethnic Study of Atherosclerosis (MESA) provides a unique opportunity to detect subjects free of CVD who may have LE-PAD in the absence of traditional risk factors. We propose to assess the prevalence of LE-PAD in the absence of the traditional risk factors, and its relationship with novel risk factors and other subclinical atherosclerotic CVD in this situation.
We hypothesized that in a low-risk subset of the MESA cohort, defined by the absence of the 4 traditional risk factors, a low ABI (<1.00) as well as an abnormally high ABI (>1.30) can still be present, and that they are both correlated with an increased prevalence of atherosclerosis in coronary and carotid arteries.
1. Methods
1.1. MESA population
MESA was initiated by the National Heart, Lung and Blood Institute, to investigate the prevalence, correlates, and progression of subclinical CVD in a multi-ethnic population-based sample of 6814 men and women aged 45–84 years without clinical CVD [13]. Participants were selected between 2000 and 2002 from six US field centers: Baltimore City and County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN. The Institutional Review Boards at all participating centers approved the study. All participants gave informed consent.
1.2. Definition of the low-risk group
Participants with any of the four traditional CVD risk factors (smoking, diabetes, hypertension and dyslipidemia) were excluded from the study. Participants were classified as smokers if they were currently smoking or had stopped smoking within the 10 years preceding the interview. Dyslipidemia was defined by a total/HDL-cholesterol ratio above 5 or use of lipid-lowering agents [14]. Diabetes was defined by fasting blood glucose above 1.26 g/L or use of anti-diabetic drugs. Systolic and diastolic blood pressures (SBP, DBP) were measured 3 times in the right arm of seated participants with a Dinamap model Pro 100 automated oscillometric sphygmonanometer (Critikon, Tampa, FL). The average of the last 2 measurements was used in the analyses. Participants were considered hypertensive if SBP was above 140 mmHg and/or DBP above 90 mmHg and/or the subject both self-reported a history of hypertension and was taking anti-hypertensive drugs.
1.3. Other clinical and socioeconomic variables
We estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation [15]. We analyzed the contribution of the pulsatile component of blood pressure [16], expressed as the pulse pressure (PP = SBP − DBP). The level of education was dichotomized at high school graduation. Participants were considered as low income if their reported annual house-hold income was below $25,000/year. The estimated 10-years risk of incident coronary heart disease, including coronary death, myocardial infarction, and angina was calculated according to the Framingham risk score [1].
1.4. Laboratory variables
Laboratory variables (blood glucose, total- and HDL-cholesterol, C-reactive protein (CRP), interleukin-6 (IL-6) and creatinine) were centrally measured on blood collected from enrollees and frozen at −70 °C. The methods of measurements are described elsewhere [13]. Glomerular filtration rate (eGFR) was estimated using the Modification of Diet in Renal Disease (MDRD) equation.
1.5. Ankle-brachial index (ABI)
After a 5-min rest in supine position, systolic blood pressures were measured in both arms and in posterior tibial (PT) and dorsalis pedis (DP) arteries of both ankles, using appropriate-sized cuffs and a continuous wave Doppler probe. The ABI was computed separately for each leg, with the numerator the highest of the PT or DP systolic pressures and the denominator the highest of the right vs. left brachial systolic pressures. For each participant, the index ABI was the lower of the right vs. left ABI. However we defined high ABI as the higher of the two sides being >1.30 and the other side being >1.00. When the ankle artery could not be occluded despite high (>250 mmHg) cuff pressure, the ABI was systematically categorized as >1.30.
1.6. Subclinical disease measures
Computed tomography of coronary arteries was performed as previously described [13,17,18]. We defined the presence of subclinical coronary disease by the presence of any coronary calcification (CAC score > 0).
Carotid ultrasound imaging protocol has been described else-where [7,13]. The images were analyzed off-line for the presence of carotid plaque, defined as any focal thickening of the carotid wall in the carotid bulbs or internal carotid arteries.
