Abstract
Friend of a friend relationships, or the indirect connections between people, influence our health, well-being, financial success and reproductive output. As with humans, social behaviours in other animals often occur within a broad interconnected network of social ties. Yet studies of animal social behaviour tend to focus on associations between pairs of individuals. With the increase in popularity of social network analysis, researchers have started to look beyond the dyad to examine the role of indirect connections in animal societies. Here, I provide an overview of the new knowledge that has been uncovered by these studies. I focus on research that has addressed both the causes of social behaviours, i.e. the cognitive and genetic basis of indirect connections, as well as their consequences, i.e. the impact of indirect connections on social cohesion, information transfer, cultural practices and fitness. From these studies, it is apparent that indirect connections play an important role in animal behaviour, although future research is needed to clarify their contribution.
Keywords: cooperation, culture, fitness, heritability, indirect exchange, social brokers, social learning, social network analysis
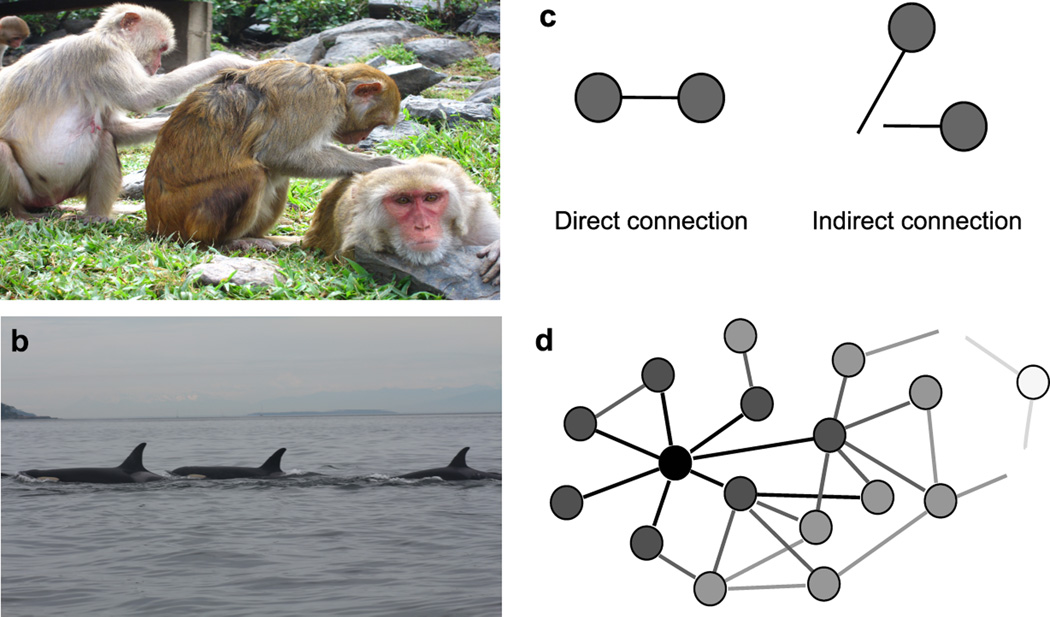
Sociality is a strategy most animals use to cope with their environments, allowing them to survive and reproduce in conditions that may not be conducive to survival and reproduction (Dunbar, 1988). To further our understanding of this essential facet of life, studies of animal behaviour have set out to determine the evolutionary forces that shape social behaviours and the proximate mechanisms that underlie their production (Mayr, 1961; Tinbergen, 1963). To date, studies have tended to focus on associations between pairs of animals: who interacts with whom and in what manner (Krause et al., 2010). However, social behaviour almost always occurs within a polyadic network of social ties (Madden et al., 2011) (Fig. 1a, b). Animals are not only connected to the individuals with whom they interact directly (direct connections), but are also tied indirectly to the partners of their social partners (indirect connections) (Croft et al., 2008; Krause et al., 2009; Sih et al., 2009; Wey & Blumstein, 2010) (Fig. 1c). Indirect connections can extend up to multiple degrees of separation (the partners of your partners’ partners’ partners’ partners) and can ultimately result in everyone in a population being connected to everyone else (Fig. 1d). In human parlance, we refer to these connections as friends of a friend (or enemies of an enemy) and these relationships have been shown to affect peoples’ health, well-being and financial success, including how happy a person feels (Fowler & Christakis, 2008), how much they weigh (Christakis & Fowler, 2007), as well as their ability to find a job (Pellizzari, 2010). Friend of a friend relationships in people have also been shown to be heritable (Fowler et al., 2009) and to influence fertility (Balbo & Barban, 2014). In humans at least, understanding the causes and consequences of sociality seems to in part depend on understanding indirect connections. We must therefore ask, are indirect connections important to other animals? And what information, if any, do researchers studying animal behaviour gain by extending their view beyond dyadic associations?
Figure 1.
Direct and indirect connections in animal social networks. Social interactions occur in a polyadic network of social ties in gregarious species such as (a) rhesus macaques, Macaca mulatta, and (b) killer whales, Orcinus orca. Indirect connections can emerge from a number of different types of association, including (a) grooming and (b) nearest-neighbour proximity. In both (a) and (b), the individual on the far left is indirectly connected to the individual on the far right via their mutual direct connections to the individual in the middle. Direct and indirect connections can be represented graphically in social networks, whereby nodes are connected via lines representing associations. Here, grey nodes represent individuals that are connected to each other directly or indirectly (c). Together, direct and indirect connections can result in every actor being connected to every other actor in a population. In the cartoon network (d), the black node is connected to all other nodes, with node darkness decreasing as social distance to the black node increases. Photos: L. J. N. Brent.
Here, I aim to demonstrate that there is mounting evidence that indirect connections are important to our understanding of animal behaviour. Social network analysis is the leading technique used to detect and quantify indirect connections. The rise in popularity of social network analysis in animal behaviour research (Brent, Lehmann, & Ramos-Fernández, 2011; Croft et al., 2008; Wey et al., 2008) has meant that the number of studies that have examined indirect connections has grown rapidly in recent years. I provide an overview of many of these studies, which I have organized into six broad sections intended to represent some of the major lines of research in which indirect connections have made, or have the potential to make, the greatest impact. These lines of research explore (1) the genetic basis of indirect connections, (2) the fitness consequences of indirect connections, (3) the association between indirect connections and social cohesion, (4) the impact of indirect connections on the transmission of information, (5) the maintenance of cooperation through indirect connections and (6) the cognitive basis of indirect connections. In each section, I attempt to highlight studies that have uncovered new and important information that would not have been revealed had the focus been solely at the level of dyadic associations. I conclude by summarizing of some of the major outstanding questions in the hopes of directing future research. I begin, however, by reviewing the different ways individuals can be indirectly connected and how those differences can be measured using social network analysis.
MEASURING INDIRECT CONNECTIONS USING SOCIAL NETWORK ANALYSIS
Social network analysis is a powerful analytical tool that allows researchers to investigate the complex webs of interconnections that exist between individual members of populations. One of the principal advantages of social network analysis is that it provides an array of measures of individual sociality, often referred to as network position or centrality, which represent the extent to which an individual is connected to others (Borgatti et al., 2009; Wasserman & Faust, 1994). This includes both direct and indirect connections and thus allows researchers to explore both types of association simultaneously.
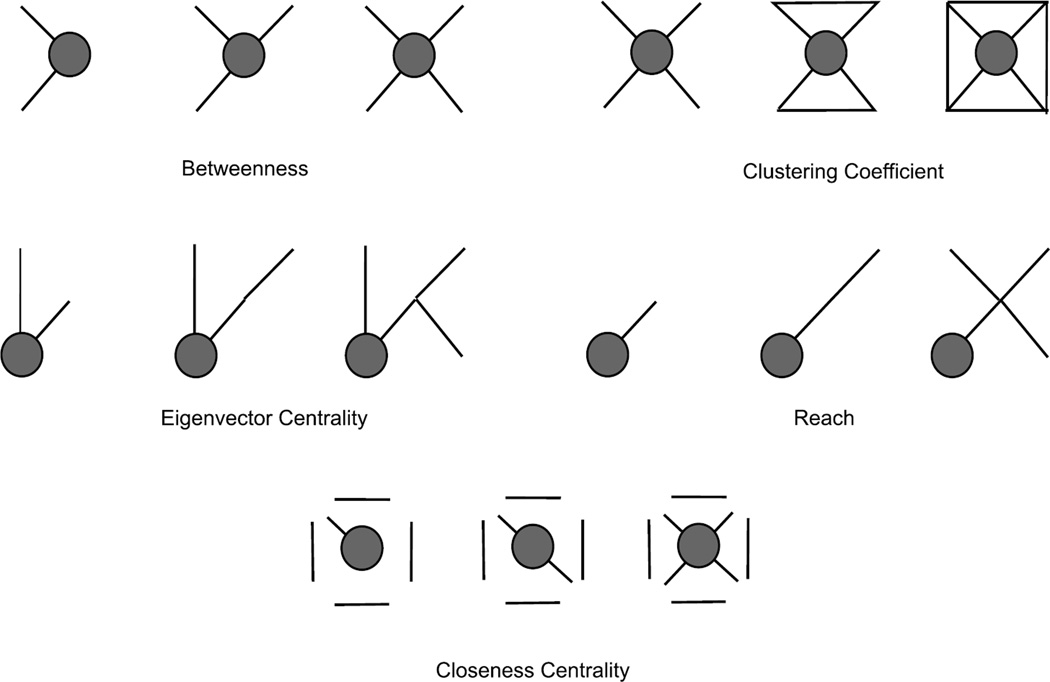
In social network analysis there are two main direct measures of centrality, called degree and strength, respectively. These measures are equivalent to those traditionally used in animal behaviour research, whereby centrality is quantified using either an individual’s number of partners (degree) and/or the amount of time they spend associating with others (strength) (Brent, Lehmann, et al., 2011; Wey et al., 2008). In addition, there are a number of social network-based measures that reflect indirect connections between individuals by taking into account both an actor’s centrality, as well as her contribution to the centrality of the others (Madden et al., 2011). In Table 1, I describe in detail the indirect measures of centrality most commonly used in animal behaviour research, which include reach, clustering coefficient, betweenness, eigenvector centrality, closeness and information centrality. Reach, for example, represents the number of degrees of separation (k) between individuals (Milgram, 1967). Individuals with high reach are connected to a large number of others who are k degrees of separation away (Fig. 2). Reach is important because it can detect behavioural contagion (Flack et al., 2006): individual A can direct aggression towards individual B, which can induce B to direct aggression towards C. Thus individual A directly impacts upon the social life of individual C, despite the fact they do not interact directly. Clustering coefficient, on the other hand, reflects the extent to which an individual’s local social network is interconnected, i.e. whether or not an individual’s social partners are partners with each other (Newman, 2003), and can be important for fission–fusion dynamics and collective foraging (Fig. 2). For example, individual A can only forage next to individuals B and C if B and C also have a relationship of mutual tolerance. Betweenness is another measure that captures the interconnectedness of subgroups. However, unlike clustering coefficient, individuals with high betweenness tend to interact with individuals who do not interact with one another (Freeman, 1977). By connecting disparate parts of the network, betweenness can be important for maintaining group cohesion, as well as influence the transfer of information, disease and resources between group members (Freeman, 1977). Measures of centrality can be based on associations that are directionless (there is no giver or receiver) and that are coded in a binary fashion (yes = an association occurred, no = no association occurred), but information on the frequency of interaction between individuals, as well as their direction (i.e. whether the actor has given or received an interaction), can often be incorporated (Opsahl, 2009; Whitehead, 2008). More comprehensive lists of these measures, along with the algorithms used to calculate them, can be found in a number of methods-based books and papers (Borgatti et al., 2002; Croft et al., 2008; Freeman, 1977; Wasserman & Faust, 1994).
