Abstract
Cordyceps sinensis, also called DongChongXiaCao (winter worm, summer grass) in Chinese, is becoming increasingly popular and important in the public and scientific communities. This study summarizes the chemical constituents and their corresponding pharmacological actions of Cordyceps sinensis. Many bioactive components of Cordyceps sinensis have been extracted including nucleoside, polysaccharide, sterol, protein, amino acid, and polypeptide. In addition, these constituents' corresponding pharmacological actions were also shown in the study such as anti-inflammatory, antioxidant, antitumour, antiapoptosis, and immunomodulatory actions. Therefore can use different effects of C. sinensis against different diseases and provide reference for the study of Cordyceps sinensis in the future.
1. Introduction
The genus Cordyceps is an important kind of medicinal fungi belonging to the Ascomycota, Pyrenomycetes, Hypocreales, and Clavicipitaceae [1–5]. Cordyceps are specific macrofungi because of their characteristic parasitic habit on larvae and pupae of insects. As a pleomorphic fungus distributed worldwide, Cordyceps is particularly abundant in tropical forests and humid temperate [3–5]. Within the genus Cordyceps, over 400 species have been described so far [4, 5], of which Cordyceps sinensis, also called as “winter worm, summer grass,” is recognized as the most famous tonic herb in traditional Chinese medicine (TCM) for centuries.
Cordyceps sinensis is an abundant resource in nature with various biological activity and has been used extensively as a tonic and health supplement for subhealth patients especially seniors in China and other Asian countries. Till now, numerous bioactive constituents have been extracted such as cordycepin, polysaccharides, ergosterol, mannitol, and adenosine [6, 7]. Meanwhile, various pharmacological actions of these chemical constituents have been reported, including antitumour effect, hepatoprotective and inflammatory effects, and antioxidant, nephroprotective, and antiapoptotic properties [8–14]. To sum up, the effect of C. sinensis may be caused by a single active ingredient or by the combined action of many active agents that existed in the extract.
Research is necessary to get an overview about the genus Cordyceps sinensis because of the increasing interest both for medicine and mycology [15, 16]. Therefore, our study has reviewed the chemical constituents and their corresponding pharmacological actions of the Cordyceps sinensis for its significant role in the development of new drugs and therapeutics for various diseases. Moreover, realizing the pharmacological action of the monomer composition could strengthen the drug efficacy through extracting a single ingredient in Cordyceps sinensis. Therefore, it is necessary to review the development on the research of C. sinensis.
2. Chemical Constituents and Their Corresponding Pharmacological Actions of Cordyceps sinensis
2.1. Nucleosides
Nucleosides, a major active component of C. sinensis, are used as a valuable chemical marker for quality control of Cordyceps [1]. Besides, nucleosides play an important role in the drug development of cancer and infectious diseases, and nucleosides and their derivatives have been widely used in anticancer and antiviral therapies. Since 3′-deoxyadenosine, namely cordycepin, was isolated from cultured Cordyceps militaris, nucleosides in Cordyceps have become a focus [1]. In succession, more than ten nucleosides and their related compounds, including adenine, adenosine, inosine, cytidine, cytosine, guanine, uridine, thymidine, uracil, hypoxanthine, and guanosine, have been isolated from Cordyceps sinensis. Almost all of the nucleotides and nucleosides in C. sinensis can be transformed reciprocally [17]. Furthermore, many scholars began to study its pharmacological effects and had a lot of achievements [18, 19]. An UPLC method for fast simultaneous determination of several nucleosides was developed this year and was also applied for the determination of the analytes in cultured Cordyceps sinensis [19, 20]. Then, a series of researches about nucleosides was carried out quickly. For example, nucleosides can adjust and control the human body physiological activities through purinergic and/or pyrimidine receptors [17]. Therefore, determination of nucleosides and their related compounds is extremely important for the pharmacological study and quality control of C. sinensis and its products.
2.1.1. Cordycepin
Early in 1950, cordycepin was first isolated from C. militaris and its structural formula was confirmed as 3′-deoxyadenosine but it is only found in natural C. sinensis with very low content and cannot be detected in the cultured ones [17]. Cordycepin is the most considerable adenosine analogue from some Cordyceps [21], which is a derivative of the nucleoside adenosine differing from the latter by the absence of oxygen in the 3′ position of its ribose entity (Figure 1). Cordycepin was separated with a mixture of acetonitrile and water (5 : 95, v/v) at a flow rate of 1.0 mL/min, which is the commonly used method to extract the composition [22].
Figure 1.
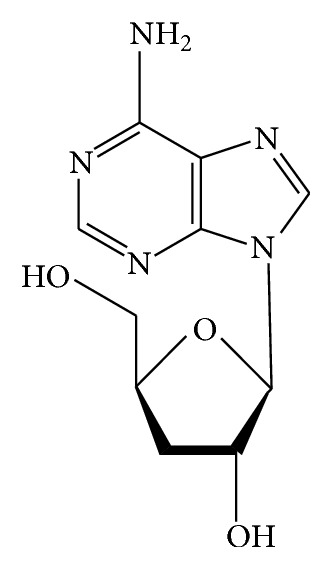
Chemical structure of cordycepin.
Cordycepin is a category of compounds that exhibits significant therapeutic potential and has many intracellular targets, including nucleic acid, apoptosis, and cell cycle. Tuli et al. researched the variety of molecular mechanisms that mediate the pharmacological effects of cordycepin. Besides, they deem that cordycepin can participate in various molecular processes in cells because of its similarity with adenosine [7]. Wang et al. investigated the effects of cordycepin in prevention of focal cerebral ischemic/reperfusion (IR) injury and suggested that cordycepin has a neuroprotective effect in the ischemic brain, which is due to the inhibition of inflammation and increase of antioxidants activity related to lesion pathogenesis [23, 24]. So cordycepin could be an attractive therapeutic candidate with oral activity against I/R-associated heart diseases such as myocardial infarction [25]. Besides, cordycepin showed the obvious analgesic effect through acetic acid-induced abdominal constrictions, hot-plate test, and neurolysin inhibition assay in mice [26]. Qian et al. considered that cordycepin is a potent anti-inflammatory and analgesic medicine. There are several studies demonstrating that C. sinensis stimulates steroidogenesis in primary mouse Leydig cell and activates apoptosis in MA-10 mouse Leydig tumor cells in dose- and time-dependent manners [27, 28]. The steroidogenic and apoptotic mechanism of cordycepin is also clear—cordycepin stimulated intracellular PLC/PKC and MAPK signal transduction pathways to induce steroidogenesis and cell death in MA-10 mouse Leydig tumor cells [29]. In addition, cordycepin stimulated the release of some cytokines of resting PBMCs and influenced proliferation of PBMCs and transcription factors in THP-1 cell line. Accordingly, cordycepin can intensively regulate the functions of human immune cells in vitro [30]. Besides, cordycepin is a broad spectrum biocidal compound possessing not only antitumor activity but also antibacteria, antivirus, and insecticidal activities [2]. To sum up, cordycepin was confirmed as a marker for C. militaris within the content profiles of nucleosides in Cordyceps product [1].
2.1.2. Adenosine
Adenosine (Figure 2), which plays an important role in biochemical process in the organism, is a major nucleoside in Cordyceps spp. [31]. The content of adenosine is much higher in cultured Cordyceps sinensis than in the natural one. Among them, cultured C. sinensis has a large number of adenosines, which are much higher than those in cultured C. militaris [20, 31]. Nucleotide named AMP can be degraded to adenosine and the source of inosine in natural C. sinensis may be the oxidative deamination of adenosine [17]. Many other adenosine analogues such as 2′-deoxyadenosine, 2′3′-dideoxyadenosine, cordycepin triphosphate, and 3′-amino-3′-deoxyadenosine have also been found in Cordyceps sinensis [1]. Yang and Li introduced three methods to extract adenosine: organic solvent pressurized liquid extraction, boiling water extraction, and ambient temperature water extraction. They found that the extraction ratio of adenosine is much affected by extracting time and natural Cordyceps sinensis may contain some enzymes which can decompose adenosine [32–34].
Figure 2.