1.7. Statistical methods
Categorical and continuous variables were compared across ABI groups within each gender using Chi-squared tests and analysis of variance, respectively. Natural-log transformation was used as needed to stabilize variance prior to comparison of the means. Log-transformed variables were back-transformed for tabular presentation but statistical testing was performed on the log-transformed values. The novel risk factors selected here were based on a previous study on risk factors for LE-PAD in MESA [19]. Logistic regression models were used to assess the association of risk factors to a low- or high-ABI separately. In the model for low ABI, those with high ABI were excluded and vice versa.
To model the prevalence of CAC or carotid plaque as a function of low or high ABI, relative risk regression was used. That is, a generalized linear model with log link, Gaussian error structure, and robust standard errors. Scatterplots with superimposed running mean smoothers were used to illustrate the unadjusted relationships. For all the tests, a p < .05 was considered as significant.
2. Results
Among the 6814 men and women free of clinical CVD enrolled in the MESA, 1932 (781 men and 1151 women) were selected for this analysis, in the absence of any of the 4 traditional risk factors. This low CVD-risk group included 841 non-Hispanic whites (43.5%), 388 African Americans (20%), 400 Hispanics (20.7%) and 303 Chinese Americans (15.6%). The mean estimated Framingham risk score of all CHD was 6.1%. In this low CVD-risk group, a low ABI (<1.0) was found in 176 cases (9.0%) and a high ABI was present in 149 cases (7.8%). Interestingly, 17.5% of MESA participants with low-ABI had no traditional CVD risk factors.
The characteristics of the 3 ABI groups in our population study are presented in Table 1. Participants in the low-ABI group were older and included a higher proportion of female subjects. The ethnic distribution also differed, with a higher prevalence of African Americans and lower prevalence of Hispanic subjects among participants with a low ABI, compared to those with a normal ABI. Although all the participants were selected according to the absence of hypertension, those with a low ABI presented on average a higher pulse pressure than in the normal ABI group. Similarly, the average values of inflammatory markers (CRP, IL-6) were higher in the low-ABI group than in the normal ABI group. Mean eGFR was slightly, but significantly, lower in the low-ABI group. Regarding the association with atherosclerosis in other vascular beds, a higher prevalence of CAC > 0 and carotid plaques were found among participant with low ABI, though the latter was of borderline significance (p = .07).
Table 1.
Characteristics of men and women without traditional cardiovascular disease risk factors, according to ankle brachial index categories (N= 1938).
| Low ABI (<1.0), N= 176 | p vs. normal | Normal (1.0–1.3), N= 1607 | p vs. normal | High (>1.3), N= 149 | |
|---|---|---|---|---|---|
| Mean ABI | 0.96 (0.05) | <0.001 | 1.13 (0.06) | <0.001 | 1.36 (0.08) |
| Age (years) | 61.4 (11.1) | 0.002 | 59.0 (9.9) | 0.97 | 59 (9.8) |
| Male gender (%) | 34 (19.3) | <0.001 | 647 (40.3) | <0.001 | 100 (67.1) |
| Ethnicity (%) | |||||
| NHW | 79 (44.9) | 679 (42.3) | 83 (55.7) | ||
| Black | 58 (33.0) | <0.001 | 307 (19.1) | 0.003 | 23 (15.4) |
| Hispanic | 16 (9.1) | 352 (21.9) | 32 (21.5) | ||
| Chinese | 23 (13.1) | 269 (16.7) | 11 (7.4) | ||
| High-school graduation (%) | 152 (86.4) | 0.72 | 1372 (85.4) | 0.02 | 138 (92.6) |
| Low income (%) | 41 (23.3) | 0.54 | 408 (25.4) | 0.002 | 21 (14.1) |
