Abstract
Objective
To determine if ECG repolarization measures can be used to detect small changes in serum potassium levels in hemodialysis patients.
Patients and Methods
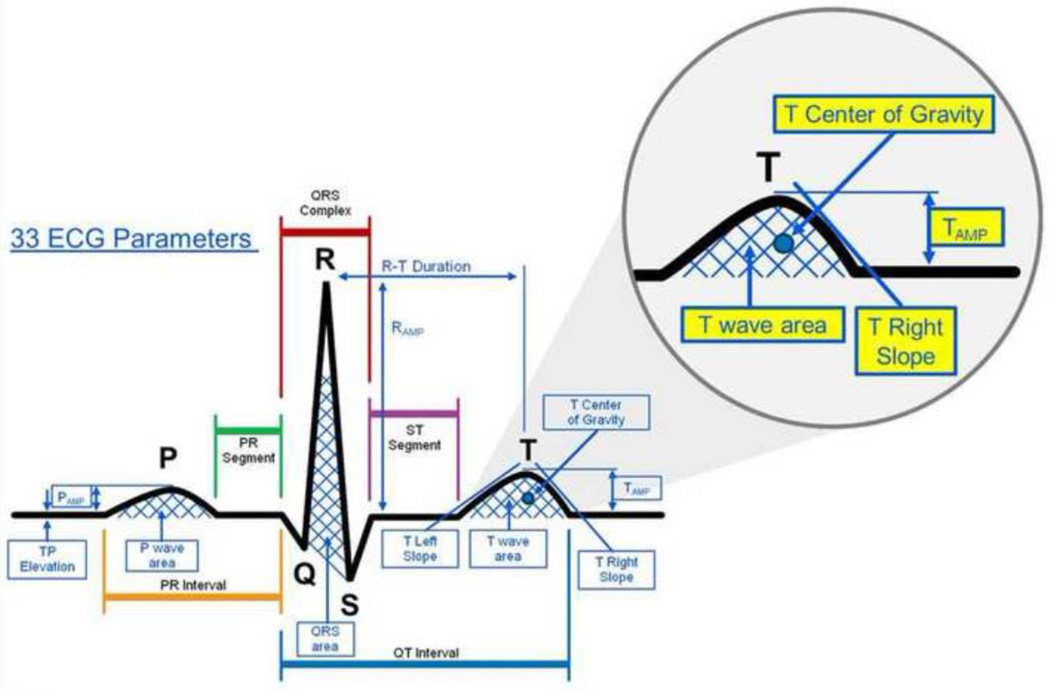
Signal-averaged ECGs were obtained from standard ECG leads in 12 patients before, during, and after dialysis. Based on physiological considerations, five repolarization-related ECG measures were chosen and automatically extracted for analysis: the slope of the T wave downstroke (T right slope), the amplitude of the T wave (T amplitude), the center of gravity (COG) of the T wave (T COG), the ratio of the amplitude of the T wave to amplitude of the R wave (T/R amplitude), and the center of gravity of the last 25% of the area under the T wave curve (T4 COG) (Figure 1).
Results
The correlations with potassium were statistically significant for T right slope (P < 0.0001), T COG (P=0.007), T amplitude (P=0.0006) and T/R amplitude (P=0.03), but not T4 COG (p=0.13). Potassium changes as small as 0.2 mmol/L were detectable.
Conclusion
Small changes in blood potassium concentrations, within the normal range, resulted in quantifiable changes in the processed, signal-averaged ECG. This indicates that non-invasive, ECG-based potassium measurement is feasible and suggests that continuous or remote monitoring systems could be developed to detect early potassium deviations among high-risk patients, such as those with cardiovascular and renal diseases. The results of this feasibility study will need to be further confirmed in a larger cohort of patients.
