Abstract
We report here the isolation and in vitro culture of bovine inner cell mass (ICM) cells and the use of ICM cells in nuclear transfer to produce totipotent blastocysts that resulted in calves born. Of 15 cell lines represented in this study, 13 were derived from immunosurgically isolated ICM of 3 in vitro produced day 9-10 bovine blastocysts, while 2 lines were derived from single blastocysts. Approximately 70% of attempted cell lines became established cell lines when started from 3 ICMs. The ability to establish cell lines was dependent on the number of ICMs starting the line. Sire differences were noted in the ability of ICMs to establish cell lines and to form blastocysts. The cell lines were cultured as a low cell density suspension in the medium CR1aa plus selenium, insulin, and transferrin (SIT) and 5% fetal calf serum (FCS) for 6-101 days before use in nuclear transfer, at which time some had multiplied to more than 2000 cells. If allowed to aggregate, cells of established cell lines formed embryoid bodies. A total of 659 nuclear transfer clones were made by fusing the ES cells into enucleated oocytes with polyethylene glycol; 460 of these fused, based on cleavage (70%). After culture of the clones for 7 days in vitro in CR1aa/SIT/5% FCS, 109 (24%) of those fused became blastocysts. Thirty-four blastocysts were transferred into uteri of 27 cows, and 13 cows (49%) became pregnant. Four of the 13 cows gave birth to 4 normal calves. DNA typing showed the calves to be derived from the respective sires of the cell lines. The calves were derived from cultures of less than 28 days.
Full text
PDF


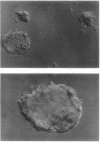
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler J. E., Anderson G. B., BonDurant R. H., Pashen R. L., Penedo M. C. Production of ovine chimeras by inner cell mass transplantation. J Anim Sci. 1987 Jul;65(1):317–324. doi: 10.2527/jas1987.651317x. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Collas P., Robl J. M. Factors affecting the efficiency of nuclear transplantation in the rabbit embryo. Biol Reprod. 1990 Nov;43(5):877–884. doi: 10.1095/biolreprod43.5.877. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- First N. L., Prather R. S. Genomic potential in mammals. Differentiation. 1991 Sep;48(1):1–8. doi: 10.1111/j.1432-0436.1991.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Gossler A., Doetschman T., Korn R., Serfling E., Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Joyner A. L. Gene targeting and gene trap screens using embryonic stem cells: new approaches to mammalian development. Bioessays. 1991 Dec;13(12):649–656. doi: 10.1002/bies.950131206. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Hagemann L. J., Doetschman T., Hagaman J. R., Huang S., Williams P. J., First N. L., Maeda N., Smithies O. Germ-line transmission of a planned alteration made in a hypoxanthine phosphoribosyltransferase gene by homologous recombination in embryonic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Badge R. H., Bygrave A., Bradley A., Robertson E., Tilly R., Cheah K. S. Tissue-specific expression of the human type II collagen gene in mice. Proc Natl Acad Sci U S A. 1987 May;84(9):2803–2807. doi: 10.1073/pnas.84.9.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlinski J. A., Gerhäuser D., Lioi B., Winking H., Illmensee K. Nuclear transfer from teratocarcinoma cells into mouse oocytes and eggs. Development. 1990 Feb;108(2):337–348. doi: 10.1242/dev.108.2.337. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarianni E., Galli C., Laurie S., Moor R. M., Evans M. J. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991;43:255–260. [PubMed] [Google Scholar]
- Notarianni E., Laurie S., Moor R. M., Evans M. J. Maintenance and differentiation in culture of pluripotential embryonic cell lines from pig blastocysts. J Reprod Fertil Suppl. 1990;41:51–56. [PubMed] [Google Scholar]
- Parrish J. J., Susko-Parrish J., Winer M. A., First N. L. Capacitation of bovine sperm by heparin. Biol Reprod. 1988 Jun;38(5):1171–1180. doi: 10.1095/biolreprod38.5.1171. [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Anderson G. B., Bondurant R. H. Influence of feeder layer type on the efficiency of isolation of porcine embryo-derived cell lines. Theriogenology. 1990 Nov;34(5):865–877. doi: 10.1016/0093-691x(90)90558-b. [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Anderson G. B., Bondurant R. H. On the isolation of embryonic stem cells: Comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990 Nov;34(5):879–901. doi: 10.1016/0093-691x(90)90559-c. [DOI] [PubMed] [Google Scholar]
- Rosenkrans C. F., Jr, First N. L. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J Anim Sci. 1994 Feb;72(2):434–437. doi: 10.2527/1994.722434x. [DOI] [PubMed] [Google Scholar]
- Sirard M. A., Parrish J. J., Ware C. B., Leibfried-Rutledge M. L., First N. L. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod. 1988 Oct;39(3):546–552. doi: 10.1095/biolreprod39.3.546. [DOI] [PubMed] [Google Scholar]
- Smith L. C., Wilmut I. Influence of nuclear and cytoplasmic activity on the development in vivo of sheep embryos after nuclear transplantation. Biol Reprod. 1989 May;40(5):1027–1035. doi: 10.1095/biolreprod40.5.1027. [DOI] [PubMed] [Google Scholar]
- Stanton B. R., Reid S. W., Parada L. F. Germ line transmission of an inactive N-myc allele generated by homologous recombination in mouse embryonic stem cells. Mol Cell Biol. 1990 Dec;10(12):6755–6758. doi: 10.1128/mcb.10.12.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Ikawa Y., Yoshida K., Shigetani Y., Takeda N., Mabuchi I., Yamamoto T., Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]