| Past smoker (%) | 69 (39.2) | 0.16 | 545 (33.9) | 0.04 | 63 (42.3) |
| BMI (kg/m2) | 27.3 (6.2) | 0.17 | 26.7 (5.1) | 0.002 | 28.1 (5.2) |
| SBP (mmHg) | 115 (13.8) | 0.30 | 114 (13.1) | 0.23 | 113.0 (12.3) |
| DBP (mmHg) | 65.9 (8.7) | <0.001 | 68.3 (8.7) | 0.73 | 68.6 (8.6) |
| PP (mmHg) | 49.3 (11.5) | <0.001 | 45.8 (10.7) | 0.08 | 44.2 (9.1) |
| Total/HDL cholesterol | 3.47 (0.80) | 0.03 | 3.61 (0.79) | 0.48 | 3.65 (0.78) |
| Glucose (mg/dL) | 86.9 (10.0) | 0.81 | 87.1 (9.8) | 0.15 | 88.3 (9.8) |
| CRP (mg/L) | 4.44 (6.6) | 0.001 | 3.15 (6.0) | 0.59 | 2.61 (3.8) |
| IL-6 (pg/mL) | 1.63 (1.5) | 0.001 | 1.26 (1.0) | 0.36 | 1.38 (1.4) |
| GFR (mL/min/1.73m2) | 79.6 (15.4) | 0.01 | 82.5 (15.0) | 0.07 | 80.2 (13.8) |
| CAC > 0 (%) | 72 (40.9) | 0.01 | 507 (31.5) | 0.01 | 62 (41.6) |
| Carotid plaque (%) | 54 (31.4) | 0.07 | 399 (25.0) | 0.82 | 36 (24.2) |
Tests for differences in average glucose, CRP and IL-6 were conducted on log-transformed values.
The high-ABI group also differed from the normal ABI-group, in that they included a higher proportion of men, had higher education levels, and a lower prevalence of participants with low-income. They also had a higher BMI than the normal ABI group. Finally, a greater prevalence of CAC was found in the high-ABI than in the normal-ABI group. In contrast, no difference was found regarding the rates of coexisting carotid plaque.
Two separate logistic regression models were performed to detect independent factors associated with low- and high-ABI. In each case, the group with normal ABI was the reference (Table 2). As compared to participants with normal ABI, those with low ABI included a higher proportion of females and African-Americans, and had higher levels of IL-6. Also, a borderline association was present with past-smoking (beyond the 10 last years), lower eGFR and Hispanic ethnicity. Regarding high ABI, only male gender and BMI were positively and significantly associated with this condition.
Table 2.
Adjusted associations of clinical characteristics with presence of low ABI and high ABI, among individuals without traditional cardiovascular disease risk factors.
| Risk factors | Low ABI |
High ABI |
||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Age (10 years) | 1.06 (0.86, 1.30) | 0.59 | 0.99 (0.80, 1.23) | 0.95 |
| Male gender | 0.35 (0.23, 0.54) | <0.001 | 3.01 (1.99, 4.56) | <0.001 |
| Race/ethnicity | ||||
| NHW | Ref. | Ref. | ||
| Black | 2.02 (1.33, 3.08) | 0.001 | 0.61 (0.36, 1.02) | 0.06 |
| Hispanic | 0.42 (0.22, 0.78) | 0.006 | 0.90 (0.54, 1.51) | 0.69 |
| Chinese | 1.04 (0.58, 1.86) | 0.90 | 0.54 (0.27, 1.10) | 0.09 |
| High-school graduation | 0.74 (0.43, 1.26) | 0.27 | 1.62 (0.77, 3.41) | 0.21 |
| Low income | 0.89 (0.58, 1.37) | 0.59 | 0.73 (0.42, 1.27) | 0.27 |
| Past smokinga | 1.39 (0.97, 1.97) | 0.07 | 1.07 (0.74, 1.54) | 0.71 |
| Total/HDL-cholesterol | 0.95 (0.76, 1.18) | 0.64 | 0.83 (0.65, 1.06) | 0.14 |
| Blood glucose (ln mg/dL) | 1.55 (0.29, 8.18) | 0.61 | 0.95 (0.16, 5.57) | 0.96 |
| BMI (/m/kg2) | 0.98 (0.95, 1.02) | 0.44 | 1.07 (1.03, 1.12) | 0.001 |
| Pulse pressure (/10mmHg) | 1.18 (0.99, 1.39) | 0.06 | 0.88 (0.72, 1.08) | 0.96 |
| CRP (ln mg/L) | 1.04 (0.87, 1.23) | 0.69 | 0.89 (0.73, 1.08) | 0.23 |
| IL-6 (ln pg/mL) | 1.42 (1.06, 1.91) | 0.02 | 1.15 (0.84, 1.58) | 0.38 |
| GFR (/10 mL/min/1.73m2) | 0.88 (0.78, 0.99) | 0.04 | 0.91 (0.79, 1.04) | 0.17 |
Each model is a logistic regression (one model for low ABI relative to normal ABI; and one model for high ABI relative to normal) including all indicated variables.