Keywords: potassium, ECG, signal processing, T-wave, dialysis, hyperkalemia
Introduction
Potassium homeostasis is impaired in patients with cardiovascular diseases due to advanced age, diabetes, and renal dysfunction.1 Evidence-based therapies including renin-angiotensin blockade, beta adrenergic blockade, and potassium sparing diuretics promote hyperkalemia. Diuretic-induced hypokalemia may promote arrhythmogenesis. There is compelling evidence that even modest potassium changes in patients with cardiovascular or renal disease may increase the risk of hospitalization and death.1–3 In patients undergoing three times weekly hemodialysis, the sudden death rate triples in the 12 hours prior to scheduled dialysis following a two day, weekend hiatus, the time at which hyperkalemia is most likely.4–6 Importantly, no method of non-invasively detecting hyperkalemia exists currently.
A tool to permit non-invasive, unobtrusive potassium assessment that integrates with current remote monitoring platforms may shorten hospitalizations, facilitate remote outpatient care, foster independent living, and ease transitions of care. Currently, all tests to assess potassium require access to blood. A bloodless test to assess potassium would be an important advance. Significant abnormalities in potassium are accompanied by marked derangements on the surface electrocardiogram (ECG).7–10 However, while dramatic ECG abnormalities in association with often life-threatening alterations in potassium have been well described, subtle ECG changes may occur from physiologic increases or decreases in potassium levels, including changes within the normal range of 3.5 to 5.5 mmol/L. These subtle ECG changes are not routinely appreciated on the surface electrocardiogram due to their small amplitude relative to background ECG noise (from skeletal muscle or skin galvanic current, often in the range of 8–10 microVolts (mV)), resulting in a poor signal-to-noise ratio. Additionally, confounding factors may influence the ECG, including heart rate, body position, individual variations in body habitus and ECG, and other metabolic or cardiovascular conditions. We have developed algorithms for acquisition, processing and analysis of ECG signals that augment the signal-to-noise ratio, correct for confounders, create an individualized template and permit determination of potassium values within the normal range.
We hypothesized that: 1) Small changes in plasma potassium are accompanied by quantifiable changes in the processed surface ECG; 2) Once an individualized baseline ECG is obtained for a known potassium value, subsequent changes in potassium can be determined noninvasively by measuring changes in the processed ECG; 3) Potassium related ECG changes can be used to track changes in potassium levels in the clinical context. To test these hypotheses we performed a pilot, feasibility study in patients undergoing hemodialysis.
Methods
Patient Cohort
Patients 18 years old or older, undergoing hemodialysis at Mayo Clinic Rochester, were eligible for study enrollment under an IRB approved protocol (IRB# 10-008249; #13-006127). Both inpatient and outpatient hemodialysis patients were enrolled.
Data acquisition
An ECG was recorded with electrodes in standard clinical positions using a Siesta 802 system (Compumedics, Charlotte, NC) with a sampling at a rate of 1024 bps. ECG acquisition commenced 15 minutes before the start of hemodialysis, and continued until its conclusion. Blood was drawn from the arterial tubing at three time points during the study: i) pre-dialysis, ii) at the mid-time point of the dialysis run after temporarily clamping the heparin line (if in use), stopping dialysate flow and decreasing the blood flow rate to 100 mL/min for at least 15 seconds and iii) post-dialysis after stopping dialysate flow and decreasing the blood flow rate to 100 ml/min for at least 15 seconds.
ECG processing
For analysis purposes the ECG recordings were “batched” into three 15 minute segments corresponding to signals acquired: i) immediately before dialysis, ii) at the time of the mid-dialysis blood sample and iii) immediately following dialysis. Analysis of the data was then performed programmatically in a numerical computing environment (Matlab, R2009b, Beer Sheba, Israel). QRS complexes were excluded from analysis based on R-R interval and amplitude deviations, and using template matching and covariance filtering to eliminate artifact and repolarization changes due to ectopy. Following these steps, the filtered complexes were signal averaged to eliminate noise. The processed signals underwent feature extraction and predictive analysis for potassium assessment.
Based on initial data and physiologic significance, we chose five ECG features for in-depth analysis: the right slope of the T wave (T right slope), the amplitude of the T wave (T amplitude), the center of gravity (COG) of the T wave (T COG), the ratio of the amplitude of the T wave to amplitude of the R wave (T/R amplitude), and the center of gravity of the last 25% of the area under the T wave curve (T4 COG) (Figure 1). T right slope was calculated as the mean first derivative of the T wave from the beginning to the end of the TpTe interval. We defined the center of gravity as the mean amplitude (mV)/mean duration (ms). Automatic algorithms to extract these features were created.