Table 1.
Social network-based measures of indirect connectedness most commonly used in animal behaviour research
| Indirect measure | Definition |
|---|---|
| Betweenness | The total number of shortest paths (routes of connections that can be followed on a graph from one actor to another) that pass through an individual linking other members of the social group to each other. Individuals with high betweenness tend to connect what would otherwise be unconnected parts of a network. High betweenness also represents individuals that have a large influence on the transfer of items through a network, based on the assumption that items follow the shortest paths (Freeman, 1977). These ‘items’ can include anything from information and disease, to cultural practices and money. As such, individuals with high betweenness are typically characterized as the ‘brokers’ of a network (Newman, 2003; Wasserman and Faust, 1994). |
| Clustering coefficient | Represents the nature of an individual’s local social network by measuring the proportion of an individual’s social partners who are partners with each other (Newman, 2003). Clustering coefficient is therefore a local measure of cliquishness or subgrouping. Clustering coefficient values range from zero to one, with zero indicating that none of an individual’s social partners are partners with each other, and one indicating that all of an individual’s social partners are also partners with one another. |
| Eigenvector centrality | A measure of both the number and quality of a subject’s social partners. Individuals with high eigenvector centrality have a large number of partners, who themselves have a large number of partners (Wasserman and Faust, 1994). This is based on the concept that connections to highly connected individuals contribute more to the score of the individual in question than do connections to poorly connected individuals, which is important in a variety of nonbiological contexts, such as the algorithm Google uses to rank webpages. |
| Reach | Represents the extent to which individuals are connected based on their degrees of separation from others. It is calculated by counting the number of nodes that each node can reach in k or less steps. For k = 1, reach is equivalent to degree centrality. Reach is perhaps most famously known from Stanley Milgram’s ‘six-degrees of separation’ study in which he demonstrated that a piece of mail was delivered to its specified recipient after passing through six individuals, only the latter of which was acquainted with the recipient (Milgram, 1967). |
| Farness centrality | The farness of an individual is defined as the sum of the shortest path lengths to all other nodes. |
| Closeness centrality | The inverse of farness centrality. Closeness can be interpreted to represent the amount of time it would take to spread an item (e.g. information) from one individual to all others (Sabidussi, 1966). |
| Information centrality | Evaluates how often an individual lies along a path between other individuals. Information centrality is similar to betweenness, but instead of following only the shortest paths, it also uses more circuitous paths (Newman, 2003; Stephenson and Zelen, 1989; Wasserman and Faust, 1994). |
Figure 2.
Toy networks representing some of the most commonly used individual-based measures of indirect connectedness. Focal individuals are grey nodes. In each case, scores increase from left to right: the grey node in the right-most network has the highest score for a given measure. These are examples of measures based on unweighted and undirected associations, although weights and directions can be applied to most measures. Descriptive definitions of each measure are given in Table 1.
Although there is some overlap between network-based measures of centrality, it is crucial to note that each measure captures a distinct aspect of the social environment. Individuals with a high score for one measure do not necessarily have a high score for another (Brent, Lehmann, et al., 2011; Sueur et al., 2011). This includes direct and indirect measures; individuals that are highly directly connected to others are not necessarily highly indirectly connected. For example, two individuals that have the same degree (i.e. the same number of social ties) do not necessarily have the same clustering coefficient; one individual may be partners with n individuals who are not partners with each other (low clustering coefficient), while another may be partners with n individuals who are also partners with each other (high clustering coefficient). Individuals with the same degree may also differ in their betweenness; one individual may be partners with n individuals who are members of the same subgroup (low betweenness), while another may be partners with n individuals who are members of different subgroups (high betweenness). The network risks splitting apart if you remove the latter individual but not if you remove the former.
Beyond theoretical examples, evidence from field-based studies demonstrates that an individual’s centrality depends on the measure used to describe it. In the association networks of sperm whales, Physeter macrocephalus, centrality not only varies between individuals but differences also exist within individuals (Lusseau et al., 2008). In Lusseau et al.’s study, whale 5703 had the highest scores for strength and eigenvector centrality of all seven group members, but had the lowest clustering coefficient (Lusseau et al., 2008). In a study of captive chimpanzees, Pan troglodytes, the individuals that were deemed the ‘most social’ because they had the greatest number of grooming partners (degree) were not the same individuals as those with the highest clustering coefficient or highest betweenness (apart from one chimpanzee who had the second-highest betweenness score and was tied for highest degree) (Kanngiesser et al., 2011). Similarly, in three groups of Gunnison's prairie dogs, Cynomys gunnisoni, the most directly connected individuals were not the most indirectly connected; in group ‘CCI’, individuals 30, 31 and 32 had the highest degree, while individuals 17, 25, 27 and 30 had the highest betweenness; and in group ‘HIS’, individual 11 had the highest degree, while individual 16 the highest betweenness (Verdolin et al., 2014).
There are therefore considerable, quantifiable, differences in the extent to which individuals are directly and indirectly connected. Individuals with strong direct connections but weak indirect connections might be able to influence their immediate social partners but have little influence on the rest of the population. In contrast, individuals with weak direct connections but strong indirect connections may be the single tie linking otherwise unconnected sections of the network and may thus be able to exert considerable influence over the population. To fully understand the role of indirect connections in animal societies, we must investigate the causes of the differences between individuals and document their consequences. Crucially, this includes exploring whether indirect connections are the product of natural selection by, for example, examining whether differences between individuals have a genetic basis (Brent & Melin, 2013; Chang et al., 2013; Fisher, 1930). In the next section, I review the growing body of evidence that suggests that differences in indirect connectedness are heritable.
ARE INDIRECT CONNECTIONS HERITABLE?
For social behaviours to evolve, they must have a genetic basis on which selection may act (Brent, Heilbronner, et al., 2013; Lea et al., 2010). Because indirect connections are partly dependent upon the interactions of pairs of third parties, actors can exert less control over them and, as a result, they may be less likely to be influenced by the actor’s genes (Lea et al., 2010). In other words, if indirect connections are mostly under the control of the social and physical environment, then an individual’s tendency to be indirectly connected should not be heritable. Indeed, in a study of yellow-bellied marmots, Marmota flaviventris, the number of partners from which an actor received interactions directly (‘in-degree’) was heritable in both affiliative and agonistic networks (affiliation in-degree h2=0.11; agonistic in-degree h2=0.11), as was a second measure of direct agonistic connections (‘attractiveness’ h2=0.18), but none of the evaluated measures of indirect connectedness (betweenness and eigenvector centrality) were heritable (Lea et al., 2010) (Fig. 3a).
Figure 3.