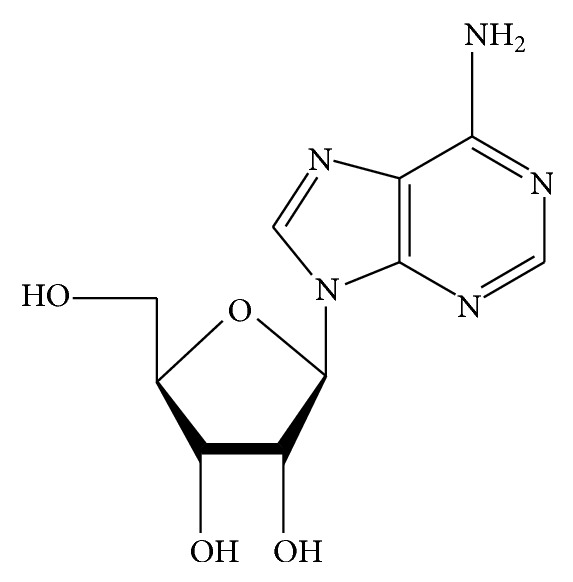
Chemical structure of adenosine.
Also, adenosine is an energy transfer and signal transductant in cells and can still exert a wide spectrum of cytoprotection or prevent tissue damage such as treating chronic heart failure, anti-inflammatory properties, and anticonvulsant activity [35–38]. In addition, adenosine is reported to suppress cell growth via diverse extrinsic and intrinsic signaling pathways. In both pathways, adenosine activates caspases in a mitochondria-dependent and/or -independent manner [39–41]. For example, Ma et al. first observed that adenosine increases ROS production in tumor cells and identified the positive feedback loop for ROS-mediated mitochondrial membrane dysfunction which amplifies the death signals in the cells [42]. However, Iannone et al. support the hypothesis that inhibition of adenosine production in tumors or inhibition of A2aR is a promising strategy to increase the effectiveness of melanoma immunotherapy, because they have done a lot of experiments proving that adenosine can limit the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model [43]. In fact, adenosine mediates its effects through activation of a family of four G-protein coupled receptors, namely A1, A2A, A2B, and A3 [44]. This nucleoside plays an important role in immunity and inflammation, and the adenosine A2A plays an important role in depression, locomotion, and anxiety [45]. In particular in skin cells this endogenous nucleoside, acting at one or more of its receptors, could participate in dermal tissue protection and repair. To sum up, adenosine and its analogues have received so much attention due to their various pharmacological effects.
2.1.3. Nucleobases
To date, six nucleobases (Figure 3), including cytosine, uracil, thymine, adenine, guanine, and hypoxanthine, were determined in natural and cultured Cordyceps sinensis. The overall content of nucleosides is much higher in cultured Cordyceps sinensis than in natural ones by comparison [32]. A method based on optimum acid hydrolysis followed by high-performance liquid chromatography (HPLC) with diode array detection was developed for quantitative determination of these bioavailable nucleosides by Fan et al. which is now the most recognized appraisal method [46]. As a result, the total purine and pyrimidine bases may be the reasonable marker for evaluation of the nutrition of the materials containing nucleosides [47]. However, the pharmacological effects on nucleobases alone have not been reported currently.
Figure 3.
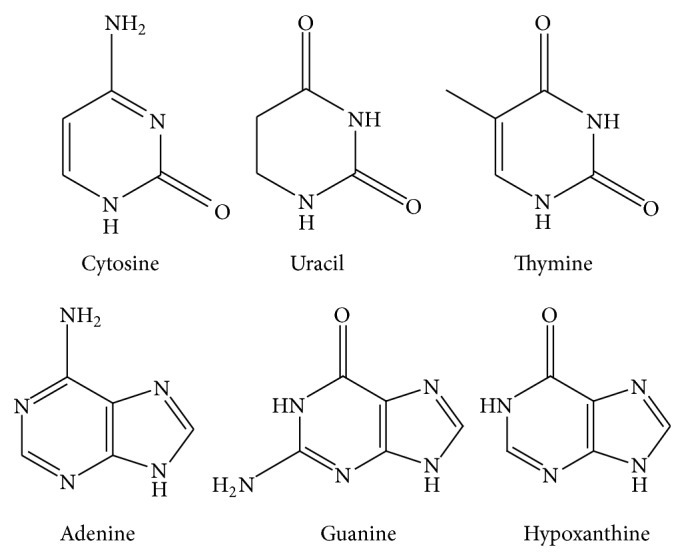
Chemical structure of six nucleosides.
2.1.4. Nucleotides
Three nucleotides, namely, uridine-5′-monophosphate (UMP), adenosine-5′-monophosphate (AMP), and guanosine-5′-monophosphate (GMP), were separated by ion-pairing reversed-phase liquid chromatography-mass spectrometry (IP-RP-LC-MS) developed by Yang et al. [17]. In the pharmacological aspect, nucleotides were reported to enhance the immune response, influence metabolism of fatty acids, help the absorption of iron gut, and improve the gastrointestinal injury after repair [17, 48]. The nucleotides such as AMP, GMP, and UMP can be degraded to adenosine, guanosine, and uridine, respectively. Actually, nucleotides could be considered as an amphoteric molecule with base and phosphoric acid. Guanosine has the highest content of all in natural and artificial Cordyceps sinensis showed by many investigations [48]. Nucleotides can inhibit urethral inflammation, promote blood circulation, and improve brain function, and their most important effect is enhancing human immunity, which has been reported in previous studies [49].
2.2. Polysaccharides
Cordyceps sinensis contains a great deal of polysaccharides, which can be in the range of 3–8% of the total weight [50, 51]. Cordyceps polysaccharides mainly include extracellular polysaccharide and intracellular polysaccharide. A large amount of experimental evidence has shown that fungal polysaccharides have a wide range of bioactivities including antitumor [52], anti-influenza virus [53], immunopotentiation [54], hypoglycemic [55], hypocholesterolemic [56], and antioxidant effects [51]. Other studies have suggested that the pharmacological activity of the polysaccharide was correlated with its characteristics. For example, it is polysaccharides' high molecular weight that determines the antitumor activity [57]. In other words, Sasaki et al. confirmed that the fungi polysaccharide's antitumor activity is related to the molecular weight, and the fungi polysaccharide has antitumor activity if its molecular weight is greater than 16000. But beyond that, ten monosaccharides, namely, rhamnose, ribose, arabinose, xylose, mannose, glucose, galactose, mannitol, fructose, and sorbose in 13 samples of natural and cultured C. sinensis, were qualitatively and quantitatively analyzed [58]. However, Cordyceps polysaccharides were usually composed of these monosaccharides and they play a prominent role in the organism. Polysaccharides are the main contributor towards the pharmacological properties of C. sinensis. Nevertheless, its application has been limited so far because of its limited supply. It is an endangered species due to the excessive harvest of the natural fungus [59, 60]. Meanwhile, cultured C. sinensis has shown as many pharmacological properties as natural C. sinensis [50].
2.2.1. EPSF
Exopolysaccharide fraction (EPSF), a heteropolysaccharide, was extracted from the cultured supernatant of C. sinensis. The cultured supernatant was collected and then treated with three times volume of 95% ethanol for precipitation. As a result, the sediment contains a large number of EPSF [60]. EPSF has a large number of pharmacological effects; two of the most important are immunomodulatory and antitumour effects [61]. Previous reports have shown that EPSF could scavenge free radical, induce differentiation of cancer cells, and enhance antitumor ability via activating different immune responses in the host [61]. Thus it can be seen that elevating immunity is much helpful in tumor therapy. In order to explore the effects of exopolysaccharide fraction (EPSF), ICR mice were treated with EPSF for 7 days at different doses after H22 tumor cells' injection. The data of these studies show that EPSF could elevate the immunocytes' activities in H22 tumor-bearing mice, which might be closely related to elevating peritoneal macrophages' and splenic lymphocytes' activity [60]. Studies have shown that mature DCs (dendritic cells) are important modulators of immune response and their ability to initiate cytotoxic T lymphocyte is very valuable in cancer immunotherapy, and maturation of DCs is a critical factor for the initiation of immune response [62]. Song et al. found that EPSF can promote DC's maturation and activation, which is probably related to the inhibition of STAT3 phosphorylation [63]. This is another mechanism of EPSF's antitumor effect. Yang et al. have investigated the effects of the EPSF on c-Myc, c-Fos, and vascular endothelial growth factor (VEGF) expression of tumor-bearing mice using Simple PCI image analysis software. The c-Myc, c-Fos, and VEGF levels in the lungs and livers of EPSF-treated mice were found to be significantly lower than those of untreated mice, which suggests that EPSF had inhibited tumor growth in the lungs and livers of mice. As a result, it might be a potential adjuvant in cancer therapy [64]. As mentioned above, the EPSF can inhibit a variety of cancer cells; moreover, it may enhance the antitumour ability of animals or humans by activating different immune responses in the host. EPS-1, which is an exopolysaccharide produced by the medicinal fungus Cordyceps sinensis, has been specifically named and widely concerned. A recent study has shown that the sulfated EPS-1 derivatives have remarkable antioxidant activities. So, sulfation was an effective and favorable strategy for improving the physicochemical properties and bioactivities of fungal polysaccharides [65], which is a good idea for the study of polysaccharides.