Figure 1. ECG parameters analyzed for correlation with potassium changes.
Large image: All measurable ECG parameters that were tested for sensitivity to potassium changes while recording ECG in dialysis patients at various potassium concentrations. Inset: Close-up view of parameters that had best correlation to changes in potassium: T center of gravity (T Cog), Tamp- Amplitude of T wave, T wave area, T right slope. T/R amplitude is calculated based on the corresponding values of the T wave area and R wave area shown in figure. T4 Cog is the latter 25% of the area under the T wave.
Principal Component Analysis and Unsupervised Optimal Fuzzy Clustering
Principle component analysis and clustering processes were performed on ECG sampled records to observe changes in the samples’ patterns. The ECG data set included records constructed from one minute of filtered and averaged ECG data. The records were represented by P dimensions of samples in the time domain, where P is the number of samples in each heart beat (P≈800, but is heart-rate dependent). Each session included 15 records that represented a measured potassium level. The clustering procedure included two major steps: principal component analysis of the records in the set and unsupervised optimal fuzzy clustering (UOFC).
A set of basis waveforms (principal components) common to all the records were computed using a covariance matrix (P × P) of the ECG matrix that was defined as cov = E{(V − μv)(V − μv)T}, where: μv is the averaged ECG vector of all 60 records. G (P × P dimensional matrix) and λi, (i = 1, 2, … ,800) were the eigenvectors and the corresponding eigenvalues of the covariance matrix. We then reduced the records to a small number of coefficients. These coefficients were used to divide the records into clusters. Utilizing principle component analysis with a mean standard error (MSE) of 1% we were able approximate the data with 10 coefficients; by increasing the MSE to 5% we can reduce the number of the eigenvectors to F=4. The coefficients matrix YF=10 was then used as the features vectors for unsupervised optimal fuzzy clustering. Data were clustered without a priori assumptions about the characteristic features of the clusters. The clustering procedure ran in two iterative steps: 1. All records were assigned to a single cluster, and the memberships in this cluster were calculated; 2. A second cluster was created to include records with the lowest memberships in the first cluster. This sequence was repeated until two validity criteria were met that were based on: a) sum of memberships within each cluster, b) standard deviation of members within the cluster.
Statistical Analysis
In order to test for association of each parameter with measured potassium values, a linear mixed model was used. Potassium values were used as the dependent variable and each individual parameter was tested one at a time for evidence of association. A random per subject intercept with a freely estimated correlation structure was used to properly account for the correlation between repeated measurements within subjects. A fixed effect corresponding to the ECG parameter was included in each model as the main effect and tested The fit of the model generated for each parameter and lead combination was determined using the Akaike Information Criterion (AIC).11 Lower AICs denote a better fit of the data. The AICs for all parameters across the 12 ECG leads were given a ranking from lowest to highest. Average rankings by lead and parameter were calculated and summarized. The ranks were compared for differences between parameters in order to determine the best parameter. This was done via one way analysis of variance on the ranks and corrected for multiple comparisons using the Turkey HSD test. A second analysis was also conducted within the mixed model framework that included a patient by ECG parameter interaction term. This term was used to test for evidence of different slopes by subject. Pearson correlation coefficients were calculated to summarize correlation between measurements of potassium and individual ECG parameters within subject. Heart rates were compared among time periods using repeated measures analysis of variance. All analyses were conducted using SAS version 9.3, Cary, NC. Two-sided p-values <0.05 were considered to be statistically significant.