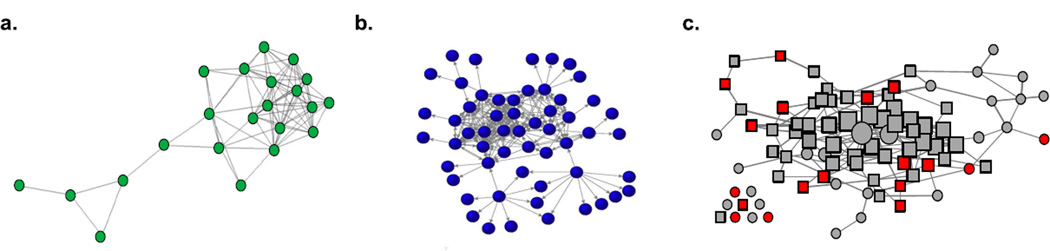
The genetic basis of indirect connections. The extent to which individuals are indirectly connected to others via affiliative interactions is not heritable in (a) yellow-bellied marmots (data from Brent, Heilbronner, et al., 2013), but is heritable in (b) humans (reprinted with permission from Fowler et al., 2009) and (c) rhesus macaques (data from Lea et al., 2010). Networks are based on grooming, play, co-foraging, greetings, spatial proximity and inspections in marmots, named friendships in humans, and grooming in rhesus macaques. Nodes represent individuals; lines represent interactions between pairs of individuals. In the rhesus macaque network (c), circles denote adult males, squares denote adult females, and red symbols denote individuals with the rare allele for two gene variants in the serotonin pathway and low eigenvector centrality.
Yet just as the social systems of species vary widely, so too may the importance of third-party relationships (Cheney, 2011). In some species, indirect connections might be more than a simple emergent property of the social network and may instead reflect a meaningful aspect of the way individuals navigate their social environment (Brent, Heilbronner, et al., 2013). For example, indirect connections may be especially important to the many species of large mammal and some birds that form highly differentiated relationships (Brent et al., 2014), recognize bonds between pairs of third parties (Cheney, 2011; Massen, Pašukonis, et al., 2014), and are sensitive to the perspectives of others (Chang et al., 2013; Cheney, 2011). In these species, individual differences in indirect connectedness may be under genetic control. To my knowledge, only two studies to date have examined the heritability of indirect connections in species for which third-party relationships are suspected to be important (Fig. 3). In a study of human friendship, the number of times a subject was named as the friend of others was heritable (in-degree h2=0.46), as were two measures of indirect connectedness, the proportion of an individual’s friends who were friends with one another (clustering coefficient h2=0.47), and the extent to which an individual connected pairs of individuals who were not friends with each other (betweenness h2=0.29) (Fowler et al., 2009). In a population of free-ranging rhesus macaques, Macaca mulatta, direct, but not indirect, measures of connectedness were heritable in the aggression network (Brent, Heilbronner, et al., 2013). However, a different pattern was found in the two affiliative networks examined (time spent grooming and time spent in proximity). Although direct measures of connectedness demonstrated nonzero heritability in the affiliation networks, betweenness and eigenvector had higher and significant heritability (Brent, Heilbronner, et al., 2013). Preliminary findings from this study also pinpoint a specific gene pathway that may underlie the expression of individual differences in indirect connectedness; rhesus macaques with low eigenvector centrality in the grooming network were more likely than other individuals to have rare alleles for two gene variants in the serotonergic pathway (Fig. 3c). That is, monkeys that were weakly indirectly connected were more likely to have gene variants associated with poor serotonergic signalling (Brent, Heilbronner, et al., 2013). No such association was found between genetic variation and measures of direct connectedness. Serotonin is associated with the regulation of mood, memory and reward (Chang et al., 2013) and is a key candidate for future studies of the genetic basis of indirect connections. Taken together, the results of these studies suggest that the social temperaments and skills that shape indirect connections may be partly genetically determined in some gregarious species but perhaps not others, although studies in a broader range of species are required to confirm this idea.
DO INDIRECT CONNECTIONS HAVE FITNESS CONSEQUENCES?
If social behaviours are favoured by selection then they should be associated with proximate measures of fitness, such as increased survival and reproductive output. In the first seminal paper to demonstrate such an association, Silk et al. (2003) showed female savannah baboons, Papio cynocephalus, that spend a greater amount of time grooming and in proximity to other females have offspring that are more likely to survive to 1 year of age. Since then, similar relationships between affiliative interactions or relationships and fitness proxies have been found in chacma baboons, Papio ursinus (Silk et al., 2009; Silk et al., 2010), rhesus macaques (Brent, Heilbronner, et al., 2013), Assamese macaques, Macaca assamensis (Schülke et al., 2010), bottlenose dolphins (Tursiops sp.) (Frere et al., 2010), feral horses, Equus caballus (Cameron et al., 2009), and yellow-bellied marmots (Lea et al., 2010). Fitness consequences have also been demonstrated for agonistic interactions in some species (Brent, Heilbronner, et al., 2013; Lea et al., 2010).
These studies have greatly advanced our understanding of the evolution of sociality based on direct connections. The fitness consequences of indirect connections, on the other hand, are less well understood. Indirect connections may reflect the social strategies of individuals; adaptive social ties may not only depend on the relationships between individuals and their immediate social partners, but also on the indirect connections between an individual and their partners’ social partners (Cheney, 2007). Indirect connections are also tightly linked to processes that can directly influence an individual’s fitness, such as the rate at which they are exposed to pathogens (Hamede et al., 2009; Weber et al., 2013), as well as their ability to obtain vital information (Bode et al., 2011; Voelkl & Noë, 2010; also see Obtaining Information Indirectly). To fully understand the adaptive value of sociality in animals, it may therefore be important to examine the relationship between indirect connections and fitness.
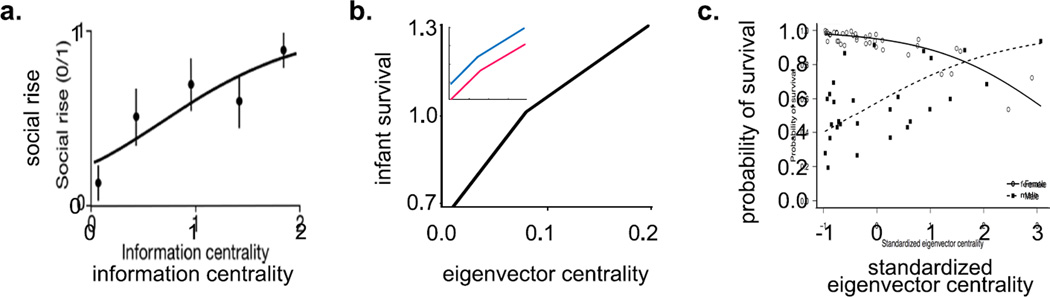
Some of the first evidence for a relationship between indirect connections and fitness comes from a study of long-tailed manakins, Chiroxiphia linearis. Manakins live in lek-mating systems where male birds perform coordinated displays with other males in order to attract mates. Despite these coordinated displays, reproductive skew is high; only alpha males tend to copulate with females and, as such, social status is a good predictor of reproductive success in this species. McDonald (2007) explored the social factors associated with a rise in social status in male manakins. He examined seven measures of centrality in the coordinate display network, including one direct measure (degree) and six indirect measures (information centrality, eigenvector centrality, power, closeness, distance weighted reach and betweenness). McDonald found that the best predictor of future dominance rank was information centrality (Fig. 4), which is similar to betweenness because it represents how often an individual lies along a path between other individuals (Stephenson & Zelen, 1989). Distance-weighted reach, which represents the weighted sum of all path lengths from an actor to all other nodes (Newman, 2003; Wasserman & Faust, 1994) was the only other significant predictor of social rise. In other words, indirect measures of connectedness predict social rise in male manakins, whereas direct measures do not (McDonald, 2007). However, in two studies of a closely related species, the wire-tailed manakin, Pipra filicauda, where similar measures of direct and indirect connectedness in the cooperative display network were explored, degree was the strongest predictor of social rise and the number of offspring sired (Ryder et al., 2008; Ryder et al., 2009).
Figure 4.
Fitness consequences of indirect connections. Indirect connections were significant predictors of the probability of (a) an increase in rank in manakins (data from Brent, Heilbronner, et al., 2013), (b) infant survival in rhesus macaques (data from McDonald, 2007) and (c) survival in juvenile male bottlenose dolphins (reprinted with permission from Stanton & Mann, 2013). In (b), the inset graph shows infant survival for males (dashed line) and females (solid line).
Indirect connections were also linked to fitness in wild male chimpanzees (Gilby et al., 2013). Like many animals, chimpanzees form coalitionary alliances, whereby pairs of actors jointly direct aggression towards third parties (Harcourt & de Waal, 1992). Although the extent to which an individual’s access to coalitionary support influences its fitness has long been hypothesized (Seyfarth, 1977; Silk, 2007), little empirical evidence exists to support this idea. To address this question, Gilby et al. (2013) examined the relationship between male chimpanzees’ position in the coalitionary network and two measures of reproductive success: whether a male sired an offspring during the current study period, and whether a male was higher ranking in the study period that immediately followed the period in question. As with manakins, alpha male chimpanzees sire the majority of offspring (Wroblewski et al., 2009), and a rise in social status is a good predictor of future reproductive success. Gilby et al. used three measures of network centrality: degree, betweenness and eigenvector centrality. Male chimpanzees with the highest betweenness were more likely to increase in rank and to sire offspring. Degree, on the other hand, was positively associated with both measures of reproductive success, but not significantly so.