2.2.2. APS
An acid polysaccharide (APS) was isolated from cultivated C. sinensis mycelia by ion-exchange and sizing chromatography. APS is composed of mannose, glucose, and galactose in an approximate molar ratio of 3.3 : 2.3 : 1 [66]. In the present study, pretreatment of PC12 cells with APS could reduce H2O2-induced cell death, which was investigated by measuring cell viability, lactate dehydrogenase (LDH) release, antioxidant enzyme activity, malondialdehyde (MDA) levels, and intracellular accumulation of reactive oxygen species (ROS) and Ca2+ [66]. In conclusion, APS possesses protective effects in PC12 cells against H2O2-induced injury [67]. However, the antioxidant mechanism of APS remains unclear and needs further investigation. In view of the fact that acid polysaccharide fraction (APSF), extracted from C. sinensis fungus, has stimulating effects on macrophages [68], Chen et al. have proved that APSF may convert M2 macrophages to M1 phenotype by activating NF-κB pathway. So APSF also has immunomodulatory effects as many other polysaccharides [69].
2.2.3. CPS-1
A water-soluble polysaccharide named CPS-1 had been isolated from C. sinensis mycelium by hot water extraction, ethanol precipitation, anion-exchange, and gel permeation chromatography [70]. CPS-1 was a glucomannogalactan with the monosaccharide composition of glucose : mannose : galactose = 2.8 : 2.9 : 1. (1 →) and (1 → 3,6) linkage of glucose composed the backbone of CPS-1. Present studies have demonstrated that CSP-1 had strong antioxidation activities, which can be used to reduce the blood glucose level [71] and treat renal failure [70]. On one hand, CPS-1 can scavenge hydroxyl radicals and reduce power- and Fe2+-chelating. That indicated a connection between antioxidant activity and reparation of renal failure. On the other hand, CPS-1 stimulates pancreatic release of insulin and/or reduces insulin metabolism, so the polysaccharide can treat diabetes. Especially, the reducing power of CPS-1 was very potent and nearly as effective as ascorbic acid [71].
2.2.4. CPS-2
CPS-2, a Cordyceps sinensis polysaccharide, was found to be mostly of α-(1 → 4)-D-glucose and α-(1 → 3)-D-mannose, branched with α-(1 → 4,6)-D-glucose every twelve residues on average (Figure 4). A monosaccharide analysis conducted by the PMP precolumn derivation method showed that CPS-2 was composed of mannose, glucose, and galactose with the ratio of 4 : 11 : 1 [72]. CPS-2, which appeared as white powder, has been demonstrated to have significant therapeutic activity against chronic renal failure. Recently, the underlying molecular mechanism has been explored by scientists. Wang et al. found that CPS-2 could reduce PDGF-BB-induced cell proliferation through the PDGF/ERK and TGF-b1/Smad pathways [73]. As a result, CPS-2 inhibits PDGF-BB-induced human mesangial cells (HMCs) proliferation in a dose-dependent manner.
Figure 4.
Predicted structure of CPS-2 isolated from the fruiting bodies of cultured Cordyceps sinensis.
2.2.5. Other Polysaccharides
A neutral mannoglucan with a molecular weight of 7.7 × 103 Da was obtained from the 0.05 M acetate buffer extract of C. sinensis mycelium. It is a branched polysaccharide with a backbone composed mainly of (1→4)- and (1→3)-linked D-glucosyl residues. Moreover, mannoglucan showed weak cytotoxicity activity against SPC-I cancer line and no obvious cytotoxicity activities against BCAP37 and SW480 cancer line [74]. Similarly, a water-soluble polysaccharide fraction, CME-1, with a molecular mass of 27.6 kDa, was prepared from Cordyceps sinensis mycelia and identified by NMR and GC-MS [75]. Wang et al. finally found that CME-1 can protect RAW264.7 cells against oxidative stress through inhibition of SMase activity and reduction of C16- and C18-ceramide levels [75]. In addition, a new component named cordyglucans was released by successive extractions with hot water and 0.05 M sodium hydroxide solutions. Cordyglucans were found to exhibit potent antitumor activity, which could be correlated to their (1→3)-β-D-glucan linkages [76]. Besides, two other polysaccharides, named CS-F10 and cordysinocan, were extracted from the cultured mycelium of Cordyceps sinensis, respectively. The former has the hypoglycemic activity, which can lower the plasma glucose level and decrease protein content of facilitative glucose transporter isoform 2 from rat liver following i.p. administration [55] and the latter can not only induce the cell proliferation, but also increase the phagocytosis activity and the enzymatic activity of acid phosphatase [77].
2.3. Sterols
Sterols components of fungus have important physiological function; they also have a variety of biological activities at the same time. So studying sterols has important theoretical significance and application prospects.
2.3.1. Ergosterol
Ergosterol (Figure 5) is a characteristic of fungi sterol and an important source of vitamin D2 [78]. Ergosterol did not get enough attention in the study of C. sinensis although it is a characteristic of fungi sterol [79]. Y. H. Li and X. L. Li determined the content of ergosterol in Cordyceps sinensis with HPLC method and obtained a high yield. They also presented that ergosterol existed in free and combined states [80]. It is important that ergosterol is a food, feed, and pharmaceutical raw material. In addition, it is an important raw material in the production of steroid hormone drugs [81]. Zheng et al. have proved the cytotoxicity and antimicrobial activity of ergosterol; it possesses weak cytotoxicity against HL-60 and BEL-7402 cell lines and moderate antimicrobial activity against the bacteria E. aerogenes and P. aeruginosa and the fungus C. albicans [82]. At present, the ergosterol biosynthesis pathway research has made great progress which will provide theoretical guidance to get high yield strains by genetic engineering [83].
Figure 5.
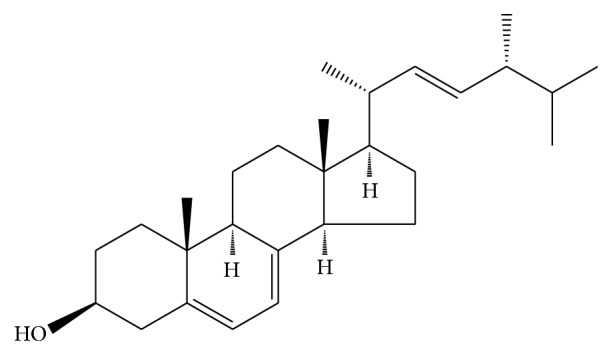
The structure of ergosterol.
2.3.2. H1-A
H1-A (Figure 6), a pure compound used in traditional Chinese medicine, has been isolated from C. sinensis. To clarify the pharmacologic properties of H1-A, a series of researches studied its effect on mesangial cell proliferation, cytotoxicity, cell cycles, and apoptosis. These findings suggest that H1-A modulates some subcellular signal transduction pathways and changes the balance between proliferation and apoptosis of mesangial cells in vitro or in vivo. H1-A may be effective in the management of autoimmune disorders, and the modulation of the signal transduction proteins Bcl-2 and Bcl-XL may represent a target for future pharmacologic interventions [84]. As early as four years ago, they have reported the effect of H1-A on inhibiting autoimmune disease in MRL lpr/lpr mice. Additionally, the structure has been analyzed with NMR by Yang et al., as shown in Figure 6. It is like an ergosterol and has been proved to be without glucocorticosteroid receptor binding ability. From another point of view, H1-A is a kind of ergosterol and its structure looks like testosterone and dehydroepiandrosterone [85]. Meanwhile, H1-A can suppress the activated HMC and alleviate IgAN (Berger's disease) with clinical and histologic improvement. Lin et al. predicted that H1-A as a therapeutic regimen might be used in the future [86].