Results
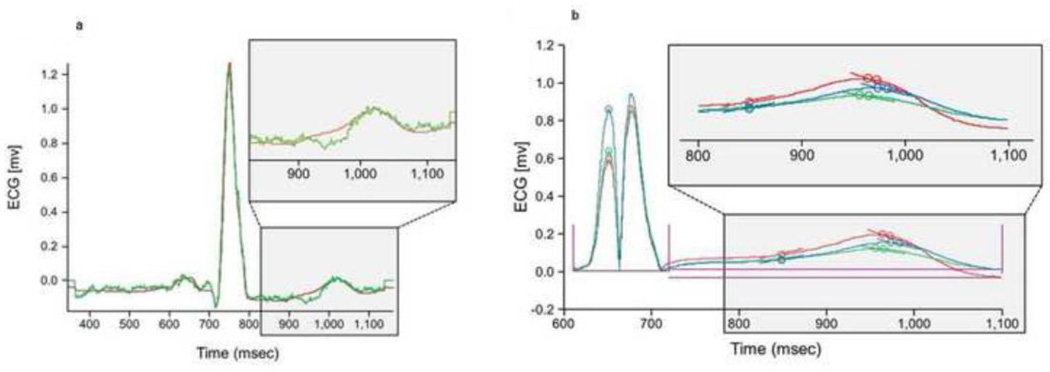
Twelve dialysis patients (58% men, 42% women) with a mean age of 55 ± 15 years were enrolled. Ten were Caucasian, one was Native American and one was Asian. All patients were in normal sinus rhythm at the time of the study. The mean heart rates (beats per minute) were 74 ± 12 before dialysis, 78 ± 13 mid-dialysis and 78 ± 13 following dialysis. The heart rates did not differ statistically among time periods (P=0.06). Figure 2A depicts the tracings acquired from a single patient showing the raw original vs. the filter, processed tracing. Figure 2B shows a signal averaged ECG from a single patient at different levels of potassium. The lead with the lowest average AIC rank was precordial lead V4, however, the leads did not differ statistically. ECG parameters were also evaluated by AIC ranks across all leads (Table 1). The parameter with the lowest average AIC rank was T right slope. T right slope was not statistically different from either T COG (P=0.99) or T4 COG (P=0.82). The average AIC ranks for T Right slope, T COG, and T4 COG were superior to both T amplitude and T/R amplitude (P<0.001).
Figure 2. Mathemathematical Processing of ECG Signals.
Panel A) Depicts the averaged raw original versus processed ECG averaged signal for a given patient. The green tracing shows difference between a raw and a processed signal; this is the pre-processed signal. The red tracing is the averaged ECG signal after ECG processing algorithm was performed by exclusion based on R-R interval and amplitude deviations, template matching, and covariance filtering to eliminate artifact and noise. Inset- zoomed in view of the T wave of both unfiltered and filtered tracings. Panel B) Depicts multiple ECG averaged signals from a single patient at various potassium concentrations. Three examples are shown that display time-aligned, rectified, and processed ECG signals at various times during dialysis in at various potassium concentrations (red, blue, green). Note that the dotted S wave is a positive transposition of the negative solid S wave in order to calculate from an absolute positive value. Inset- zoomed in version of T wave segment of all three ECG tracings. T-wave changes are clearly visible after processing, despite relatively minor potassium changes.
Table 1.