In addition to coalitionary alliances and mating displays, other types of association, such as grooming and spatial association, require dyadic coordination and are likely to be under the influence of network structure. Indeed, there is mounting evidence that indirect connections for these types of associations may also have fitness consequences. In adult male and female rhesus macaques, both the amount of grooming individuals received from others (grooming in-strength) as well as the amount of time they spent in proximity to others (proximity strength) were significant linear predictors of reproductive success, as measured by the relative proportion of infants that survived to 1 year of age (Brent, Heilbronner, et al., 2013). But the best predictor of infant survival in this study was an individual’s eigenvector centrality in the proximity network (selection differential of 3.64 compared to 1.18 for proximity strength and 0.002 for grooming in-strength) (Fig. 4b). In other words, while it is beneficial for rhesus macaques to spend a lot of time grooming and in proximity to others, the best strategy is to spend a lot of time near group mates who themselves spend a lot of time near others. This study revealed similar findings for aggressive interactions. Controlling for dominance rank and the sex of the actor, the amount of aggression rhesus macaques gave to others (out-strength) was significantly related to infant survival in a quadratic fashion, but the strongest relationship was between the quadratic of infant survival and eigenvector centrality. Therefore, there are two routes to success in rhesus macaques when it comes to aggressive interactions: rhesus macaques who engage in high rates of aggression with partners who themselves engage in high rates of aggression have increased infant survival, but so too do passive individuals who engage in low rates of aggression with partners who themselves engage in low rates of aggression (Brent, Heilbronner, et al., 2013).
In juvenile bottlenose dolphins, Tursiops truncatus, Stanton and Mann (2012) investigated the relationship between an individual’s survival to age 10 and their centrality in the spatial association network, as measured by degree, strength, weighted betweenness, eigenvector centrality and clustering coefficient. The model that best predicted survival included eigenvector centrality, strength and sex. Although the relationship between centrality and survival was not significant for either strength or eigenvector centrality on their own, the interaction between eigenvector centrality and sex was a significant predictor of survival. Specifically, eigenvector centrality was a positive linear predictor of survival for juvenile males, but not females (Fig. 4c). In other words, the tendency for males to associate with individuals who themselves associate with many others are more likely to live to age 10 (Stanton & Mann, 2012). Juvenile male dolphins receive a disproportionate amount of harassment compared to females from older juvenile dolphins (Stanton & Mann, 2012). Juvenile males that are more socially connected might therefore be less frequent targets of harassment, which may help them to survive.
Yet despite growing evidence for a relationship between individual differences in indirect connectedness and fitness, the direction of causality underlying this relationship and precisely how it comes about remains unclear. In the chimpanzee example, one possible explanation for betweenness’ relationship with fitness is that instead of forming coalitions with everyone, male chimpanzees with high betweenness may form the ‘right’ coalitions (Cheney, 2007; Gilby et al., 2013; Noë, 1992). This political power could afford males with increased influence over others (de Waal, 1982), including the ability of others to attain high dominance rank, which could in turn lead to increased reproductive success for the male in question. However, this explanation implicitly suggests that males are cognizant of who forms coalitions with whom and that they have information regarding their own network position. Although there is some evidence to suggest that some species have this ability (see Thinking Beyond the Dyad: Indirect Connections and Cognition), simpler explanations (e.g. betweenness arises as an emergent property of the network and its association to fitness is a by-product of its association to other traits with direct ties to reproductive success; Gilby et al., 2013) must also be ruled out. In addition to continuing to explore the relationship between indirect connections and fitness in a broad range of species, future research should therefore also focus on unraveling the mechanisms that underlie this relationship.
FRIENDS HELP FRIENDS OF FRIENDS STICK TOGETHER
Whether to avoid predators or facilitate the acquisition of resources, living in groups is one of the main ways that gregarious animals cope with challenges in their environment (Krause & Ruxton, 2002). Maintaining cohesion amongst members of a social group is therefore critical to the success of the individuals that live within those groups (Krause & Ruxton, 2002). As such, understanding the principles and consequences of social cohesion, how it is maintained and what happens when it is not, is crucial to the understanding of the evolution of sociality. Since the cohesion of groups depends not only on the relationships between pairs of individuals but also on the connections between all group members (Sih et al., 2009), significant advances might come about via the inclusion of measures of indirect connections in studies of social cohesion.
The measure of indirect connectedness that has received the most attention to date in studies of social cohesion is betweenness. Individuals with high betweenness are often referred to as ‘brokers’ because they connect otherwise isolated clusters of a network (Freeman, 1979). When brokers are removed, networks can become fragmented and even split apart. Network fragmentation following the removal of individuals with high betweenness has been demonstrated in rodents (Manno, 2008) and primates (Kanngiesser et al., 2011; Lehmann et al., 2010; Lehmann & Dunbar, 2009). For example, in a study of 11 species of Old World monkeys, Lehmann et al. (2010) found that simulated (i.e. statistical) removal of individuals with the highest betweenness resulted in grooming networks that were significantly less well connected. In contrast, Lusseau and Newman (2004) found that simulated removal of individuals with high betweenness did not destroy connectivity in the association network of bottlenose dolphins. Indeed, the largest component of the dolphin network shrunk only slightly faster than it would if randomly selected individuals were removed (Lusseau & Newman, 2004). Together, the results of these studies suggest that indirect connections are important to the robustness of the networks of some species but not others.
However, most studies that have explored the impact of indirect connections on social cohesion have only examined the consequences of removing individuals with high betweenness and have not simultaneously investigated the removal of individuals with high scores for other measures of centrality, including direct measures. One exception is the study of dolphins by Lusseau and Newman (2004), where the authors obtained qualitatively similar results when either individuals with high betweenness or high degree centrality were removed. Differences in the outcome of the removal of individuals with strong direct or indirect ties may be isolated to networks characterized by specific properties. For instance, in networks with a relatively even distribution of ties and with few cliques or clusters, betweenness is more normally distributed amongst individuals and more tightly correlated with degree. In these cases, of which the dolphin association network is a good example, the removal of individuals with high betweenness will be roughly equivalent to the removal of individuals with high degree. In contrast, networks that are more highly clustered have betweenness scores that are more highly skewed across individuals and less related to degree. In these cases, as in the grooming networks of Old World monkeys investigated by Lehmann et al. (2010), the removal of individuals with high betweenness compared to the removal of individuals with high degree is likely to have a greater impact on network cohesion. Nevertheless, although theoretically demonstrable, the role of indirect connections in the cohesion of social networks with different structural properties has yet to be shown via rigorous empirical means.
Although social cohesion is important, dispersal is often necessary in order to reduce competition and maintain genetic diversity within populations (Krause & Ruxton, 2002). Dispersal can be a dangerous undertaking, with an increased risk of injury and death occurring in many species (Bonte et al., 2012). Thus, decisions about when and where to disperse can be critical. Whether individuals succeed in dispersing can be influenced by network connections. In a study of network centrality in Gunnison’s prairie dogs, Verdolin et al. (2014) found that males tended to have high degree, while females tended to have high betweenness. Because females tend to disperse within populations in this species, connections to members of other cliques (i.e. high betweenness) may facilitate dispersal (Verdolin et al., 2014). Males, on the other hand, which tend to disperse between populations, may be better off adopting a strategy that capitalizes on relationships in their immediate social environment (i.e. high degree). Overall, the difference in dispersal strategies between male and female prairie dogs may explain (or be explained by) differences in the extent to which the sexes are directly and indirectly connected. These differences would not have been revealed had the authors restricted their analyses to direct connections alone. Social cohesion and dispersal not only have consequences for conservation strategies and population management, but can also influence animals’ ability to communicate. The transfer of information between members of a population is therefore also likely to be influenced by indirect connections.
OBTAINING INFORMATION INDIRECTLY
Many gregarious animals rely on information to effectively use their habitat, find resources, avoid predators, and make decisions about when and where to move as a group (Conradt & Roper, 2003; Couzin, 2009). A clearer understanding of the processes and consequences of collective knowledge and collective cognition will therefore greatly advance our understanding of one of the main ways animals cope with their environments (Couzin et al., 2005). Here, too, indirect connections may be crucial because information makes its way across networks through a series of paths, using not only direct but also indirect connections between individuals (Bode et al., 2011). In humans, the importance of indirect connections to the flow of information is well documented (Bond et al., 2012; Milgram, 1967). In nonhuman animals, only a few studies have traced the spread of information through social networks (Bode et al., 2011; Cantor & Whitehead, 2013) and still fewer of these have investigated the importance of indirect connections. One such study examined the breeding songs of humpback whales, Megaptera novaeangliae. Humpback songs are socially learned; males sing the same song as other members of their population, which differs from the songs of other populations (Cantor & Whitehead, 2013). Songs change over time, with all individuals in a population maintaining the changes. In the South Pacific, humpback songs were shown to develop in a geographically sequential manner; songs heard in the westernmost breeding ground in one year were heard in the adjacent breeding ground the following year and in the breeding ground 2000 km to the east 2 years later (Noad et al., 2000). Introduction of the new song was suggested to be the result of a small number of migrants travelling east (Noad et al., 2000). Although the identities of the potential migrants was not known, males moving from population A may have introduced the new song to population B. Having learnt the new song, males from population B could have transported it to population C. If we consider populations as the actors in this scenario, then population B can be designated as the broker between populations A and C. That is, population A influences the songs of population C indirectly, through mutual connections of both A and C to B.
The role of indirect connections in the spread of cultural practices has been demonstrated in other cetaceans. In the bottlenose dolphins of Shark Bay, Australia, some individuals use sponges as hunting tools while others do not. Mann et al. (2012) found that sponge use is related to social associations. Compared to females that do not use sponges, sponge-using females preferentially associate and form more cliquish subgroups, i.e. they tend to associate with females who are themselves associates (Mann et al., 2012). This finding suggests that the transmission of cultural practices may be determined by (and/or may determine) the indirect connections between individuals. Comparable results were found in a study of another population of bottlenose dolphins where individuals differ in their tendency to engage in two potentially culturally transmitted behaviours, side flops and upside-down lob tails (Lusseau, 2006). Exploring the social networks of these dolphins, Lusseau (2006) found that individuals that performed these behaviours did not differ in their number of social associates (degree) from individuals that did not perform these behaviours, but they did have significantly greater betweenness. Combined with the finding that degree and betweenness were only weakly correlated (Lusseau, 2006), these results suggest that betweenness, but not degree, plays a role in the spread of cultural practices. In other words, whether or not an actor is exposed to and adopts a cultural practice does not depend on how many social partners it has but may depend on how its social partners are themselves connected.