Figure 6.
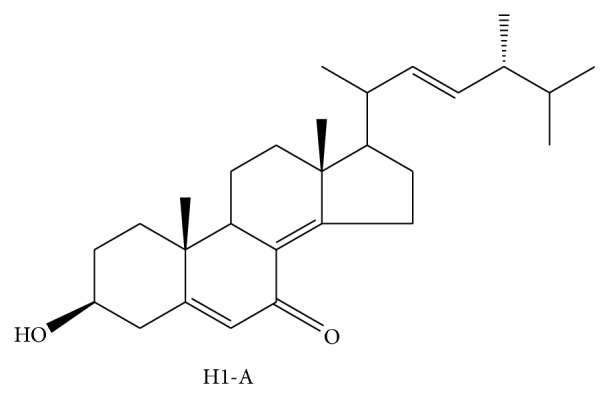
Chemical structure of compound H1-A.
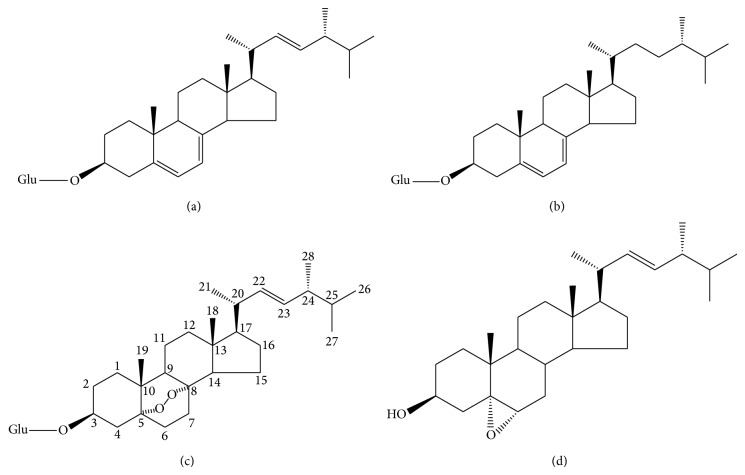
2.3.3. Other Sterols
Two identified compounds, ergosteryl-3-O-β-D-glucopyranoside (a) and 22,23-dihydroergosteryl-3-O-β-D-glucopyranoside (b), were isolated during the fractionation of the methanol extract of C. sinensis [87]. Besides, 5α,8α-epidioxy-24(R)-methylcholesta-6,22-dien-3β-D-glucopyranoside (c) and 5α,6α-epoxy-24(R)-methylcholesta-7,22-dien-3β-ol (d) are two glycoside derivatives of a sterol and they also exist in the methanol extract of C. sinensis [87, 88]. These are the other four important sterol compounds (Figure 7), which are sterol derivatives, and their structure as shown in Figure 7. Moreover, Matsuda et al. have confirmed through a large number of experiments that the latter two have the anticancer activity, but the first two do not [88]. The glycosylated form of ergosterol peroxide was found to be a greater inhibitor to the proliferation of K562, Jurkat, WM-1341, HL-60, and RPMI-8226 tumor cell lines [88].
Figure 7.
Four important sterol compounds.
2.4. Protein
Most of the proteins in C. sinensis are enzymes, including the intracellular proteases and extracellular proteases.
2.4.1. CSDNase
A new acid deoxyribonuclease (DNase) that acted at an acidic pH without divalent ions was extracted from C. sinensis and designated CSDNase. Although acid DNase was first studied biochemically in the 1960s [89], its structure has not been elucidated. DNases may be broadly divided into two classes: DNase I and DNase II. CSDNase belongs to the latter. The protein was purified by (NH4)2SO4 precipitation and a series of chromatographic separations. It was found to be single-chained with an apparent molecular mass of 34 kDa and act on both dsDNA and ssDNA as a deoxyribonuclease but act preferentially on dsDNA. The activity of CSDNase was primarily expressed during fungal mycelium growth and it was an endocellular enzyme [90]. Furthermore, CSDNase was an endonuclease, which was found to hydrolyze DNA and to generate 3-phosphate and 5-OH termini. These results indicated that the nucleolytic properties of CSDNase were essentially the same as those of other well characterized acid DNases.
2.4.2. CSP
A novel serine protease with fibrinolytic activity named CSP was purified from the culture supernatant of the fungus Cordyceps sinensis. CSP is a single polypeptide chain with an apparent molecular weight of 31 kD. It is a novel extracellular protease with a free cysteine residue near the active site. It also can hydrolyse bovine serum albumin (BSA) and human serum albumin (HSA) to a lesser extent. Li et al. have found that CSP was a plasmin-link protease but not a plasminogen activator, and it preferentially cleaved the Aα chain of fibrinogen and the α-chain of fibrin. In conclusion, the presence of CSP possibly linked C. sinensis to its pharmacological use for cardiovascular disease, which will provide a new insight into the protein engineering of new thrombolytic agents [91].
2.5. Amino Acid and Polypeptide
C. sinensis contains many amino acids and polypeptides, which played an important role in clinical trials. For example, some polypeptide macromolecule in C. sinensis could significantly reduce the mean arterial pressure of rats and induce a direct endothelium-dependent vasorelaxant effect by stimulating the production of nitric oxide and endothelium-derived hyperpolarizing factor [92]. Thus, it could be used for the treatment of hypertension. As a result, it is necessary to explore the pharmacological effects of amino acid and polypeptide in C. sinensis.
2.5.1. Cordymin
Cordymin is a peptide from the medicinal mushroom Cordyceps sinensis with the putative beneficial effect on diabetic osteopenia in diabetic rats. The relationship between diabetes and osteoporosis is widely studied [93, 94]. However, the mechanism of cordymin for the treatment of diabetic osteopenia is complicated. To sum up, the significant effect of cordymin on diabetic osteopenia might be directly through weakening ALP and TRAP activity and mediately through recovery of β cells and lowering the concentration of serum glucose, which subsequently triggered a lower extent of oxidative stress in diabetic rats [95]. All those findings indicated a major breakthrough for the treatment of diabetic osteopenia using monomer composition—cordymin.
2.5.2. Cordycedipeptide A
A new cyclodipeptide named cordycedipeptide A was isolated from the culture liquid of Cordyceps sinensis (Figure 8). Its structure was elucidated as 3-acetamino-6-isobutyl-2,5-dioxopiperazine. Jia et al. have reported the cytotoxic activities of the constituent to L-929, A375, and Hela and its better effect on several tumor cell lines [96]; another pharmacological action of cordycedipeptide A remains to be further researched however.
Figure 8.
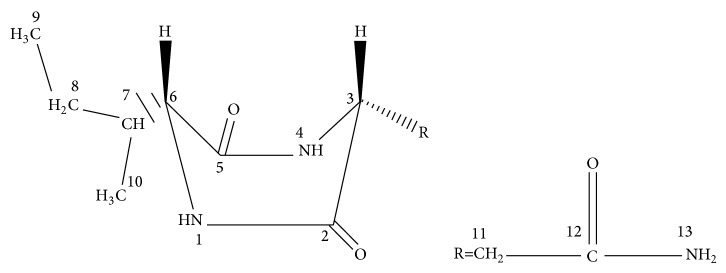
The structure of cordycedipeptide A.
2.5.3. Cordyceamides A and B
Two new aurantiamides named as cordyceamides A and B were isolated from the culture liquid of Cordyceps sinensis (Figure 9). Their structures were elucidated as N-benzoyl-L-tyrosinyl-L-phenylalaninol acetate and N-benzoyl-L-tyrosinyl-L-p-hydroxyphenylalaninol acetate by NMR techniques. Previous studies suggested that both Cordyceamides A and B had cytotoxic effects on L929, A375 and Hela cell lines. A showed better effect than B on L929 cell and A375 cell, but on Hela cell B showed better effect [97].
Figure 9.