Past smoking refers to any smoking prior to 10 years previous. Other smokers who stopped smoking were excluding from the study population, along with the current smokers.
The association between the ABI and the presence of coronary artery calcification and carotid plaque are presented in Figs. 1 and 2. The association between the ABI and the probability for CAC score > 0 presented a U-shaped curve, with the lowest CAC prevalence for ABI values within the 1.10–1.20 interval. Both low- and high-ABI were positively correlated with the presence of CAC (CAC score > 0) in the unadjusted models (Table 3). In models adjusted for age, sex, ethnicity and risk factors significantly associated with a low or high ABI, the association remained significant for low ABI, but became non-significant for high ABI (Table 3).
Fig. 1.
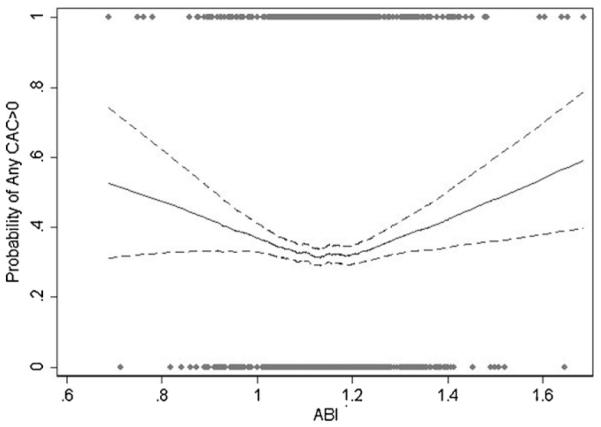
Probability of CAC > 0 according to the ABI values.
Fig. 2.
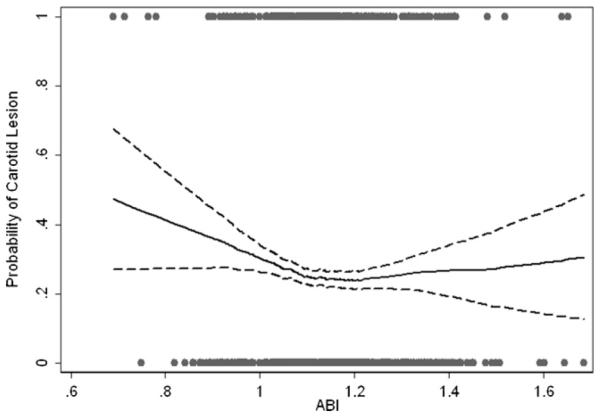
Probability of presence of carotid plaque according to the ABI values.
Table 3.
Associations of the ankle-brachial index categories with the presence of coronary artery calcium and carotid plaque.
