Abstract
Muscle contraction results from cyclic interactions between the contractile proteins myosin and actin, driven by the turnover of adenosine triphosphate (ATP). Despite intense studies, several molecular events in the contraction process are poorly understood, including the relationship between force-generation and phosphate-release in the ATP-turnover. Different aspects of the force-generating transition are reflected in the changes in tension development by muscle cells, myofibrils and single molecules upon changes in temperature, altered phosphate concentration, or length perturbations. It has been notoriously difficult to explain all these events within a given theoretical framework and to unequivocally correlate observed events with the atomic structures of the myosin motor. Other incompletely understood issues include the role of the two heads of myosin II and structural changes in the actin filaments as well as the importance of the three-dimensional order. We here review these issues in relation to controversies regarding basic physiological properties of striated muscle. We also briefly consider actomyosin mutation effects in cardiac and skeletal muscle function and the possibility to treat these defects by drugs.
1. Introduction
Muscle contraction is the result of cyclic interactions between the contractile proteins myosin and actin, driven by the turnover of adenosine triphosphate (ATP) [1–8]. In vertebrate striated muscle (heart and skeletal muscle), actin and myosin are organized with several accessory proteins in highly ordered sets of interdigitating thin and thick filaments, respectively, forming repetitive 2.0–2.5 μm long sarcomeres [2]. The functional units of muscle are the half-sarcomeres. These are connected in series to each other forming ~1 μm wide myofibrils (Figures 1(a) and 1(b)) that run the entire length of the muscle cell (muscle fiber) and in parallel over the muscle fiber cross-section. During muscle contraction, globular myosin motor domains (heads) extend from the thick filaments to interact cyclically with actin binding sites on the thin filaments forming so-called cross-bridges (Figure 1(b)). The ordered arrangement on different hierarchical levels in muscles is highly beneficial to the effectiveness of the contractile process which is reflected in the independent evolution [9] of similar sarcomere organizations in phylogenetically distant organisms such as mammalians and Cnidaria (e.g., jellyfish). Some of the advantages of this arrangement are obvious, such as effective summation of length changes produced by sarcomeres arranged in series and forces over the muscle cross-section. However, there are likely additional, subtle benefits and even inbuilt imperfections of the ordered arrangement have been found to be of physiological importance [10–12].
Figure 1.
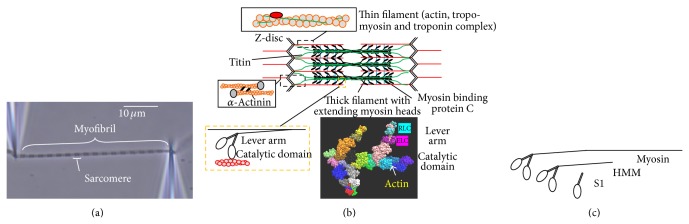
Hierarchical organization of myofibril. (a) Segment of myofibril captured between two microneedles for force measurements or application of length changes. (b) The sarcomere (structure between two Z-discs), with key protein components schematically illustrated. The resting length of the sarcomere is approximately 2.0 μm in the human heart and 2.5 μm in human skeletal muscle. Insets: the thin filament (top); critical molecular arrangement of the Z-disc (middle); extending myosin catalytic domain and lever arm interacting with actin filament (bottom; left) and 4 molecules of myosin subfragment 1 interacting with actin filament in the absence of ATP (PDB 1MQ8; bottom right). Regulatory light chain (RLC) and essential light chain (ELC) stabilize the lever arm. (c) Schematic illustration of myosin molecule (approximately to scale; see also (b)) and soluble motor fragments, heavy meromyosin (HMM) and subfragment 1 (S1) obtained by proteolytic cleavage under different conditions [356, 357].