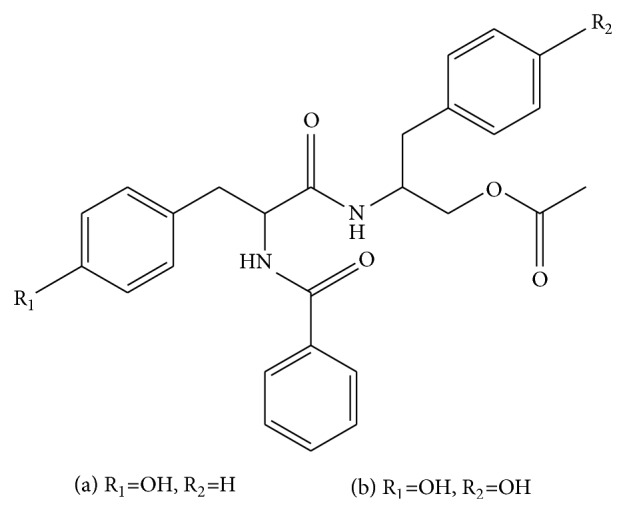
The structure of (a) and (b). (a) Cordyceamides A. (b) Cordyceamides B.
2.5.4. Tryptophan
There are 18 kinds of amino acids in C. sinensis and they mainly played a sedative hypnotic effect. Tryptophan is the most effective ingredient among them. Tryptophan is the precursor of serotonin material, which has close relationship with animals' insomnia [98]. Otherwise, glutamic acid has the effect of immune inhibition. Due to the performance of the combined effect being more complex, more research needs to be explored.
2.6. Others
C. sinensis contains a lot of D-mannitol, also known as cordycepic acid, and the content in insect body is higher than that in stroma. Its structure has been determined as 1,3,4,5-tetrahydroxy-cyclohexanoic acid, isomeric with quinic acid. It differs mainly from the natural quinic acid in being dextrorotatory and not forming the lactone [99]. Cordycepic acid played a significant role in treating liver fibrosis of hepatic stellate cells. Liver fibrosis is a variety of factors involved in complex process [100]. Cordycepic acid ameliorates the LPs-induced inflammatory phenotype and TGFβ1-induced fibrogenic response of cultured HSCs, which are the drug's therapeutic mechanisms to inhibit and resolve liver fibrosis [101]. Additionally, cordycepic acid in C. sinensis has effects on diuretic, improving the plasma osmotic pressure and anti-free radical according to pharmacological research and clinical reports [102, 103]. So it is regarded as one of the active ingredients in C. sinensis.
A new monosaccharide saponin, whose structure is 3-O-glucopyranoside, was isolated and identified from the mycelia of C. sinensis. It displayed very good antitumor activity, but the content of the mycelia was very low [104].
Moreover, there are many other ingredients in the fungi C. sinensis, for example, alkane, polyamine, vitamin, and microelement. Each of these has its own pharmacological effects. More constituents need to be separated and studied in the future research work, however.
3. Conclusion
C. sinensis, a macro fungus of biomedical importance, contains a number of bioactive components (Table 1). Many of them are biological response modifiers which activate our immune systems for a multitude of defensive functions. The immunomodulating effects are associated with its antitumour activity, which is the most proverbial effect of C. sinensis. Many ingredients in C. sinensis have the antitumour activity as shown above, such as cordycepin, adenosine, EPSF, cordyglucans, and monosaccharide saponins. As investigation into this fungus continues, more bioactive constituents with potential therapeutic value will be isolated. However, new methods and technologies need to be adopted to extract and analyse the components, requiring evaluation along the modern scientific line. Overall, so far, we know only a little of the wonders of this creature and it still has many secrets for us to discover. More research is needed on the herbal medicine and its related species.
Table 1.
Chemical constituents and their corresponding pharmacological actions of C. sinensis.
| Chemical constituents of C. sinensis | Pharmacological effects | References |
|---|---|---|
| Cordycepin | Anti-inflammatory effect | [17, 21–30] |
| Analgesic effect | ||
| Stimulates steroidogenesis | ||
| Enhances immunity | ||
| Antitumor activity | ||
| Antibacteria, antivirus, and insecticidal activities | ||
|
| ||
| Adenosine | Anticonvulsant activity | [20, 31–46] |
| Inhibits cancer cell growth | ||
| Anti-inflammatory effect | ||
|
| ||
| EPSF | Immunomodulatory effect | [60–65] |
| Antitumour effect | ||
| Antioxidant effect | ||
|
| ||
| APS | Antioxidant effect | [66–69] |
| Immunomodulatory effects | ||
|
| ||
| CPS-1 | Antioxidant effect | [70, 71] |
|
| ||
| CPS-2 | Inhibits cell proliferation | [72, 73] |
|
| ||
| Mannoglucan | Cytotoxicity activity | [74] |
|
| ||
| CME-1 | Antioxidant effect | [75] |
|
| ||
| Cordyglucans | Antitumour effect | [76] |
|
| ||
| CS-F10 | Hypoglycemic activity | [55, 77] |
|
| ||
| Cordysinocan | Induces cell proliferation | [77] |
|
| ||
| Ergosterol | Cytotoxicity | [78–83] |
| Antimicrobial activity | ||
|
| ||
| H1-A | Immunoregulation | [84–86] |
|
| ||
| CSDNase | Hydrolyzes DNA | [89, 90] |
| Nucleolytic properties | ||
|
| ||
| CSP | Fibrinolytic activity | [91] |
|
| ||
| Cordymin | Antidiabetic | [93–95] |
|
| ||
| Tryptophan | Sedative hypnotic effect | [98] |
|
| ||
| Cordycepic acid | Treating liver fibrosis diuretic | [99–103] |
| Improving the plasma osmotic pressure | ||
| Anti-free radical | ||
|
| ||
| Monosaccharide saponins | Antitumor activity | [104] |
Acknowledgments
This work was supported by the Foundation of Jinan Science and Technology Development Program (201303055), Project of Shandong Province Higher Educational Science and Technology Program (J14LK61), and National Natural Science Foundation of China (81373780) and (81373968).