Mean AIC ranks across all leads, by parameter (N = 12 patients)
| Parameter | Mean Rank |
|---|---|
| T right slope | 17.3 * |
| T COG (mv/ms) | 18.5 * |
| T4 COG (mv/ms) | 21.7 * |
| T amplitude | 41.5 # |
| T/R amplitude | 53.5 |
Akaike Information Criterion (AIC); center of gravity (COG); mean amplitude (mV); mean duration (ms)
P < 0.001 vs. T amplitude and T/R amplitude
P =0.04 vs. T/R amplitude
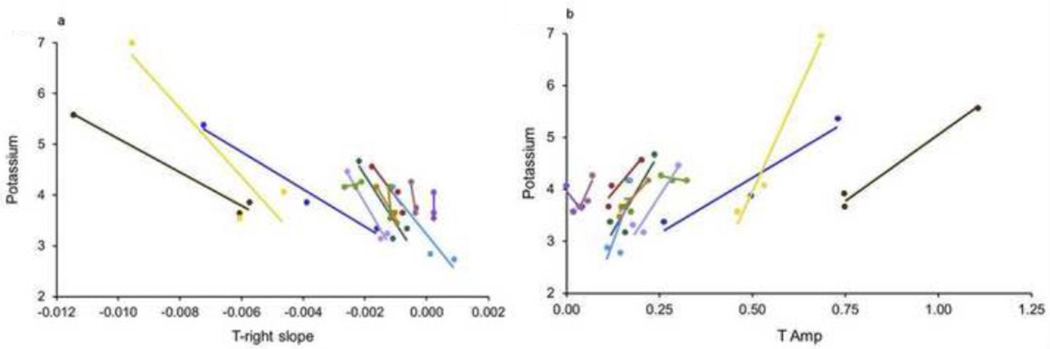
The correlations between potassium and each ECG parameter, in precordial lead V4, are shown in Table 2. The correlations with potassium were statistically significant for T right slope (P < 0.0001), T COG (P=0.007), T amplitude (P=0.0006) and T/R amplitude (P=0.03), but not T4 COG (p=0.13). To determine whether there was significant individual variability in the rate of ECG feature response to potassium changes, we tested whether there was evidence for individual patient slopes. Differences among the slopes of the individual-patient regression lines for potassium versus each parameter in V4 were statistically significant only for T amplitude (P=0.05) and T/R amplitude (P=0.0005), suggesting that the other parameters may be more universal in response slopes. Table 3 shows the AIC and statistical significance for T right slope (the preferred parameter) across leads. The presence of statistically significant correlations in leads V2-V6 and in the inferior leads indicates that the T right slope parameter is relatively insensitive to lead position across the precordium as well as in the inferior leads. Figures 3A and 3B show the individual patient values and individual patient regression lines for the parameters with the greatest statistical significance in lead V4, T right slope and T amplitude. For T right slope, a negative correlation with potassium was present (meaning that the downslope became steeper with increasing potassium) for 10 of the 12 patients, with correlation coefficients between −0.86 and −0.99. The single, positive correlation (R=0.75) occurred in a patient in whom the potassium range was small (4.2–4.3). The second patient with aberrant correlation had no variability in the T right slope measurements and a correlation could not be calculated. For T amplitude, a positive correlation with potassium was present for 9 of the 12 patients, with correlation coefficients between 0.77 and 0.99. The three, aberrant, negative correlations (R=−0.84, −0.71, −0.03) also occurred patients in whom the potassium ranges were small (4.2–4.3, 3.6–4.1, 3.6–4.2).
Table 2.
Correlations between potassium and each parameter in lead V4 (N = 12 patients)
| Parameter | Slope estimate | AIC | P |
|---|---|---|---|
| T right slope (mv/ms) | −198 | 55 | < 0.0001 |
| T COG (mv/ms) | 3117 | 64 | 0.007 |
| T4 COG (mv/ms) | 2850 | 69 | 0.13 |
| T amplitude (mv) | 1.8 | 74 | 0.0006 |
| T/R amplitude | 0.74 | 87 | 0.03 |
Center of gravity (COG); mean amplitude (mV); mean duration (ms)
Table 3.
Correlations between potassium and T right slope parameter across all leads (N = 12 patients)
| Lead | Slope Estimate (meq/L/mv) |
AIC | P |
|---|---|---|---|
| V3 | −243 | 52 | <0.0001 |
| V4 | −198 | 55 | <0.0001 |
| III | −184 | 61 | <0.0001 |
| aVF | −185 | 61 | <0.0001 |
| II | −166 | 63 | 0.0002 |
| V5 | −180 | 63 | 0.0003 |
| V6 | −210 | 65 | 0.0005 |
| V2 | −185 | 72 | 0.002 |
| aVL | 225 | 74 | 0.14 |
| aVR | 132 | 75 | 0.11 |
| I | −159 | 75 | 0.17 |
| V1 | 32 | 77 | 0.70 |
Figure 3. Changes in Potassium Concentration Correlate with Subtle Changes in Processed ECG.