Generally, there has been formidable progress [6, 13–16] in the understanding of striated muscle function since the elucidation of its basic principles [2, 17–19]. Initially, this progress relied mainly on mechanical and ultrastructural studies of muscle cells and biochemical studies of isolated actin and soluble myosin motor fragments. Key developments in the late eighties and early nineties transformed the field substantially with a shift of focus to a more reductionist approach (reviewed in [13]) and with complementary insights gained from studies of a range of newly discovered nonmuscle myosins. One of the major technical developments in this period was the in vitro motility assay [7, 20] where fluorescent actin filaments are observed [21] while being propelled by myosin or more often proteolytic myosin motor fragments (Figure 1(c)) such as subfragment 1 (S1) or heavy meromyosin (HMM). The latter contains two myosin heads, that is, two complete catalytic domains each with lever arm and two light chains, connected to a coiled-coil tail domain. Other key developments include (i) single molecule mechanics (optical tweezers based [22–24]) and single molecule fluorescence microscopy techniques [25] related to the in vitro motility assays, (ii) crystallization of actin [26] and the myosin motor domain (S1) [5, 27] allowing determination of their molecular structure with atomic resolution, and, finally, (iii) development of techniques for genetic engineering of myosin [28, 29]. While recent technical progress allowed a number of long-standing issues to be settled (cf. [30]), several important features of muscle contraction remain incompletely understood. This includes central issues such as (1) the molecular basis for the high maximum power-output [31, 32], (2) the mechanism of effective resistance to stretch of active muscle [33–35], and (3) the mechanisms by which myopathy mutations [36–43] and certain drugs affect muscle function.
The difficulties in addressing the problems (1)–(3) are due to limited understanding of important molecular mechanisms of the actomyosin interaction. This limitation is, in turn, attributed to challenges when integrating information derived from studies on different levels of hierarchical order (e.g., muscle cell versus single molecules) or using different techniques (e.g., biochemical solution studies versus muscle cell mechanics or single molecule mechanics). In this paper, we will review incompletely understood aspects of the actomyosin interaction. Other general aspects of muscle physiology and regulation are not included—the reader is instead referred to previous comprehensive reviews [15, 44, 45] and references therein.
2. The Molecular Basis of Muscle Contraction: Current View
Molecular motors may be classified as processive or nonprocessive depending on whether they take several steps or only one step along their track before detaching. A typical example of a processive motor is the nonmuscular myosin V with roles in certain forms of intracellular transport, for example, in the nervous system [46]. This motor is characterized by a slow and strongly strain-dependent detachment from the actin filament and appropriate coordination of its two motor domains (heads). Such characteristics allow the motor to move processively several steps along an actin filament.
The myosin II motor of muscle, generally denoted by “myosin” below, is, on the other hand, classified as nonprocessive. Thus, each myosin II motor domain spends most of its ATPase cycle time detached from actin and a single myosin motor is believed to take only one single step along an actin filament before detaching. Efficient operation of muscle therefore relies on a large assembly of myosin II motors working together. Consequently, the production of force and displacement by actomyosin in striated muscle is the result of cyclic interactions of billions of myosin motor domains with actin filaments. The efficiency and control of this process are optimized by the assembly of actin, myosin, and accessory (e.g., regulatory) proteins into highly ordered structures on different levels of hierarchical organization (Figure 1). The force-generating interaction cycles between actin and myosin are powered by the turnover of MgATP (denoted by ATP below) and are, except in response to rapid perturbations of length or tension [47–49], asynchronous between different motors as a basis for smooth shortening and force-development.