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Xiao J. H., Qi Y., Xiong Q. Nucleosides, a valuable chemical marker for quality control in traditional Chinese medicine Cordyceps . Recent Patents on Biotechnology. 2013;7(2):153–166. doi: 10.2174/1872208311307020007. [DOI] [PubMed] [Google Scholar]
- 2.Ng T. B., Wang H. X. Pharmacological actions of Cordyceps, a prized folk medicine. The Journal of Pharmacy and Pharmacology. 2005;57(12):1509–1519. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha B., Sung J.-M. Notes on Cordyceps species collected from the central region of Nepal. Mycobiology. 2005;33(4):235–239. doi: 10.4489/myco.2005.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong K. L., So E. C., Chen C. C., Wu R. S. C., Huang B.-M. Regulation of steroidogenesis by Cordyceps sinensis mycelium extracted fractions with (hCG) treatment in mouse Leydig cells. Archives of Andrology. 2007;53(2):75–77. doi: 10.1080/01485010600915236. [DOI] [PubMed] [Google Scholar]
- 5.Yu H. M., Wang B.-S., Huang S. C., Duh P.-D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. Journal of Agricultural and Food Chemistry. 2006;54(8):3132–3138. doi: 10.1021/jf053111w. [DOI] [PubMed] [Google Scholar]
- 6.Yue K., Ye M., Zhou Z., Sun W., Lin X. The genus Cordyceps: a chemical and pharmacological review. Journal of Pharmacy and Pharmacology. 2013;65(4):474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 7.Tuli H. S., Sharma A. K., Sandhu S. S., Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sciences. 2013;93(23):863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Zhong F., Liu X., Zhou Q., et al. 1H NMR spectroscopy analysis of metabolites in the kidneys provides new insight into pathophysiological mechanisms: applications for treatment with Cordyceps sinensis . Nephrology Dialysis Transplantation. 2012;27(2):556–565. doi: 10.1093/ndt/gfr368. [DOI] [PubMed] [Google Scholar]
- 9.Kuo C.-F., Chen C.-C., Lin C.-F., et al. Abrogation of streptococcal pyrogenic exotoxin B-mediated suppression of phagocytosis in U937 cells by Cordyceps sinensis mycelium via production of cytokines. Food and Chemical Toxicology. 2007;45(2):278–285. doi: 10.1016/j.fct.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.-C., Huang Y.-L., Huang B.-M. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. The International Journal of Biochemistry and Cell Biology. 2005;37(1):214–223. doi: 10.1016/j.biocel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi N., Yoshida J., Ren L. J., et al. Augmentation of various immune reactivities of tumor-bearing hosts with an extract of Cordyceps sinensis . Biotherapy. 1990;2(3):199–205. doi: 10.1007/bf02173520. [DOI] [PubMed] [Google Scholar]
- 12.Lo H.-C., Hsu T.-H., Tu S.-T., Lin K.-C. Anti-hyperglycemic activity of natural and fermented Cordyceps sinensis in rats with diabetes induced by nicotinamide and streptozotocin. The American Journal of Chinese Medicine. 2006;34(5):819–832. doi: 10.1142/s0192415x06004314. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K., Konoha K., Yamaguchi Y., Kagota S., Shinozuka K., Kunitomo M. Combined effects of Cordyceps sinensis and methotrexate on hematogenic lung metastasis in mice. Receptors and Channels. 2003;9(5):329–334. doi: 10.3109/713745176. [DOI] [PubMed] [Google Scholar]
- 14.Jordan J. L., Hirsch G. M., Lee T. D. G. C. sinensis ablates allograft vasculopathy when used as an adjuvant therapy with cyclosporin A. Transplant Immunology. 2008;19(3-4):159–166. doi: 10.1016/j.trim.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda R., Nishimura M., Sun Y., Wada M., Nakashima K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomedical Chromatography. 2008;22(6):630–636. doi: 10.1002/bmc.980. [DOI] [PubMed] [Google Scholar]
- 16.Guan J., Zhao J., Feng K., Hu D.-J., Li S.-P. Comparison and characterization of polysaccharides from natural and cultured Cordyceps using saccharide mapping. Analytical and Bioanalytical Chemistry. 2011;399(10):3465–3474. doi: 10.1007/s00216-010-4396-y. [DOI] [PubMed] [Google Scholar]
- 17.Yang F. Q., Li D. Q., Feng K., Hu D. J., Li S. P. Determination of nucleotides, nucleosides and their transformation products in Cordyceps by ion-pairing reversed-phase liquid chromatography-mass spectrometry. Journal of Chromatography A. 2010;1217(34):5501–5510. doi: 10.1016/j.chroma.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 18.Furuya T., Hirotani M., Matsuzawa M. A study on the composition of Cordyceps militaris extract and mycelium. Phytochemistry. 1983;22:2509–2512. doi: 10.1016/0031-9422(83)80150-2. [DOI] [Google Scholar]
- 19.Dong C.-H., Yao Y.-J. Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture. Journal of Applied Microbiology. 2005;99(3):483–492. doi: 10.1111/j.1365-2672.2005.02640.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang F. Q., Guan J., Li S. P. Fast simultaneous determination of 14 nucleosides and nucleobases in cultured Cordyceps using ultra-performance liquid chromatography. Talanta. 2007;73(2):269–273. doi: 10.1016/j.talanta.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Paterson R. R. M. Cordyceps—a traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008;69(7):1469–1495. doi: 10.1016/j.phytochem.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda R., Nishimura M., Sun Y., Wada M., Nakashima K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomedical Chromatography. 2008;22(6):630–636. doi: 10.1002/bmc.980. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Liu Y.-M., Cao W., Yao K.-W., Liu Z.-Q., Guo J.-Y. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metabolic Brain Disease. 2012;27(2):159–165. doi: 10.1007/s11011-012-9282-1. [DOI] [PubMed] [Google Scholar]
- 24.Ying X. Z., Peng L., Chen H., Shen Y., Yu K., Cheng S. Cordycepin prevented IL-β-induced expression of inflammatory mediators in human osteoarthritis chondrocytes. International Orthopaedics. 2014;38(7):1519–1526. doi: 10.1007/s00264-013-2219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park E.-S., Kang D.-H., Yang M.-K., et al. Cordycepin, 3′-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovascular Toxicology. 2014;14(1):1–9. doi: 10.1007/s12012-013-9232-0. [DOI] [PubMed] [Google Scholar]
- 26.Qian G.-M., Pan G.-F., Guo J.-Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis . Natural Product Research. 2012;26(24):2358–2362. doi: 10.1080/14786419.2012.658800. [DOI] [PubMed] [Google Scholar]
- 27.Pan B.-S., Lin C.-Y., Huang B.-M. The effect of cordycepin on steroidogenesis and apoptosis in MA-10 mouse Leydig tumor cells. Evidence-Based Complementary and Alternative Medicine. 2011;2011:14. doi: 10.1155/2011/750468.750468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leu S.-F., Poon S. L., Pao H.-Y., Huang B.-M. The in vivo and in vitro stimulatory effects of cordycepin on mouse Leydig cell steroidogenesis. Bioscience, Biotechnology and Biochemistry. 2011;75(4):723–731. doi: 10.1271/bbb.100853. [DOI] [PubMed] [Google Scholar]
- 29.Pao H. Y., Pan B. S., Leu S. F., Huang B. M. Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor cells through the protein kinase C pathway. Journal of Agricultural and Food Chemistry. 2012;60(19):4905–4913. doi: 10.1021/jf205091b. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X. X., Luo L. P., Dressel W. K., et al. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis . The American Journal of Chinese Medicine. 2008;36(5):967–980. doi: 10.1142/s0192415x08006387. [DOI] [PubMed] [Google Scholar]
- 31.Yang F. Q., Li S. P., Li P., Wang Y. T. Optimization of CEC for simultaneous determination of eleven nucleosides and nucleobases in Cordyceps using central composite design. Electrophoresis. 2007;28(11):1681–1688. doi: 10.1002/elps.200600416. [DOI] [PubMed] [Google Scholar]
- 32.Yang F.-Q., Ge L., Yong J. W. H., Tan S. N., Li S.-P. Determination of nucleosides and nucleobases in different species of Cordyceps by capillary electrophoresis-mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2009;50(3):307–314. doi: 10.1016/j.jpba.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Huang L.-F., Liang Y.-Z., Guo F.-Q., Zhou Z.-F., Cheng B.-M. Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militarris by LC/ESI-MS. Journal of Pharmaceutical and Biomedical Analysis. 2003;33(5):1155–1162. doi: 10.1016/s0731-7085(03)00415-1. [DOI] [PubMed] [Google Scholar]
- 34.Yang F. Q., Li S. P. Effects of sample preparation methods on the quantification of nucleosides in natural and cultured Cordyceps . Journal of Pharmaceutical and Biomedical Analysis. 2008;48(1):231–235. doi: 10.1016/j.jpba.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Kitakaze M., Hori M. Adenosine therapy: a new approach to chronic heart failure. Expert Opinion on Investigational Drugs. 2000;9(11):2519–2535. doi: 10.1517/13543784.9.11.2519. [DOI] [PubMed] [Google Scholar]