Compared to studies based on observational data alone, the diffusion of information within social groups can be investigated more rigorously in experimental settings. For instance, single individuals can be given information on how to solve a novel foraging task and the transmission of that information throughout the population can be documented. Network-based diffusion analysis (Hoppitt et al., 2010; Nightingale et al., 2015) can be used to examine whether the probability that a naïve individual will learn from a skilled individual is partly determined by its social relationship as defined either by direct (e.g. Allen et al., 2013; Kendal et al., 2010) or indirect connections. In one study of three tit species (Paridae), researchers explored the social transmission of information via indirect connections on the location of a new feeder (Aplin et al., 2012). To do this, the authors constructed a social network based on whether or not pairs of birds visited the same feeder within 30 s of one another. The authors found that whether or not individuals discovered a new feeder was best predicted by their betweenness centrality (Aplin et al., 2012). Birds were more likely to discover a new feeder if they tended to associate with individuals that were from disparate parts of the social network. This is an important discovery since the spread of novel information throughout a group has been hypothesized to be related to the betweenness of the individual with whom the information arises (Brent, 2010; Freeman, 1979). Note, however, that this study examined the role of indirect but not direct connectedness (Aplin et al., 2012) and thus the relative contribution of one type of connection compared to other is not known.
Finally, studies of fission–fusion dynamics and the decisions animals make when moving collectively are also likely to be influenced by indirect connections (Couzin, 2006; Croft et al., 2004). However, most models of collective movement published to date generally do not consider network structure or indirect connections (Bode et al., 2011), although there are some exceptions (e.g. Croft et al., 2004; King et al., 2011; Sueur & Petit, 2008). For example, individual chacma baboons with the highest eigenvector centrality in their grooming network are more likely to lead group movement (King et al., 2011). Yet, as with studies of the transmission of information, studies of collective motion in a social network context have tended not to examine both measures of direct and indirect connections simultaneously. It is therefore currently difficult to draw definitive conclusions regarding the role of indirect connections in collective knowledge and collective cognition, and future research should explore this relationship in detail.
INDIRECT COOPERATION: YOU SCRATCH MY BACK, I’LL SCRATCH SOMEONE ELSE’S
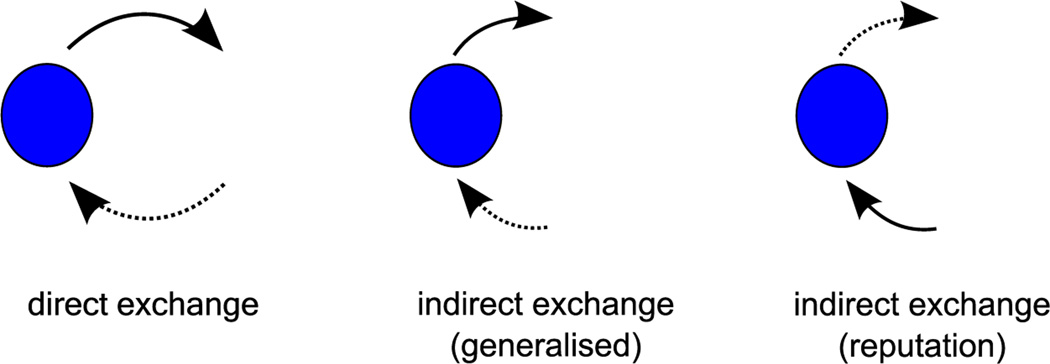
Cooperation among nonrelatives is widespread in humans and other animals. Unlike cooperation between relatives that can be explained by kin selection (Hamilton, 1964), how cooperation is maintained between nonkin is a long-standing evolutionary puzzle. One main hypothesis is that cooperation is based on direct exchange (Fig. 5), whereby individuals give services to nonkin from whom they have received services (Trivers, 1971). Although many studies present evidence in support of direct exchange (Raihani & Bshary, 2011), pairs of potential partners do not exist in a social vacuum and the distribution of services may also be determined by information contained in the broader network of the group (Mohtashemi & Mui, 2003). For example, individuals may give to others based on interactions with third parties (i.e. based on indirect connections, rather than direct exchange; Alexander, 1987; Nowak & Sigmund, 2005). There are currently two main types of indirect exchange: generalized and reputation-based exchange. In generalized exchange (Fig. 5), individuals act positively towards others if they themselves have been the subject of positive interactions: individual A helps individual B, which results in B helping others (Nowak & Sigmund, 2005). In reputation-based exchange (Fig. 5), individuals act positively with the individuals who act positively with others: individual A helps individual B, which results in C helping A (Nowak & Sigmund, 2005).
Figure 5.
The maintenance of cooperation by direct and indirect interactions. In direct exchange, individuals cooperate with those from whom they have received cooperative acts. In generalized indirect exchange, individuals cooperate with others if they have previously received cooperative interactions from anyone: A (lower open dot) cooperates with B (solid dot), and then B cooperates with others (upper open dot). In reputation-based indirect exchange, individuals cooperate with the individuals who cooperate with others: individual A (solid dot) cooperates with B (upper open dot), which results in C (lower open dot) cooperating with A.
Indirect forms of exchange are proposed to have played an important role in the evolution of human cooperation (Nowak & Roch, 2007; Nowak & Sigmund, 1998), with both types having been demonstrated in empirical studies (e.g. generalized: Bartlett & DeSteno, 2006; Berkowitz & Daniels, 1964; reputation: Milinski et al., 2001; Semmann et al., 2004; Wedekind & Milinski, 2000). In contrast, only a handful of studies have examined indirect exchange in nonhumans. For generalized exchange, laboratory-housed rats are more likely to pull a lever to produce food for a partner if they have previously received food, irrespective of the identity of the donor (Rutte & Taborsky, 2007, 2008). In contrast, captive chimpanzees were not more likely to give food to their cage-mates after they received grooming from a third party (de Waal, 1997). For reputation-based exchange, nonhumans may lack some of the necessary skills, such as the ability to retain large amounts of information on the relationships of others and to use complex language to share information regarding the actions of others (Nowak, 2006; Panchanathan & Boyd, 2003). However, calculated bookkeeping and complex language are not absolute requirements, and individuals may be able to construct the reputations of others by emotional bookkeeping (Schino & Aureli, 2009) and visual observation (Brent, 2010; Kundey et al., 2011). Recent research suggests that simple examples of reputation formation exist in disparately related taxa. Domestic dogs, Canis familiaris, prefer to take treats from human experimenters they previously observed sharing a treat with another person compared to humans who were seen withholding treats (Kundey et al., 2011). Chimpanzees, but not bonobos, Pan paniscus, gorillas, Gorilla gorilla, or orang-utans, Pongo pygmaeus, sit significantly longer next to humans they observed being ‘nice’ to another human who was begging for food by giving them a grape compared to humans who did not give grapes to beggars (Russell et al., 2008; Subiaul et al., 2008). Male song sparrows, Melospiza melodia, retaliate against males that they believe intruded into a third male’s territory during playback experiments (Akçay et al., 2010). Finally, reef fish spend more time in proximity to cooperative cleaner fish (Labroides dimidiatus) than they do to cleaner fish whose tendency to cooperate they do not know (Bshary & Grutter, 2006). Cleaner fish are also less likely to provide a poor service (i.e. by feeding on their client’s mucus instead of their ectoparasites) when bystander client fish are present relative to when they are not being observed (although this is probably an example of indirect pseudoreciprocity (i.e. image scoring resulting in the choice of a cooperative partner for interactions that are mutually beneficial: Bshary & Grutter, 2006; Pinto et al., 2011). Taken together these results suggest members of some species may have the capacity to use indirect forms of exchange, although the extent to which indirect exchange shapes their cooperative interactions is unclear.
Although not framed in explicit network terms, it is easy to envisage the consequences of indirect exchange using network-based measures of indirect connectedness. Cooperative interactions originating from individuals with high reach, for example, could quickly cascade through a network via generalized exchange; upon receiving a service from an individual with high reach, the individual’s direct partners could, in turn, give a service to their own direct partners who could, in turn, give a service to their own direct partners, and so on. The use of network-based measures may also advance our understanding of how cooperative interactions are maintained in polyadic networks. For instance, individuals may prefer to give services to those who spend less time giving services to others (i.e. low out-strength), but who are highly indirectly connected. Such a situation would arise if the exchange of services was not only more likely among direct partners but also among friends of friends (Sih et al., 2009). Future research should examine how an individual’s level of direct and indirect connectedness affects partner choice in nonhuman animals.