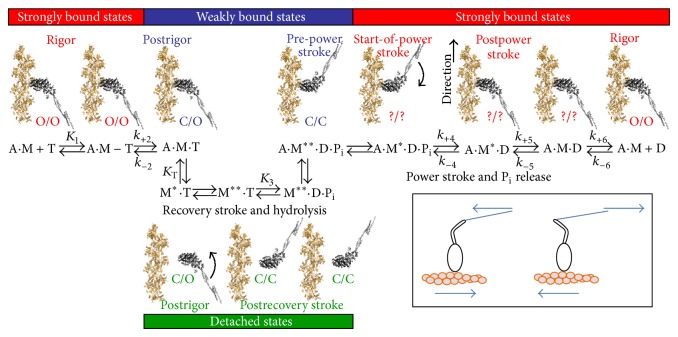
The basic principles of the force-generating cross-bridge cycle in striated muscle have been elucidated on basis of biochemical, mechanical, and structural data [1, 2, 4–6, 23, 27, 47, 50–68]. Binding of ATP to the myosin motor domain first causes a structural change with a swing of the myosin lever arm (a “recovery stroke,” bottom Figure 2) that prepares the myosin head for executing a force-generating power stroke upon the next binding to actin. This event is also associated with altered disposition of loops (switch 1, switch 2, and P-loop) at the catalytic site (see further below). Subsequently, ATP is hydrolyzed to ADP and inorganic phosphate (Pi) but the hydrolysis products remain bound to the active site of myosin. The subsequent, critical step in the force-generating cycle is the binding of the myosin head to the actin filament, forming a so-called cross-bridge. The initial binding is nonspecific [69, 70] and dynamically disordered with a range of azimuthal and axial angles of both motor domain and the light chain binding lever arm [71–73] relative to the actin filament. Furthermore, this initial weak binding is mainly electrostatic in nature [69, 70] with attached and detached states in rapid equilibrium. The transition from the initial weakly and non-stereo-specifically bound state of the myosin head to a stereospecifically bound state has been suggested to involve an average rolling transition of the myosin head on the actin filament [72–74] followed by locking in the stereospecifically attached state. This “roll and lock” transition may both increase the effective attachment rate [75] and contribute to the translation of the thin filaments [71] as well as the tension recovery following rapid length steps [72, 74].
Figure 2.
Simplified schematic representation of the predominant biochemical and structural states of the actomyosin ATPase cycle. Actin is depicted in orange; the myosin motor domain with artificial lever arm (X-ray structure PDB:1G8X [358]) is shown in grey colors (A = actin, M = myosin motor domain, T = ATP, D = ADP, and P = Pi). The open (O) or closed (C) conformation of the active site elements switch 1 and switch 2 is indicated with switch 1 designated as the first. The power stroke corresponds to the switch 2 closed-to-open transition while the motor domain is bound to actin. The recovery stroke occurs in the detached state. It is assumed that the two heads of myosin act independently from each other and only one head is shown. Equilibrium constants and rate constants are denoted by upper case and lower case letters, respectively. Inset: Schematic illustration of tension in lever arm that causes muscle shortening (left) and that resists shortening (right).
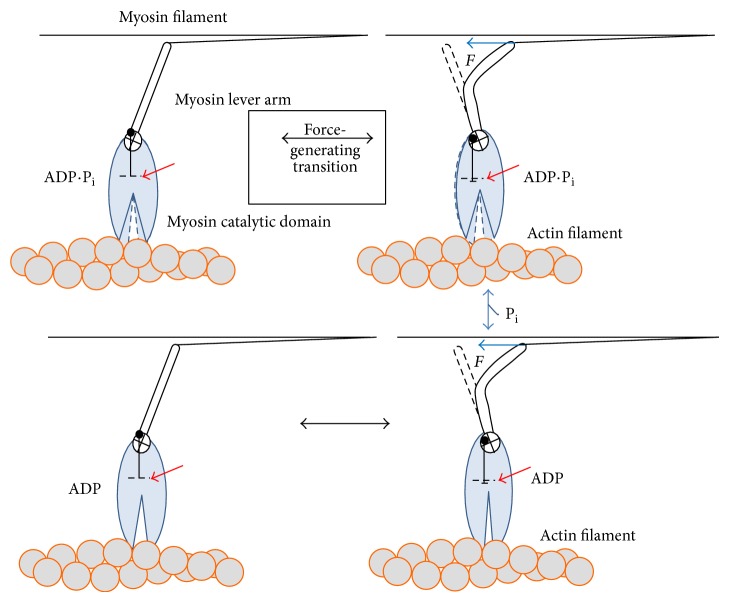
Binding of the myosin head to an actin filament causes ~100-fold activation of the rate of Pi release. In the absence of actin, the Pi release, or rather a preceding conformational transition related to the reversal of the recovery stroke, is rate limiting for ATP turnover by myosin. The release of Pi from actin-bound myosin is associated with a strongly increased actomyosin affinity and a large drop in free energy. Further, there is an appreciable structural change that, in the absence or presence of a counteracting load, causes a swing of the light chain binding myosin lever arm or a tendency for such a swing, respectively. This swing of the lever arm, often termed the power stroke (Figure 2, step 4), is the basis for force-generation of muscle and the myosin induced sliding motion between the thin and thick filaments in the sarcomere. The type of structural change that actually occurs in this process depends on stretching elastic elements in the myosin head and elsewhere and, as just mentioned, the magnitude of the structural change varies depending on the external load on the cross-bridge (see further below).