| Unadjusted |
Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|---|
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| Coronary artery calcium | ||||||
| Normal ABI | Ref. | Ref. | Ref. | |||
| Low ABI (≤1.00) | 1.30 (1.07, 1.57) | 0.008 | 1.21 (1.03, 1.41) | 0.019 | 1.19 (1.02, 1.39) | 0.026 |
| High ABI (≥1.30) | 1.32 (1.08, 1.62) | 0.008 | 1.07 (0.90, 1.27) | 0.429 | 1.10 (0.93, 1.30) | 0.280 |
| Carotid plaque | ||||||
| Normal ABI | Ref. | Ref. | Ref. | |||
| Low ABI (≤ 1.00) | 1.26 (0.99, 1.59) | 0.06 | 1.09 (0.86, 1.39) | 0.46 | 1.04 (0.84, 1.30) | 0.70 |
| High ABI (≥1.30) | 0.97 (0.72, 1.30) | 0.82 | 0.91 (0.70, 1.20) | 0.51 | 0.93 (0.71, 1.20) | 0.56 |
Model 1 is adjusted for age, sex and ethnicity. Model 2 is additionally adjusted for past smoking, high school graduation, pulse pressure, GFR, BMI, cholesterol to HDL ratio, and IL-6.
The association between ABI and the presence of carotid plaque was nearly linear for ABI value >1.10 (Fig. 2). Below the ABI value of 1.10, the probability for the presence of carotid plaque increased progressively with decreasing ABI values. Using the regression models, only a borderline association was found between ABI < 1.00 and carotid plaque. No significant association of ABI with presence of carotid plaque was found in adjusted models (Table 3).
3. Discussion
This study confirms that even in a population free of any clinical CVD and without traditional CVD risk factors, a significant proportion of the population has subclinical LE-PAD, defined by abnormal ABI values. In our pre-selected low-risk sub-sample of the MESA baseline cohort, a low ABI (<1.00) was found in 9% of participants, and additionally 7.8% of participants had an elevated (>1.30) ABI. Almost one-fifth of the MESA participants with low (<1.00) ABI had no traditional CVD risk factors as defined above. Despite this, participants with a low ABI had a higher prevalence of subclinical coronary artery disease, compared to those with a normal ABI.
Interestingly, we found a bordeline (p = .07) association between low ABI and past smoking, despite the “past smoking” definition of at least 10 years prior. Smoking is a major risk for LE-PAD and is associated with LE-PAD progression [20]. Smoking cessation is one of the most efficient methods to slow the disease progression and improve clinical prognosis in LE-PAD patients [21]. Our finding suggests that even 10 years after smoking cessation, ABI may still be lower in patients who previously smoked.
Among the non-traditional risk factors, we found higher IL-6 levels and lower eGFR levels in participants with PAD. An association between PAD and inflammatory markers such as IL-6 has been already shown in several epidemiological studies [20,22]. This is to our knowledge the first report showing that inflammation is associated with low ABI in a selected population free of CVD risk factors. Similarly, chronic kidney disease (CKD) is frequently associated with LE-PAD [23], but this report highlights the importance of CKD as a potential risk factor for the presence of subclinical PAD in the absence of CVD risk factors.
The strong association between female gender and low ABI in this setting suggests that a low ABI is more frequent in women than men in this sub-group, suggesting that novel risk factors might be more contributive for LE-PAD in women. However, neither in the whole MESA cohort [19] nor in other epidemiological studies [10] have any interactions between novel risk factors and gender been reported. Recent findings in the MESA baseline cohort suggest that ABI values may be intrinsically lower in women than men, even after taking into account a series of anthropometric, biological and socio-economic variables [24]. Yet, a lower “normal” ABI in women than in men could partially explain why female gender is positively associated with low (<1.00), and male gender with high (>1.30) ABI. Similarly, it has been suggested that African Americans have an intrinsically lower ABI than non-Hispanic whites, and this may partly explain higher odds for a low ABI and lower odds for a high ABI, independent of other confounding variables [24].
The second major finding is that a low ABI even in the absence of traditional risk factors for CVD remains a marker of general atherosclerosis, especially coronary artery atherosclerosis. In an earlier study in the whole baseline population of MESA, McDermott et al. found that ABI values as high as 1.00 were associated with coronary and carotid atherosclerosis [7]. Interestingly, a linear relationship was found between ABI <1.0 and the probability of prevalent coronary calcification, so that the ABI remains useful to predict the presence of coronary artery disease even in the absence of traditional risk factors. This association might explain why even in patients with low Framingham risk score, a low-ABI <1.0 was associated with higher mortality rates in the ABI collaboration meta-analysis in general population [9].