THINKING BEYOND THE DYAD: INDIRECT CONNECTIONS AND COGNITION
For indirect connections to influence the lives of group-living animals, individuals may require information on the relationships between pairs of third parties. Although perhaps cognitively demanding, there is mounting evidence that some animals have some understanding of third-party relationships (Brent et al., 2014; Cheney, 2011). For example, many animals have been shown to use transitive inference, whereby known relationships between pairs of others are used to deduce unknown relationships (Grosenick et al., 2007). Male cichlid fish (Astatotilapia burtoni) infer the relative dominance status of pairs of males indirectly by using the males’ relationships with others (Grosenick et al., 2007). That is, by observing agonistic interactions indicating that A is dominant to B, and that B is dominant to C, cichlids can infer that A is dominant to C. In addition to transitive inference, many animals behave as though they have an understanding of the relative ranks of pairs of others. Many primate species solicit agonistic support from individuals that are higher ranking than their aggressors (Perry et al., 2004; Range & Noe, 2005; Schino et al., 2006). In an experimental setting, chacma baboons and vervet monkeys, Chlorocebus aethiops, looked longer in the direction of playback speakers when played calls that represented a subordinate monkey winning an agonistic encounter against a dominant monkey compared to cases where the individuals’ roles were reversed (Bergman et al., 2003; Borgeaud et al., 2013). That is, these monkeys seem to recognize the dominance relationship between individuals and to be surprised to hear calls that suggest it had been overturned. Similarly, ravens, Corvus corax, reacted differently to playbacks of vocal interactions that either confirmed or violated the relative social status of familiar pairs of third parties (Massen, Pašukonis, et al., 2014), which the authors suggest is based on information attained by subjects observing interactions between others. Animals also recognize related pairs of third parties. For example, following aggression between chacma baboons (P. hamadryas ursinus), victims will respond to reconciliatory grunts either from their attacker or from a close relative of their attacker, but will show no such response to reconciliatory grunts from individuals unrelated to the attacker (Wittig et al., 2007).
Kin relationships and relative dominance ranks might be easy to keep track of as they are relatively stable and, in the case of the latter, based on clear, binomially expressed interactions (win/loses). In comparison, relationships based on sexual or sociopositive interactions, might be more subtle, changeable, and difficult to track. In a study of wild geladas, Theropithecus gelada, bachelor males were played sounds that simulated copulations between breeding males and female members of their unit, and sounds that simulated copulations between breeding males and females from other units. Bachelors responded the same in both cases. This suggests that a female mating outside her unit did not violate the bachelors’ expectations and may indicate that bachelors do not have knowledge about relationships between males and females (le Roux & Bergman, 2012). In contrast, chacma baboon males responded more strongly playback vocalizations that suggested that a female was mating with a male that was not her current consort partner compared to their response to vocalizations suggesting a female had mated with her current consort (Crockford et al., 2007). This result suggests that baboons eavesdrop on the mating activities of others, potentially to facilitate their access to sneak mating attempts. Pair-bonded ravens were to shown to intervene in affiliative interactions between pairs of others who appeared to be initiating a bond (Massen, Szipl, et al., 2014). This finding suggests that ravens not only have an understanding of relationships between their competitors, but that they execute a strategy whereby they actively attempt to disrupt the relationships of third parties. Although this result suggests that ravens recognize the types of behaviours that might lead to the formation of a pair bond, recognizing affiliative relationships that have already formed might be more difficult. However, another member of the corvid genus, rooks, Corvus frugilegus, redirect aggression to the affiliative partners of their aggressors (Emery et al., 2007), suggesting they understand established bonds between pairs of others.
In addition to associations between third parties, it is also possible that some animals have some understanding of the global properties of their networks. However, this would require the ability to retain and continually update large amounts of information on multiagent interactions and may be beyond the capabilities or indeed necessities of most animals. Yet, whether consciously or not, animals appear to be influenced by and to influence the associations between pairs of others. A logical extension of this exciting research would be to explore whether animals recognize and/or respond differently to individuals who are highly indirectly connected or who occupy network positions with high levels of influence (e.g. with high betweenness).
CONCLUDING REMARKS
In some species an individual’s level of indirect connectedness is heritable and has been related to their survival and reproductive success (Brent, Heilbronner, et al., 2013; Gilby et al., 2013; Lea et al., 2010; Stanton & Mann, 2012). Individuals can sometimes recognize relationships between third parties and may engage in behavioural strategies to manipulate those relationships (Cheney, 2011; Massen, Szipl, et al., 2014). Indirect connections may also be important to the transfer of information and cultural practices, and to the maintenance of cooperation within animal groups (Bode et al., 2011; Nowak & Sigmund, 2005). Indirect connections may also play a role in the transmission of disease (Hamede et al., 2009; VanderWaal et al., 2014; Weber et al., 2013), in the expression of sexual behaviours (Formica et al., 2012), and may influence how individuals respond physiologically to the social environment (Brent, Semple, et al., 2011). There are also burgeoning areas of research into topics that are conceptually dependent upon multiagent social interactions, such as the impact of the metagenome (i.e. the genomes of others) on cultural evolution, friendship and gene expression (Christakis & Fowler, 2014; Slavich & Cole, 2013; Wolf et al., 1998). In addition, other areas of animal behaviour research could benefit from looking beyond dyadic associations, including studies of mate choice, hybridization and personality (Krause et al., 2010; Sih et al., 2009; Wilson & Krause, 2015).
Yet despite considerable progress in our understanding of the role of indirect connections in animal social behaviour, a great many questions remain unanswered. For example, how much information do animals have regarding the relationships of third parties and how often do they use this information to control, change or influence those relationships? In addition, we need to ask the extent to which the findings outlined in this review are generalizable. Will similar results be generated in many of the species that live in closed social groups, or are they restricted only to species with particular structural properties or cognitive abilities?
Future research that uses measures of indirect centrality should also avoid certain methodological pitfalls. Centrality measures are often intercorrelated (Sueur et al., 2011; Wey & Blumstein, 2012) and researchers must have a firm handle on the relationships between those measures in their study before conducting further analyses. To cope with intercorrelations, researchers should either select only measures that capture statistically independent aspects of centrality (Wey & Blumstein, 2012), or collapse variance among dependent measures using statistical procedures (e.g. principal component analysis). Regardless of how this issue is approached, the level of correlation between centrality measures should be reported to allow the interpretation of results. Nevertheless, measures of indirect connections can never be wholly independent from direct connections because the former cannot exist without the latter. It is sometimes possible to determine whether results generated using the aspect of a measure that captures only indirect connections differ from results generated using the entirety of the measure, e.g. by subtracting an individual’s degree from their reach (Brent, MacLarnon, et al., 2013; Flack et al., 2006), but whether or not this is conceptually necessary is debatable. In addition, animal behaviour researchers using network analysis should be aware of sampling biases introduced during data collection. In particular, it is an order of magnitude easier to omit an indirect connection compared to a direct connection, both of which can skew results. This is especially true for studies that infer social relationships from social association data, which is more prone to errors compared to direct observations of interactions (Croft et al., 2011). Finally, permutations and bootstrapping techniques are often required to correctly analyse network data (Brent, Lehmann, et al., 2011; Croft et al., 2011). However, these techniques have become increasingly popular in animal behaviour research because of the increased power and flexibility they afford, and thus this requirement is not likely to be a barrier to future studies.
Overall, the question regarding whether indirect connections are important to the lives of social animals remains open. By examining indirect connections, some studies have revealed new information that would not have been uncovered had the focus been at the level of the dyad alone, while other studies have not. To fully establish the role of indirect connections in animal behaviour, additional research that compares results generated using direct and indirect connections is required. Nevertheless, the number of studies that have demonstrated that indirect connections may be an important component of sociality has become too great to ignore and scientists engaged in animal behaviour research should continue along this path for the foreseeable future.
Highlights.
Indirect connections are heritable and predictors of fitness in some species.
Some animals recognize and may strategically manipulate third-party relationships.
Indirect connections may influence social cohesion, communication and cooperation.
Researchers should continue to explore indirect connections in animal societies.