Under certain physiological conditions, the muscle does not produce any mechanical power in spite of active cross-bridge cycles, such as during isometric contractions (without length changes), equivalent to isovolumetric contraction in a cardiac muscle contracting against closed valves. Further, eccentric contraction, when the muscle is stretched during activity, is associated with work done on the muscle rather than by the muscle [76]. Such eccentric contractions occur physiologically in skeletal and cardiac muscle [77]. During eccentric contractions, there is formation of actomyosin cross-bridges but the biochemical cycle in Figure 2 is not completed, as evident from the very low ATP turnover under these conditions [78]. Instead, the myosin cross-bridges are forcibly broken [34, 79–82] after pulling their elastic element backwards (corresponding to counterclockwise turning of lever arm in Figure 2). Thus, eventually, the backward pull leads to higher tension in the cross-bridge than sustainable by the actomyosin bond [81, 83–87]. The myosin head then detaches from actin, without release of ADP and subsequent rebinding of ATP, in contrast to the situation during shortening and isometric contraction. Indeed, cross-bridge detachment is also quite slow during isometric contraction, associated with key properties of the AM∗ADP state in Figure 2. This state has long been inferred in skeletal muscle [88–91] but its detailed properties were first studied more directly using different slow, nonstriated muscle myosins [92–99]. However, more recently a state with similar properties was found in skeletal muscle [61, 100]. It has also been essential to include in models of striated muscle contraction to accommodate findings that the ADP release occurs in two steps where the first step is an isomerization reaction that is accelerated by negative strain in the myosin lever arm (corresponding to clockwise turning in Figure 2). This results in opening of the nucleotide binding pocket (with a strain (x)-dependent rate constant k +5(x); cf. Figure 2) before ADP leaves rapidly with rate constant k +6.
The numerical value of k +5(x) is believed to be high for low x-values (dominating during rapid shortening), intermediate at intermediate x-values (dominating in isometric contraction), and very low for large x-values (dominating during forcible lengthening). Clearly, the AM∗ADP state and the strain-dependent transition k +5(x) are responsible for differences in cross-bridge kinetics between different conditions. For instance, at high physiological ATP concentration, if k +6 ≫ k +2 [101] and if k +5(x) is large (k +5(x) ≫ k +2), the overall dissociation constant k off(x, [ATP]) is given by
| (1) |
This applies for myosin heads that are brought into the drag-stroke region (x < 0 nm) during shortening where they resist sliding. In contrast, during isometric and eccentric contraction, when k +5(x) is small, then
| (2) |
In this connection it is of interest to consider the concept of duty ratio [102], f, that is, the fraction of the ATP turnover time that myosin molecules spend attached to actin. This ratio (see further [103, 104]) that is close to 1 for processive motors and <0.5 for nonprocessive motors is approximated by the following expression:
| (3) |
where k on and k off are the cross-bridge attachment and detachment rate constant, respectively. Together with (1)-(2), this suggests that the duty ratio for myosin II while low during rapid shortening (e.g., ~0.05 in unloaded shortening) may be quite high in isometric and eccentric contraction as well as very slow shortening (with low k off).