- 36.Nakav S., Chaimovitz C., Sufaro Y., et al. Anti-inflammatory preconditioning by agonists of adenosine A1 receptor. PLoS ONE. 2008;3(5) doi: 10.1371/journal.pone.0002107.e2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manfredi J. P., Sparks H. V., Jr. Adenosine's role in coronary vasodilation induced by atrial pacing and norepinephrine. The American Journal of Physiology—Endocrinology and Metabolism. 1982;243(4):H536–H545. doi: 10.1152/ajpheart.1982.243.4.H536. [DOI] [PubMed] [Google Scholar]
- 38.Ontyd J., Schrader J. Measurement of adenosine, inosine, and hypoxanthine in human plasma. Journal of Chromatography. 1984;307(2):404–409. doi: 10.1016/s0378-4347(00)84113-4. [DOI] [PubMed] [Google Scholar]
- 39.Tsai Y.-J., Lin L.-C., Tsai T.-H. Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of Cordyceps sinensis in rat. Journal of Agricultural and Food Chemistry. 2010;58(8):4638–4643. doi: 10.1021/jf100269g. [DOI] [PubMed] [Google Scholar]
- 40.Yang D., Yaguchi T., Yamamoto H., Nishizaki T. Intracellularly transported adenosine induces apoptosis in HuH-7 human hepatoma cells by downregulating c-FLIP expression causing caspase-3/-8 activation. Biochemical Pharmacology. 2007;73(10):1665–1675. doi: 10.1016/j.bcp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Yang D., Yaguchi T., Lim C.-R., Ishizawa Y., Nakano T., Nishizaki T. Tuning of apoptosis-mediator gene transcription in HepG2 human hepatoma cells through an adenosine signal. Cancer Letters. 2010;291(2):225–229. doi: 10.1016/j.canlet.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y. F., Zhang J., Zhang Q., et al. Adenosine induces apoptosis in human liver cancer cells through ROS production and mitochondrial dysfunction. Biochemical and Biophysical Research Communications. 2014;448(1):8–14. doi: 10.1016/j.bbrc.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Iannone R., Miele L., Maiolino P., Pinto A., Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. American Journal of Cancer Research. 2014;4(2):172–181. [PMC free article] [PubMed] [Google Scholar]
- 44.Gessi S., Merighi S., Borea P. A. Targeting adenosine receptors to prevent inflammatory skin diseases. Experimental Dermatology. 2014;23(8):553–554. doi: 10.1111/exd.12474. [DOI] [PubMed] [Google Scholar]
- 45.Coelho J. E., Alves P., Canas P. M., et al. Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Frontiers in Psychiatry. 2014;5, article 67 doi: 10.3389/fpsyt.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan H., Yang F. Q., Li S. P. Determination of purine and pyrimidine bases in natural and cultured Cordyceps using optimum acid hydrolysis followed by high performance liquid chromatography. Journal of Pharmaceutical and Biomedical Analysis. 2007;45(1):141–144. doi: 10.1016/j.jpba.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 47.Struck-Lewicka W., Kaliszan R., Markuszewski M. J. Analysis of urinary nucleosides as potential cancer markers determined using LC-MS technique. Journal of Pharmaceutical and Biomedical Analysis. 2014;101:50–57. doi: 10.1016/j.jpba.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Li S. P. The nucleosides contents and their variation in natural Cordyceps sinensis and cultured Cordyceps mycelia. Journal of Chinese Pharmaceutical Sciences. 2001;10:175–179. [Google Scholar]
- 49.Zhou X., Gong Z., Su Y., Lin J., Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. Journal of Pharmacy and Pharmacology. 2009;61(3):279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 50.Li S. P., Li P., Dong T. T. X., Tsim K. W. K. Anti-oxidation activity of different types of natural Cordyceps sinensis and cultured Cordyceps mycelia . Phytomedicine. 2001;8(3):207–212. doi: 10.1078/0944-7113-00030. [DOI] [PubMed] [Google Scholar]
- 51.Li S. P., Su Z. R., Dong T. T. X., Tsim K. W. K. The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine. 2002;9(4):319–324. doi: 10.1078/0944-7113-00134. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y.-J., Shiao M.-S., Lee S.-S., Wang S.-Y. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sciences. 1997;60(25):2349–2359. doi: 10.1016/s0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- 53.Ohta Y., Lee J.-B., Hayashi K., Fujita A., Dong K. P., Hayashi T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. Journal of Agricultural and Food Chemistry. 2007;55(25):10194–10199. doi: 10.1021/jf0721287. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura K., Yamaguchi Y., Kagota S., Shinozuka K., Kunitomo M. Activation of in vivo Kupffer cell function by oral administration of Cordyceps sinensis in rats. The Japanese Journal of Pharmacology. 1999;79(4):505–508. doi: 10.1254/jjp.79.505. [DOI] [PubMed] [Google Scholar]
- 55.Kiho T., Ookubo K., Usui S., Ukai S., Hirano K. Structural features and hypoglycemic activity of a polysaccharide (CS- F10) from the cultured mycelium of Cordyceps sinensis . Biological and Pharmaceutical Bulletin. 1999;22(9):966–970. doi: 10.1248/bpb.22.966. [DOI] [PubMed] [Google Scholar]
- 56.Koh J.-H., Kim J.-M., Chang U.-J., Suh H.-J. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis . Biological and Pharmaceutical Bulletin. 2003;26(1):84–87. doi: 10.1248/bpb.26.84. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X., Gong Z., Su Y., Lin J., Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. Journal of Pharmacy and Pharmacology. 2009;61(3):279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 58.Guan J., Yang F.-Q., Li S.-P. Evaluation of carbohydrates in natural and cultured Cordyceps by pressurized liquid extraction and gas chromatography coupled with mass spectrometry. Molecules. 2010;15(6):4227–4241. doi: 10.3390/molecules15064227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J., Zhang W., Lu T., Li J., Zheng Y., Kong L. Morphological and genetic characterization of a cultivated Cordyceps sinensis fungus and its polysaccharide component possessing antioxidant property in H22 tumor-bearing mice. Life Sciences. 2006;78(23):2742–2748. doi: 10.1016/j.lfs.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W., Li J., Qiu S., Chen J., Zheng Y. Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia. 2008;79(3):168–173. doi: 10.1016/j.fitote.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Sheng L., Chen J., Li J., Zhang W. An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Applied Biochemistry and Biotechnology. 2011;163(5):669–678. doi: 10.1007/s12010-010-9072-3. [DOI] [PubMed] [Google Scholar]
- 62.Movassagh M., Spatz A., Davoust J., et al. Selective accumulation of mature DC-Lamp + dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Research. 2004;64(6):2192–2198. doi: 10.1158/0008-5472.can-03-2969. [DOI] [PubMed] [Google Scholar]
- 63.Song D., Lin J. Y., Yuan F. J., Zhang W. Ex vivo stimulation of murine dendritic cells by an exopolysaccharide from one of the anamorph of Cordyceps sinensis . Cell Biochemistry and Function. 2011;29(7):555–561. doi: 10.1002/cbf.1787. [DOI] [PubMed] [Google Scholar]
- 64.Yang J., Zhang W., Shi P., Chen J., Han X., Wang Y. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathology Research and Practice. 2005;201(11):745–750. doi: 10.1016/j.prp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Yan J. K., Wang W. Q., Ma H. L., Wu J. Y. Sulfation and enhanced antioxidant capacity of an exopolysaccharide produced by the medicinal fungus Cordyceps sinensis . Molecules. 2013;18(1):167–177. doi: 10.3390/molecules18010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen W. B., Song D., Wu J. Y., Zhang W. Protective effect of a polysaccharide isolated from a cultivated Cordyceps mycelia on hydrogen peroxide-induced oxidative damage in PC12 cells. Phytotherapy Research. 2011;25(5):675–680. doi: 10.1002/ptr.3320. [DOI] [PubMed] [Google Scholar]
- 67.Li S. P., Zhao K. J., Ji Z. N., et al. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sciences. 2003;73(19):2503–2513. doi: 10.1016/s0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- 68.Chen W., Zhang W., Shen W., Wang K. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cellular Immunology. 2010;262(1):69–74. doi: 10.1016/j.cellimm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Chen W., Yuan F., Wang K., Song D., Zhang W. Modulatory effects of the acid polysaccharide fraction from one of anamorph of Cordyceps sinensis on Ana-1 cells. Journal of Ethnopharmacology. 2012;142(3):739–745. doi: 10.1016/j.jep.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Wang M., Ling Y., Fan W., Yin H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis . The American Journal of Chinese Medicine. 2009;37(5):977–989. doi: 10.1142/s0192415x09007387. [DOI] [PubMed] [Google Scholar]