Panel A) shows subtle how ECG changes in response to changes in potassium concentration, as its correlation with T-right slope. Individual patient values and individual patient regression lines for the T right slope are shown using processed T-right slope values in each of the individual dialysis patients. Each color represents data from a single patient. The lines are the individual-patient, linear regression lines. (P < 0.0001 for correlation between T right slope and potassium). Panel B) shows subtle changes in potassium concentration correlates with changes in T Amp. Individual patient values and individual patient regression lines for the T Amp are shown using processed T Amp slope values in each of the individual dialysis patients. Each color represents data from a single patient. The lines are the individual-patient, linear regression lines. (P = 0.0006 for correlation between T amplitude and potassium)
Principle component analysis and unsupervised optimal fuzzy clustering were used to define the magnitude of potassium change that resulted in automated segregation of ECG changes. With this approach changes in the ECG morphology were detected (clustered) when the potassium measure changed by as little as 0.2 mmol/L.
Discussion
In this feasibility study we demonstrate the proof of a novel concept: identifying changes in an individual’s potassium level by recording the body’s physiologic response those changes, rather than by extracting blood and subjecting it to chemical processes. The availability of chronically wearable and subcutaneously injectable ECG recording systems that transmit wirelessly and securely to the internet opens the door to ambulatory, non-obtrusive, frequent assessment of potassium as a clinical reality.12 A key aspect of this strategy is the use of personalization to acquire an individual’s physiologic template at a known potassium value, and detect changes from the personalized template to register potassium changes – a form of individualized medicine. A second important aspect was advanced signal processing and filtering to eliminate noise, ectopy, and other potential confounders. Moreover, by using principal component analysis we were able to compress the data to facilitate its use in mobile platforms, and through unsupervised optimal fuzzy clustering demonstrated that even very small changes in blood potassium levels (down to 0.2 mmol/L) result in algorithmically identifiable changes in the surface ECG. The ability to detect small changes is important – trends can be identified and interventions taken before life-threatening hyperkalemia or hypokalemia develops. With this technology integrated into a remote-monitoring system, patient alerts ranging from dietary and medication reminders to instruction to seek care could be generated automatically.
A large body of evidence supports the clinical significance of detecting abnormalities of blood potassium. First, mild hyperkalemia, usually under-detected due to an absence of signs and symptoms, increases the one-day odds of death.2 Second, following the RALES study, potassium–sparing diuretic use increased hospitalization for hyperkalemia by 3 to 5 fold and doubled hyperkalemia-related deaths.3 Third, in ambulatory patients with cardiovascular disease and chronic kidney disease (eGFR < 60 ml/min/1.73 m2), the occurrence of a single hyperkalemic event (potassium > 5.0 mEq/L) doubles all-cause mortality.13 As noted above, the risk for sudden death among hemodialysis patients triples during the 12 hours each week that hyperkalemia is most likely.4–6 After a single episode of hyperkalemia, 50% of patients will have a second episode within one year2. Hyperkalemia usually lacks signs and symptoms, a noninvasive means of identifying it is currently lacking.
We chose to study hemodialysis patients since: they have predictable potassium level changes that decrease during treatment and increase in-between dialysis runs, we could easily obtain several potassium levels for validation, and we could record the ECG at multiple potassium levels in a relatively short time span. Additionally, patients receiving dialysis may benefit most from integration of our refined ECG algorithm into remote monitoring systems. However, we anticipate this form of monitoring would have applicability in wide range of patients with cardiovascular and renal disease. For example, the ability to monitor potassium would facilitate life prolonging renin-angiotensin system antagonist therapies in heart failure patients at risk for hyperkalemia,14–18 in whom it is currently withheld.