Acknowledgments
Thank you to Robert Seyfarth and Dorothy Cheney, to whom I am indirectly connected by one academic degree of separation, for the invitation to participate in the symposium and to contribute to this Special Issue. Thanks also to Darren Croft and Robert Heathcote for helpful discussion. Funding provided by Natural Environment Research Council (NE/K01286X/1) and the National Institutes of Mental Health (R01-MH096875).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akçay Ç, Reed VA, Campbell SE, Templeton CN, Beecher MD. Indirect reciprocity: song sparrows distrust aggressive neighbours based on eavesdropping. Animal Behaviour. 2010;80:1041–1047. [Google Scholar]
- Alexander RD. The biology of moral systems. New York, NY: Aldine de Gruyter; 1987. [Google Scholar]
- Allen J, Weinrich M, Hoppitt W, Rendell L. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science. 2013;340:485–488. doi: 10.1126/science.1231976. [DOI] [PubMed] [Google Scholar]
- Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. Social networks predict patch discovery in a wild population of songbirds. Proceedings of the Royal Society B: Biological Sciences. 2012;279:4199–4205. doi: 10.1098/rspb.2012.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbo N, Barban N. Does fertility behavior spread among friends? American Sociological Review. 2014;79:412–431. [Google Scholar]
- Bartlett MY, DeSteno D. Gratitude and prosocial behavior. Psychological Science. 2006;17:319–325. doi: 10.1111/j.1467-9280.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- Berkowitz L, Daniels LR. Affecting the salience of the social-responsibility norm: effects of past help on the response to dependency relationships. Journal of Abnormal and Social Psychology. 1964;68:275–281. doi: 10.1037/h0040164. [DOI] [PubMed] [Google Scholar]
- Bode NF, Wood AJ, Franks D. Social networks and models for collective motion in animals. Behavioral Ecology and Sociobiology. 2011;65:117–130. [Google Scholar]
- Bond RM, Fariss CJ, Jones JJ, Kramer ADI, Marlow C, Settle JE, et al. A 61-million-person experiment in social influence and political mobilization. Nature. 2012;489:295–298. doi: 10.1038/nature11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, et al. Costs of dispersal. Biological Reviews. 2012;87:290–312. doi: 10.1111/j.1469-185X.2011.00201.x. [DOI] [PubMed] [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. UCINET for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- Borgatti SP, Mehra A, Brass DJ, Labianca G. Network analysis in the social sciences. Science. 2009;323:892–895. doi: 10.1126/science.1165821. [DOI] [PubMed] [Google Scholar]
- Borgeaud C, van de Waal E, Bshary R. Third-party ranks knowledge in wild vervet monkeys (Chlorocebus aethiops pygerythrus) PLoS One. 2013;8:e58562. doi: 10.1371/journal.pone.0058562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN. Investigating the causes and consequences of sociality in adult female rhesus macaques using a social network approach (Doctoral thesis) London, U.K.: University of Roehampton; 2010. [Google Scholar]
- Brent LJN, Chang SWC, Gariépy J-F, Platt ML. The neuroethology of friendship. Annals of the New York Academy of Sciences. 2014;1316:1–17. doi: 10.1111/nyas.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, et al. Genetic origins of social networks in rhesus macaques. Scientific Reports. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Lehmann J, Ramos-Fernández G. Social network analysis in the study of nonhuman primates: a historical perspective. American Journal of Primatology. 2011;73:720–730. doi: 10.1002/ajp.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, MacLarnon A, Platt ML, Semple S. Seasonal changes in the structure of rhesus macaque social networks. Behavioral Ecology and Sociobiology. 2013;67:349–359. doi: 10.1007/s00265-012-1455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent LJN, Semple S, Dubuc C, Heistermann M, MacLarnon A. Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiology & Behavior. 2011;102:76–83. doi: 10.1016/j.physbeh.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Brent LN, Melin A. The genetic basis of primate behavior: genetics and genomics in field-based primatology. International Journal of Primatology. 2013;35:1–10. doi: 10.1007/s10764-013-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R, Grutter AS. Image scoring and cooperation in a cleaner fish mutualism. Nature. 2006;441:975–978. doi: 10.1038/nature04755. [DOI] [PubMed] [Google Scholar]
- Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor M, Whitehead H. The interplay between social networks and culture: theoretically and among whales and dolphins. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368:20120340. doi: 10.1098/rstb.2012.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Brent LJN, Adams GK, Klein JT, Pearson JM, Watson KK, et al. Neuroethology of primate social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(Suppl. 2):10387–10394. doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL. Extent and limits of cooperation in animals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10902–10909. doi: 10.1073/pnas.1100291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Baboon metaphysics: The evolution of a social mind. Chicago, IL: Chicago University Press; 2007. [Google Scholar]
- Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. New England Journal of Medicine. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. Friendship and natural selection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10796–10801. doi: 10.1073/pnas.1400825111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L, Roper TJ. Group decision-making in animals. Nature. 2003;421:155–158. doi: 10.1038/nature01294. [DOI] [PubMed] [Google Scholar]
- Couzin ID. Behavioral ecology: social organization in fission-fusion societies. Current Biology. 2006;16:R169–R171. doi: 10.1016/j.cub.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Couzin ID. Collective cognition in animal groups. Trends in Cognitive Sciences. 2009;13:36–43. doi: 10.1016/j.tics.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Couzin ID, Krause J, Franks NR, Levin SA. Effective leadership and decision-making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Seyfarth RM, Cheney DL. Baboons eavesdrop to deduce mating opportunities. Animal Behaviour. 2007;73:885–890. [Google Scholar]
- Croft DP, James R, Krause J. Exploring animal social networks. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- Croft DP, Krause J, James R. Social networks in the guppy (Poecilia reticulata) Proceedings of the Royal Society B: Biological Sciences. 2004;271(Suppl.):S516–S519. doi: 10.1098/rsbl.2004.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, Madden JR, Franks DW, James R. Hypothesis testing in animal social networks. Trends in Ecology & Evolution. 2011;26:502–507. doi: 10.1016/j.tree.2011.05.012. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. Chimpanzee politics: power and sex among apes. New York, NY: Harper & Row; 1982. [Google Scholar]
- de Waal FBM. The chimpanzee's service economy: food for grooming. Evolution and Human Behavior. 1997;18:375–386. [Google Scholar]
- Dunbar RIM. Primate social systems. Ithaca, NY: Cornell University Press; 1988. [Google Scholar]
- Emery NJ, Seed AM, von Bayern AMP, Clayton NS. Cognitive adaptations of social bonding in birds. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. The genetical theory of natural selection. Oxford, U.K.: Clarendon; 1930. [Google Scholar]
- Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- Flombaum JI, Santos LR. Rhesus monkeys attribute perceptions to others. Current Biology. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Formica VA, Wood CW, Larsen WB, Butterfield RE, Augat ME, Hougen HY, et al. Fitness consequences of social network position in a wild population of forked fungus beetles (Bolitotherus cornutus) Journal of Evolutionary Biology. 2012;25:130–137. doi: 10.1111/j.1420-9101.2011.02411.x. [DOI] [PubMed] [Google Scholar]
- Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham heart study. British Medical Journal. 2008;337:a2338. doi: 10.1136/bmj.a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1720–1724. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977;40:35–41. [Google Scholar]
- Freeman LC. Centrality in social networks: conceptual clarification. Social Networks. 1979;1:215–239. [Google Scholar]
- Frere CH, Krutzen M, Mann J, Connor RC, Bejder L, Sherwin WB. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19949–19954. doi: 10.1073/pnas.1007997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Hamede RK, Bashford J, McCallum H, Jones M. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecology Letters. 2009;12:1147–1157. doi: 10.1111/j.1461-0248.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. Journal of Theoretical Biology. 1964;7:1–51. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Harcourt AH, de Waal FBM. Coalitions and alliances in humans and other animals. Oxford, U.K.: Oxford University Press; 1992. [Google Scholar]
- Hoppitt W, Boogert NJ, Laland KN. Detecting social transmission in networks. Journal of Theoretical Biology. 2010;263:544–555. doi: 10.1016/j.jtbi.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Kanngiesser P, Sueur C, Riedl K, Grossmann J, Call J. Grooming network cohesion and the role of individuals in a captive chimpanzee group. American Journal of Primatology. 2011;73:758–767. doi: 10.1002/ajp.20914. [DOI] [PubMed] [Google Scholar]
- Kendal R, Custance D, Kendal J, Vale G, Stoinski T, Rakotomalala N, et al. Evidence for social learning in wild lemurs (Lemur catta) Learning & Behavior. 2010;38:220–234. doi: 10.3758/LB.38.3.220. [DOI] [PubMed] [Google Scholar]
- King AJ, Sueur C, Huchard E, Cowlishaw G. A rule-of-thumb based on social affiliation explains collective movements in desert baboons. Animal Behaviour. 2011;82:1337–1345. [Google Scholar]
- Krause J, James R, Croft DP. Personality in the context of social networks. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:4099–4106. doi: 10.1098/rstb.2010.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, James R, Lusseau D. Animal social networks: an introduction. Behavioral Ecology and Sociobiology. 2009;63:967–973. [Google Scholar]
- Krause J, Ruxton GD. Living in groups. Oxford, U.K.: Oxford University Press; 2002. [Google Scholar]
- Kundey SMA, De Los Reyes A, Royer E, Molina S, Monnier B, German R, et al. Reputation-like inference in domestic dogs (Canis familiaris) Animal Cognition. 2011;14:291–302. doi: 10.1007/s10071-010-0362-5. [DOI] [PubMed] [Google Scholar]
- le Roux A, Bergman TJ. Indirect rival assessment in a social primate, Theropithecus gelada . Animal Behaviour. 2012;83:249–255. [Google Scholar]
- Lea AJ, Blumstein DT, Wey TW, Martin JGA. Heritable victimization and the benefits of agonistic relationships. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21587–21592. doi: 10.1073/pnas.1009882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Andrews K, Dunbar RIM. Social networks and social complexity in female-bonded primates. In: Dunbar RIM, Gamble C, Gowlett JA, editors. Social brain, distributed mind. Oxford, U.K.: Oxford University Press; 2010. pp. 57–83. [Google Scholar]