- 71.Li S. P., Zhang G. H., Zeng Q., et al. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordycepsmycelia. Phytomedicine. 2006;13(6):428–433. doi: 10.1016/j.phymed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Yin H., Lv X., Gao H., Wang M. Protection of chronic renal failure by a polysaccharide from Cordyceps sinensis . Fitoterapia. 2010;81(5):397–402. doi: 10.1016/j.fitote.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y., Liu D., Zhao H., et al. Cordyceps sinensis polysaccharide CPS-2 protects human mesangial cells from PDGF-BB-induced proliferation through the PDGF/ERK and TGF-β1/Smad pathways. Molecular and Cellular Endocrinology. 2014;382(2):979–988. doi: 10.1016/j.mce.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y. L., Hu N., Pan Y. J., Zhou L., Zhou X. Isolation and characterization of a mannoglucan from edible Cordyceps sinensis mycelium. Carbohydrate Research. 2007;342(6):870–875. doi: 10.1016/j.carres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Wang S.-H., Yang W.-B., Liu Y.-C., et al. A potent sphingomyelinase inhibitor from Cordyceps mycelia contributes its cytoprotective effect against oxidative stress in macrophages. Journal of Lipid Research. 2011;52(3):471–479. doi: 10.1194/jlr.m011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yalin W., Ishurd O., Cuirong S., Yuanjiang P. Structure analysis and antitumor activity of (1→3)-β-D-glucans (cordyglucans) from the mycelia of Cordyceps sinensis . Planta Medica. 2005;71(4):381–384. doi: 10.1055/s-2005-864111. [DOI] [PubMed] [Google Scholar]
- 77.Cheung J. K. H., Li J., Cheung A. W. H., et al. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: signaling cascade and induction of cytokines. Journal of Ethnopharmacology. 2009;124(1):61–68. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 78.Seitz L. M. Ergosterol as a measure of fungal growth. Phytopathology. 1979;69(11):1202–1206. doi: 10.1094/phyto-69-1202. [DOI] [Google Scholar]
- 79.Osswald W. F., Holl W., Elstner E. F. Ergosterol as a biochemical indicator of fungal infection in spruce and fir needles from different sources. Zeitschrift für Naturforschung. 1986;41:542–544. [Google Scholar]
- 80.Li Y. H., Li X. L. Determination of ergosterol in Cordyceps sinensis and Cordycepsblack-bone chicken capsules by HPLC. Acta Pharmaceutica Sinica. 1991;26(10):768–771. [PubMed] [Google Scholar]
- 81.Kitchawalit S., Kanokmedhakul K., Kanokmedhakul S., Soytong K. A new benzyl ester and ergosterol derivatives from the fungus Gymnoascus reessii . Natural Product Research. 2014;28(14):1045–1051. doi: 10.1080/14786419.2014.903478. [DOI] [PubMed] [Google Scholar]
- 82.Zheng J., Wang Y., Wang J. F., Liu P., Li J., Zhu W. Antimicrobial ergosteroids and pyrrole derivatives from halotolerant Aspergillus flocculosus PT05-1 cultured in a hypersaline medium. Extremophiles. 2013;17(6):963–971. doi: 10.1007/s00792-013-0578-9. [DOI] [PubMed] [Google Scholar]
- 83.Rajput S. B., Karuppayil S. M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans . SpringerPlus. 2013;2(1) article 26 doi: 10.1186/2193-1801-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L.-Y., Huang W. J., Hsieh H.-G., Lin C.-Y. H1-A extracted from Cordyceps sinensis suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. Journal of Laboratory and Clinical Medicine. 2003;141(1):74–83. doi: 10.1067/mlc.2003.6. [DOI] [PubMed] [Google Scholar]
- 85.Yang L.-Y., Chen A., Kuo Y.-C., Lin C.-Y. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. Journal of Laboratory and Clinical Medicine. 1999;134(5):492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 86.Lin C.-Y., Ku F.-M., Kuo Y.-C., et al. Inhibition of activated human mesangial cell proliferation by the natural product of Cordyceps sinensis (H1-A): an implication for treatment of IgA mesangial nephropathy. Journal of Laboratory and Clinical Medicine. 1999;133(1):55–63. doi: 10.1053/lc.1999.v133.a94239. [DOI] [PubMed] [Google Scholar]
- 87.Bok J. W., Lermer L., Chilton J., Klingeman H. G., Towers G. H. N. Antitumor sterols from the mycelia of Cordyceps sinensis . Phytochemistry. 1999;51(7):891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 88.Matsuda H., Akaki J., Nakamura S., et al. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of Cordyceps sinensis . Chemical and Pharmaceutical Bulletin. 2009;57(4):411–414. doi: 10.1248/cpb.57.411. [DOI] [PubMed] [Google Scholar]
- 89.Bernardi G., Appella E., Zito R. Studies on acid deoxyribonuclease. III. Physical and chemical properties of hog spleen acid deoxyribonuclease. Biochemistry. 1965;4(9):1725–1729. doi: 10.1021/bi00885a006. [DOI] [Google Scholar]
- 90.Ye M. Q., Hu Z., Fan Y., He L., Xia F., Zou G. Purification and characterization of an acid deoxyribonuclease from the cultured mycelia of Cordyceps sinensis . Journal of Biochemistry and Molecular Biology. 2004;37(4):466–473. doi: 10.5483/bmbrep.2004.37.4.466. [DOI] [PubMed] [Google Scholar]
- 91.Li H.-P., Hu Z., Yuan J.-L., et al. A novel extracellular protease with fibrinolytic activity from the culture supernatant of Cordyceps sinensis: purification and characterization. Phytotherapy Research. 2007;21(12):1234–1241. doi: 10.1002/ptr.2246. [DOI] [PubMed] [Google Scholar]
- 92.Chiou W.-F., Chang P.-C., Chou C.-J., Chen C.-F. Protein constituent contributes to the hypotensive and vasorelaxant activities of Cordyceps sinensis . Life Sciences. 2000;66(14):1369–1376. doi: 10.1016/s0024-3205(00)00445-8. [DOI] [PubMed] [Google Scholar]
- 93.Vestergaard P., Rejnmark L., Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcified Tissue International. 2009;84(1):45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed L. A., Joakimsen R. M., Berntsen G. K., Fønnebø V., Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromsø study. Osteoporosis International. 2006;17(4):495–500. doi: 10.1007/s00198-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 95.Qi W., Zhang Y., Yan Y.-B., et al. The protective effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, on diabetic osteopenia in alloxan-induced diabetic rats. Evidence-based Complementary and Alternative Medicine. 2013;2013:6. doi: 10.1155/2013/985636.985636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jia J.-M., Ma X.-C., Wu C.-F., Wu L.-J., Hu G.-S. Cordycedipeptide A, a new cyclodipeptide from the culture liquid of Cordyceps sinensis (BERK.) SACC. Chemical and Pharmaceutical Bulletin. 2005;53(5):582–583. doi: 10.1248/cpb.53.582. [DOI] [PubMed] [Google Scholar]
- 97.Jia J.-M., Tao H.-H., Feng B.-M. Cordyceamides A and B from the culture liquid of Cordyceps sinensis (Berk.) sacc. Chemical and Pharmaceutical Bulletin. 2009;57(1):99–101. doi: 10.1248/cpb.57.99. [DOI] [PubMed] [Google Scholar]
- 98.Zhang S. S., Zhang D. S., Zhu T. J., Chen X. Y. A pharmacological analysis of the amino acid components of Cordyceps sinensis SACC. Acta Pharmaceutica Sinica. 1991;26(5):326–330. [PubMed] [Google Scholar]
- 99.Chatterjee R., Srinivasan K. S., Maiti P. C. Cordyceps sinesis (Berkeley) Saccardo: structure of cordycepic acid. Journal of the American Pharmaceutical Association. 1957;46(2):114–118. doi: 10.1002/jps.3030460211. [DOI] [PubMed] [Google Scholar]
- 100.Guo J., Friedman S. L. Hepatic fibrogenesis. Seminars in Liver Disease. 2007;27(4):413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 101.Ouyang Y. Y., Zhang Z., Cao Y. R., et al. Effect of cordyceps acid and cordycepin on the inflammatory and fibrogenic response of hepatic stellate cells. Chinese Journal of Hepatology. 2013;21(4):275–278. doi: 10.3760/cma.j.issn.1007-3418.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 102.Nomani A. Z., Nabi Z., Rashid H., et al. Osmotic nephrosis with mannitol: review article. Renal Failure. 2014;36(7):1169–1176. doi: 10.3109/0886022x.2014.926758. [DOI] [PubMed] [Google Scholar]
- 103.Sahmeddini M. A., Zahiri S., Khosravi M. B., Ghaffaripour S., Eghbal M. H., Shokrizadeh S. Effect of mannitol on postreperfusion cardiac output and central venous oxygen saturation during orthotopic liver transplant: a double-blind randomized clinical trial. Progress in Transplantation. 2014;24(2):121–125. doi: 10.7182/pit2014483. [DOI] [PubMed] [Google Scholar]
- 104.Zhu Z.-Y., Yao Q., Liu Y., et al. Highly efficient synthesis and antitumor activity of monosaccharide saponins mimicking components of Chinese folk medicine Cordyceps sinensis . Journal of Asian Natural Products Research. 2012;14(5):429–435. doi: 10.1080/10286020.2012.670220. [DOI] [PubMed] [Google Scholar]