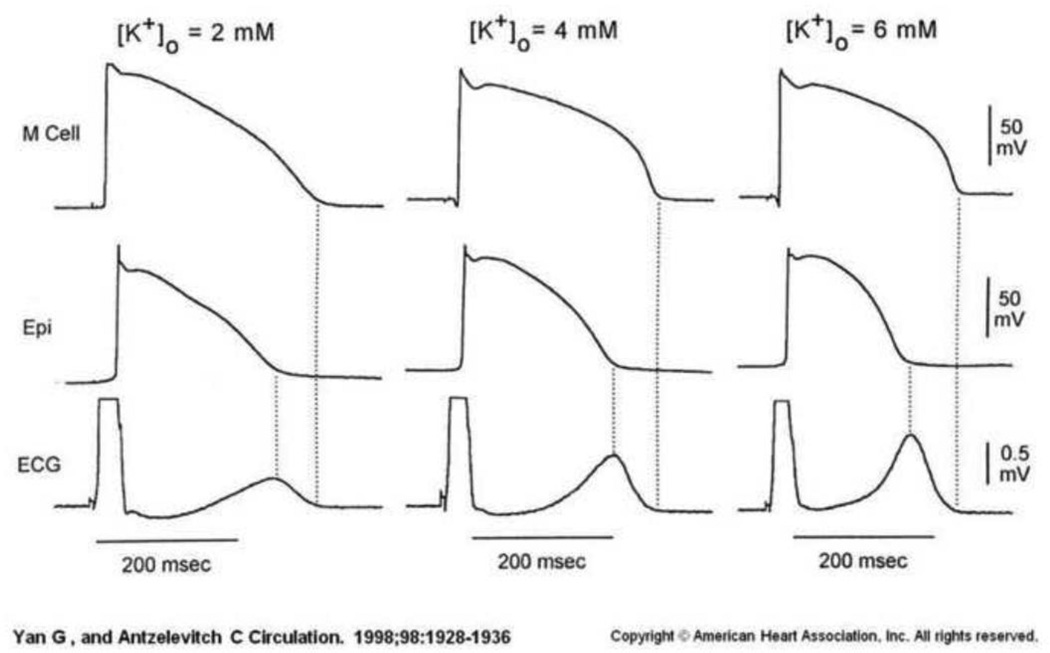
While we found that all ECG leads provide useful information for potassium prediction, we focused on V4 and a small number of ECG parameters for both physiological and practical purposes. In order to monitor potassium remotely, a small unobtrusive recording device—one with a limited number of recording channels-- is necessary to ensure adherence. The fact that findings were robust in multiple precordial leads (Table 3) suggests an insensitivity to lead position across the chest. The fact that V4 and surrounding leads had the best AIC score for location, and that T right slope did for feature, is consistent with the mechanisms by which potassium changes affect the surface ECG. As elegantly demonstrated by Antzelevitch et al in a wedge preparation,19 extracellular potassium differentially affects the action potential duration in mid-myocardial as compared to endocardial and epicardial myocytes (Figure 4). This is reflected predominantly on the surface ECG as the T wave. T wave characteristics are significantly affected by potassium flow through voltage gated ion channels, and these channels themselves are exquisitely sensitive to extracellular potassium concentrations. This makes T wave parameters ideal “micro-sensors” of potassium levels. The finding that the best performing ECG parameter was ‘T right slope’ supports the concept that we are detecting the sum of potassium changes at the cellular level, accounting for our unique fidelity in detecting subtle changes.
Figure 4. ECG signature of T wave changes are due to underlying cellular electrophysiologic changes in potassium channels in the presence of various potassium concentrations.
Reproduced with permission from Anzelevitch et al. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. Alterations in Action potential and T wave morphology are shown, and are dependent on potassium concentration. These signals are dependent on specific cardiac tissue layer; mid-myocardium (M cells) or epicardial layer. The resultant surface ECG recorded reflects a transmural repolarization change between the tissues.
Our results are best interpreted in the context of their limitations. During hemodialysis, the concentrations of analytes other than potassium (e.g. magnesium, calcium, phosphorus) change, as well as fluid status, change. We focused initially on potassium because of its importance to morbidity and mortality, and it is known impact on the ECG. Since other analytes exert known and different changes in the ECG, it may be possible to process recordings to determine which ECG changes are attributable to which analyte, recognizing that some overlap will exist and that sophisticated analysis will be required. In this preliminary study, we have only compared potassium at three time point’s peri-dialysis, and as such, our results may not be applicable to other circumstances. We are performing animal studies to permit modulation of single analytes to better define these issues. Additionally, this was an exploratory feasibility study involving a small number of patients, and will thus require confirmation in a larger cohort of patients. Further validation of these algorithms in a larger cohort could provide the ability to make this a wearable technology that can be adapted to any recording device, implantable recorder, or any web-based platform in an automated, user-friendly fashion.