- Lehmann J, Dunbar RIM. Network cohesion, group size and neocortex size in female-bonded Old World primates. Proceedings of the Royal Society B: Biological Sciences. 2009;276:4417–4422. doi: 10.1098/rspb.2009.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D. Evidence for a social role in a dolphin social network. Evolutionary Ecology. 2006;21:357–366. [Google Scholar]
- Lusseau D, Newman MEJ. Identifying the role that animals play in their social networks. Biology Letters. 2004;271(Suppl.):S477–S481. doi: 10.1098/rsbl.2004.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D, Whitehead H, Gero S. Incorporating uncertainty into the study of animal social networks. Animal Behaviour. 2008;75:1809–1815. [Google Scholar]
- Madden JR, Drewe JA, Pearce GP, Clutton-Brock TH. The social network structure of a wild meerkat population: 3. Position of individuals within networks. Behavioral Ecology and Sociobiology. 2011;65:1857–1871. [Google Scholar]
- Mann J, Stanton MA, Patterson EM, Bienenstock EJ, Singh LO. Social networks reveal cultural behaviour in tool-using using dolphins. Nature Communications. 2012;3:980. doi: 10.1038/ncomms1983. [DOI] [PubMed] [Google Scholar]
- Manno TG. Social networking in the Columbian ground squirrel, Spermophilus columbianus . Animal Behaviour. 2008;75:1221–1228. [Google Scholar]
- Massen JJM, Pašukonis A, Schmidt J, Bugnyar T. Ravens notice dominance reversals among conspecifics within and outside their social group. Nature Communications. 2014;5:3679. doi: 10.1038/ncomms4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen JJM, Szipl G, Spreafico M, Bugnyar T. Ravens intervene in others’ bonding attempts. Current Biology. 2014;24:2733–2736. doi: 10.1016/j.cub.2014.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. Cause and effect in biology: kinds of causes, predictability, and teleology are viewed by a practicing biologist. Science. 1961;134:1501–1506. doi: 10.1126/science.134.3489.1501. [DOI] [PubMed] [Google Scholar]
- McDonald DB. Predicting fate from early connectivity in a social network. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10910–10914. doi: 10.1073/pnas.0701159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram S. The small-world problem. Psychology Today. 1967;1:61–67. [Google Scholar]
- Milinski M, Semmann D, Bakker TCM, Krambeck HJ. Cooperation through indirect reciprocity: image scoring or standing strategy? Proceedings of the Royal Society B: Biological Sciences. 2001;268:2495–2501. doi: 10.1098/rspb.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtashemi M, Mui L. Evolution of indirect reciprocity by social information: the role of trust and reputation in evolution of altruism. Journal of Theoretical Biology. 2003;223:523–531. doi: 10.1016/s0022-5193(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Newman MEJ. The structure and function of complex networks. Siam Review. 2003;45:167–256. [Google Scholar]
- Nightingale G, Boogert NJ, Laland KN, Hoppitt W. Quantifying diffusion in social networks: a Bayesian approach. In: Krause J, James R, Franks DW, Croft DP, editors. Animal social networks. Oxford, U.K.: Oxford University Press; 2015. pp. 38–52. [Google Scholar]
- Noad MJ, Cato DH, Bryden MM, Jenner MN, Jenner KCS. Cultural revolution in whale songs. Nature. 2000;408:537. doi: 10.1038/35046199. [DOI] [PubMed] [Google Scholar]
- Noë R. Alliance formation among male baboons: shopping for profitable partners. In: Harcourt AH, de Waal FBM, editors. Coalitions and alliances in humans and other animals. Oxford, U.K.: Oxford University Press; 1992. pp. 285–321. [Google Scholar]
- Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Roch S. Upstream reciprocity and the evolution of gratitude. Proceedings of the Royal Society B: Biological Sciences. 2007;274:605–610. doi: 10.1098/rspb.2006.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Evolution of indirect reciprocity by image scoring. Nature. 1998;393:573–577. doi: 10.1038/31225. [DOI] [PubMed] [Google Scholar]
- Nowak MA, Sigmund K. Evolution of indirect reciprocity. Nature. 2005;437:1291–1298. doi: 10.1038/nature04131. [DOI] [PubMed] [Google Scholar]
- Opsahl T. Structure and evolution of weighted networks. London, U.K.: University of London; 2009. [Google Scholar]
- Panchanathan K, Boyd R. A tale of two defectors:the importance of standing for evolution of indirect reciprocity. Journal of Theoretical Biology. 2003;224:115–126. doi: 10.1016/s0022-5193(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Pellizzari M. Do friends and relatives really help in getting a good job? Industrial and Labor Relations Review. 2010;63:494–510. [Google Scholar]
- Perry S, Barrett HC, Manson JH. White-faced capuchin monkeys show triadic awareness in their choice of allies. Animal Behaviour. 2004;67:165–170. [Google Scholar]
- Pinto A, Oates J, Grutter A, Bshary R. Cleaner wrasses Labroides dimidiatus are more cooperative in the presence of an audience. Current Biology. 2011;21:1140–1144. doi: 10.1016/j.cub.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Raihani NJ, Bshary R. Resolving the iterated prisoner's dilemma: theory and reality. Journal of Evolutionary Biology. 2011;24:1628–1639. doi: 10.1111/j.1420-9101.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- Range F, Noe R. Can simple rules account for the pattern of triadic interactions in juvenile and adult female sooty mangabeys? Animal Behaviour. 2005;69:445–452. [Google Scholar]
- Russell YI, Call J, Dunbar RIM. Image scoring in great apes. Behavioural Processes. 2008;78:108–111. doi: 10.1016/j.beproc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M. Generalized reciprocity in rats. PLoS Biology. 2007;5:1421–1425. doi: 10.1371/journal.pbio.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutte C, Taborsky M. The influence of social experience on cooperative behaviour of rats (Rattus norvegicus): direct vs generalised reciprocity. Behavioral Ecology and Sociobiology. 2008;62:499–505. [Google Scholar]
- Ryder TB, McDonald DB, Blake JG, Parker PG, Loiselle BA. Social networks in the lek-mating wire-tailed manakin (Pipra filicauda) Proceedings of the Royal Society B: Biological Sciences. 2008;275:1367–1374. doi: 10.1098/rspb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder TB, Parker PG, Blake JG, Loiselle BA. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proceedings of the Royal Society B: Biological Sciences. 2009;76:2377–2384. doi: 10.1098/rspb.2009.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabidussi G. The centrality index of a graph. Psychometrika. 1966;31:581–603. doi: 10.1007/BF02289527. [DOI] [PubMed] [Google Scholar]
- Schino G, Aureli F. Reciprocal altruism in primates: partner choice, cognition, and emotions. Advances in the Study of Behavior. 2009;39:45–69. [Google Scholar]
- Schino G, Tiddi B, Di Sorrentino EP. Simultaneous classification by rank and kinship in Japanese macaques. Animal Behaviour. 2006;71:1069–1074. [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Current Biology. 2010;20:1–4. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Semmann D, Krambeck HJ, Milinski M. Strategic investment in reputation. Behavioral Ecology and Sociobiology. 2004;56:248–252. [Google Scholar]
- Seyfarth RM. A model of social grooming among adult female monkeys. Journal of Theoretical Biology. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Sih A, Hanser SF, McHugh KA. Social network theory: new insights and issues for behavioral ecologists. Behavioral Ecology and Sociobiology. 2009;63:975–988. [Google Scholar]
- Silk JB. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, et al. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clinical Psychological Science. 2013;1:331–348. doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton MA, Mann J. Early social networks predict survival in wild bottlenose dolphins. PLoS One. 2012;7:e47508. doi: 10.1371/journal.pone.0047508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson K, Zelen M. Rethinking centrality: methods and examples. Social Networks. 1989;11:1–37. [Google Scholar]
- Subiaul F, Vonk J, Okamoto-Barth S, Barth J. Do chimpanzees learn reputation by observation? Evidence from direct and indirect experience with generous and selfish strangers. Animal Cognition. 2008;11:611–623. doi: 10.1007/s10071-008-0151-6. [DOI] [PubMed] [Google Scholar]
- Sueur C, Jacobs A, Amblard F, Petit O, King AJ. How can social network analysis improve the study of primate behavior? American Journal of Primatology. 2011;73:703–719. doi: 10.1002/ajp.20915. [DOI] [PubMed] [Google Scholar]
- Sueur C, Petit O. Organization of group members at departure is driven by social structure in Macaca . International Journal of Primatology. 2008;29:1085–1098. [Google Scholar]
- Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410–433. [Google Scholar]
- Trivers RL. Evolution of reciprocal altruism. Quarterly Review of Biology. 1971;46:35–57. [Google Scholar]
- VanderWaal KL, Atwill ER, Isbell LA, McCowan B. Quantifying microbe transmission networks for wild and domestic ungulates in Kenya. Biological Conservation. 2014;169:136–146. [Google Scholar]
- Verdolin JL, Traud AL, Dunn RR. Key players and hierarchical organization of prairie dog social networks. Ecological Complexity. 2014;19:140–147. [Google Scholar]
- Voelkl B, Noë R. Simulation of information propagation in real-life primate networks: longevity, fecundity, fidelity. Behavioral Ecology and Sociobiology. 2010;64:1449–1459. [Google Scholar]
- Wasserman S, Faust K. Social network analysis: Methods and applications. Cambridge, U.K.: Cambridge University Press; 1994. [Google Scholar]
- Weber N, Carter SP, Dall SRX, Delahay RJ, McDonald JL, Bearhop S, et al. Badger social networks correlate with tuberculosis infection. Current Biology. 2013;23:R915–R916. doi: 10.1016/j.cub.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Milinski M. Cooperation through image scoring in humans. Science. 2000;288:850–852. doi: 10.1126/science.288.5467.850. [DOI] [PubMed] [Google Scholar]
- Wey TW, Blumstein DT. Social cohesion in yellow-bellied marmots is established through age and kin structuring. Animal Behaviour. 2010;79:1343–1352. [Google Scholar]
- Wey T, Blumstein D. Social attributes and associated performance measures in marmots: bigger male bullies and weakly affiliating females have higher annual reproductive success. Behavioral Ecology and Sociobiology. 2012;66:1075–1085. [Google Scholar]
- Wey T, Blumstein DT, Shen W, Jordan F. Social network analysis of animal behaviour: a promising tool for the study of sociality. Animal Behaviour. 2008;75:333–344. [Google Scholar]
- Whitehead H. Precision and power in the analysis of social structure using associations. Animal Behaviour. 2008;75:1093–1099. [Google Scholar]
- Wilson ADM, Krause J. Personality and social network analysis in animals. In: Krause J, James R, Franks DW, Crof DP, editors. Animal social networks. Oxford, U.K.: Oxford University Press; 2015. pp. 53–60. [Google Scholar]
- Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Kin-mediated reconciliation substitutes for direct reconciliation in female baboons. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1109–1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JB, Brodie ED, III, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends in Ecology & Evolution. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii . Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]