The lack of association between low ABI and carotid plaque after adjustments is surprising. One possibility is that coronary calcification may occur at an earlier stage of atherosclerosis progression than a carotid plaque visualized by ultrasound. In line with this hypothesis, the overall rate of carotid plaque was lower than CAC > 0 in our population study, so the statistical power to detect any difference was lower.
In this large cross-sectional study in a population free of clinical cardiovascular disease and classified at low risk for CVD events in the absence of any traditional risk factor, we found that still 16.8% of the population had an abnormal ABI (9% with low- and 7.8% with high ABI). Renal disease and inflammation represented respectively by the eGFR and IL-6 were associated with low (<1.0) ABI, while body-mass index was positively associated with elevated (>1.30) ABI. The presence of coronary calcium was associated with a low-ABI, although after adjustments for confounders. This study suggests that the ankle-brachial index may be still be a useful diagnostic marker for coronary atherosclerosis, even in the absence of traditional risk factors.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- [1].Wilson PF, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- [2].Grundy SM, Pasternak R, Greenland P, Smith S, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations. A statement of healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–92. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- [3].De Backer G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: third joint task force of European and other societies on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil. 2003;10:S1–10. doi: 10.1097/01.hjr.0000087913.96265.e2. [DOI] [PubMed] [Google Scholar]
- [4].Dormandy JA, Rutherford RB. TransAtlantic Inter-Society Consensus (TASC). Management of peripheral arterial disease (PAD) J Vasc Surg. 2000;31(1):S1–296. Pt 2. [PubMed] [Google Scholar]
- [5].Criqui MH, Denenberg JO, Langer RD, Fronek A. The epidemiology of peripheral arterial disease: importance of identifying the population at risk. Vasc Med. 1997;2:221–6. doi: 10.1177/1358863X9700200310. [DOI] [PubMed] [Google Scholar]
- [6].Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med. 1998;3:241–5. doi: 10.1177/1358836X9800300311. [DOI] [PubMed] [Google Scholar]
- [7].McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- [8].Weitz JI, Byrne J, Clagett P, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–49. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- [9].Ankle Brachial Index Collaboration Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality. A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease. The San Diego population study. Circulation. 2005;112:2703–7. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- [11].Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis. A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- [12].Whiteman MC, Deary IJ, Fowkes FG. Personality and social predictors of atherosclerotic progression: Edinburgh artery study. Psychosom Med. 2000;62:703–14. doi: 10.1097/00006842-200009000-00015. [DOI] [PubMed] [Google Scholar]
- [13].Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [14].Natarajan S, Glick H, Criqui M, et al. Cholestrol measures to identify and treat individuals at risk of coronary heart disease. Am J Prev Med. 2003;25:50–7. doi: 10.1016/s0749-3797(03)00092-8. [DOI] [PubMed] [Google Scholar]
- [15].National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl. 1):S1–266. [PubMed] [Google Scholar]
- [16].Safar ME, London GM. The arterial system in human hypertension. In: Swales JD, editor. Textbook of hypertension. Blackwell Scientific; London, UK: 1994. pp. 85–102. [Google Scholar]
- [17].Carr JJ, Crouse JR, Goff DC, et al. Evaluation of sub-second gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. Am J Radiol. 2000;174:915–21. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- [18].Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- [19].Allison MA, Criqui MH, McClelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:1190–7. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- [20].Aboyans V, Criqui MH, Denenberg JO, et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–9. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- [21].Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33:S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [22].Tzoulaki I, Murray GD, Lee AJ, et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population. Edinburgh artery study. Circulation. 2005;112:976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- [23].Wattanakit K, Folsom AR, Selvin E, et al. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–36. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- [24].Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA) J Vasc Surg. 2007;45:319–27. doi: 10.1016/j.jvs.2006.10.032. [DOI] [PubMed] [Google Scholar]