In conclusion, we found that small changes in blood potassium concentrations, in or near the normal potassium range, result in quantifiable and measurable changes in the processed, signal-averaged ECG. Additionally, the processing can be performed in a computationally efficient manner to permit a mobile, non-obtrusive monitoring system to be developed. These findings will support the development of “bloodless blood-tests,” that is, acquiring biological information that in the past has required access to blood, with potentially large implications for patients with cardiovascular and renal diseases.
Highlights.
Serum potassium can be reliably estimated using mathematical algorithms for processing signal-averaged ECGs
Potassium changes as small as 0.2 mmol/L were detectable
Potassium could be measured within and near the normal range
Initial, individual correlation of potassium with the ECG is required
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Mayo Clinic has filed a patent application around this technology naming Charles Bruce, John Dillon, Kevin Bennet, Michael Ackerman, Paul Friedman, Sam Asirvatham, Virend Somers, Dan Sadot, Yehu Sapir and Amir Geva as inventors. This patent application has not been licensed and neither Mayo Clinic nor the inventors have received any financial benefits for the patent filing to date. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. American Journal of Cardiology. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Archives of Internal Medicine. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. New England Journal of Medicine. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 4.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney International. 2006;69:2268–2273. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 5.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney International. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 6.Perl J, Chan CT. Timing of sudden death relative to the hemodialysis procedure. Nature Clinical Practice Nephrology. 2006;2:668–669. doi: 10.1038/ncpneph0345. [DOI] [PubMed] [Google Scholar]
- 7.Dittrich KL, Walls RM. Hyperkalemia: ECG manifestations and clinical considerations. Journal of Emergency Medicine. 1986;4:449–455. doi: 10.1016/0736-4679(86)90174-5. [DOI] [PubMed] [Google Scholar]
- 8.Levine HD. Electrolyte imbalance and the electrocardiogram. Modern Concepts of Cardiovascular Disease. 1954;23:246–249. [PubMed] [Google Scholar]
- 9.Webster A, Brady W, Morris F. Recognising signs of danger: ECG changes resulting from an abnormal serum potassium concentration. Emergency Medicine Journal. 2002;19:74–77. doi: 10.1136/emj.19.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrenn KD, Slovis CM, Slovis BS. The ability of physicians to predict hyperkalemia from the ECG. Annals of Emergency Medicine. 1991;20:1229–1232. doi: 10.1016/s0196-0644(05)81476-3. [DOI] [PubMed] [Google Scholar]
- 11.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Second ed. New York: Springer; 2002. Information and Likelihood Theory: A Basis for Model Selection and Inference; pp. 49–96. [Google Scholar]
- 12.Vogt J, Bruce C, Somers V, et al. Role of physical activity in recurrence of atrial fibrillation: results from a novel, prolonged-use and wearable ECG patch. J Am Coll Cardiol. 2014;63:12_S. [Google Scholar]
- 13.Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg A, Weidmann P, Shaw S, Gnadinger M. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. The American journal of medicine. 1988;85:507–512. doi: 10.1016/s0002-9343(88)80086-x. [DOI] [PubMed] [Google Scholar]
- 15.Chapagain A, Ashman N. Hyperkalaemia in the age of aldosterone antagonism. QJM : monthly journal of the Association of Physicians. 2012;105:1049–1057. doi: 10.1093/qjmed/hcs106. [DOI] [PubMed] [Google Scholar]
- 16.Khanna A, White WB. The management of hyperkalemia in patients with cardiovascular disease. The American journal of medicine. 2009;122:215–221. doi: 10.1016/j.amjmed.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? Journal of the American Society of Nephrology : JASN. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 18.Weiner ID, Linas S, Wingo CS. Disorders of Potassium Metabolism. In: Johnson RFJ, Feehally J, editors. Comprehensive Clinical Nephrology. Philadelphia, PA: Saunders Elsevier; 2010. pp. 118–129. [Google Scholar]
- 19.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]