3. Poorly Understood Phenomena in Muscle Contraction
Because muscle contraction is due to the action of a very large ensemble of actomyosin motors it is necessary to use statistical models to relate contractile properties, such as force-velocity relationships, to actomyosin interaction kinetics and mechanics [1, 105–108]. Several models of this type have been developed, often incorporating the above-mentioned principles [31, 49, 109–111] and the additional assumption that all myosin heads act independently. The latter assumption deserves clarification. In most current cross-bridge models, it is assumed that (i) the myosin heads (even the two heads of one myosin molecule) do not compete for the same site on actin and (ii) the binding of one head does not affect the kinetics (nor its strain-dependence) for any transition involving another head (whether belonging to the same myosin molecule or not). This view is similar to the definition outlined in detail by Hill [105, 106]. It means that observable properties of muscle fibers can be readily obtained from mean values calculated from a given number of state probabilities without changing the model parameters between different conditions. For instance, if the myosin heads are assumed to act independently, it means that neither propagating structural changes in the thin filaments nor sequential actions of the two partner head are assumed to alter any parameter value such as attachment rate. Importantly, however, this independence does not prevent myosin heads from interacting (cooperating) by collectively altering the strain of other heads that are attached to a given surface or thick filament past which actin filaments move [1, 49, 112–114].
Statistical cross-bridge models with independent force-generators based on a kinetic scheme similar to Figure 2, but where each biochemical state exists for a range of elastic strains, account well for several contractile phenomena. This includes the information-rich relationship between velocity and load on a muscle (the load-velocity or force-velocity relationship; Figure 3; [115]). However, some key aspects of muscle function cannot be explained in a straightforward way by the most recent cross-bridge models. For instance, if the low myosin attachment rate to actin, suggested by the rate of rise of isometric force, is plugged into a statistical model (e.g., [1] or [31]), a velocity lower than that observed experimentally is predicted for shortening against intermediate loads. Thus, the high maximal power-output during muscle shortening [116] is not accounted for ([31, 32] and references therein; see also Figure 3). Neither is a low attachment rate consistent with the high steady-state resistance to forcible lengthening in eccentric contractions [79, 80, 110, 117]. Related to the difficulty in accounting for the high power-output during shortening, it is also difficult to account for the rapid repriming of the working stroke after a rapid length step, that is, the fact that the amplitude of the tension relaxation upon a rapid release recovers appreciably faster than tension after a priming release step. These phenomena may be reproduced by modifying existing models (e.g., [110]) to make the attachment rate velocity dependent so that it is low during isometric contraction, intermediate during shortening, and fast during lengthening [33–35, 79, 80, 110]. However, a velocity dependent attachment rate is not physically reasonable. Therefore, several alternative mechanisms have been proposed to account for the apparent velocity dependence of this rate [31, 35, 75, 110, 118–124]. Some of the alternative suggestions have kept the idea of independent myosin heads but appreciably increased the number of states and/or included the possibility for myosin heads to rapidly “jump” between neighboring sites on the actin filaments. Others have instead assumed important roles of cooperativity between the two heads of a given myosin molecule. However, there is still no consensus about these models. A third possibility is that the apparent velocity dependence of the attachment rate is related to myosin- or tension-induced structural changes along the actin filament [21, 125–133]. These could cooperatively alter the myosin binding properties of neighboring or even distant actin sites. The above considered alternative explanations are addressed in further detail below.
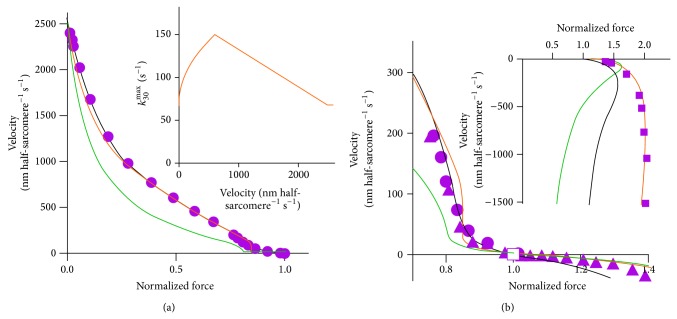
Figure 3.
Force-velocity (load-velocity) relationship of frog muscle fiber. (a) Force-velocity relationship for shortening (positive velocity). Inset: assumed velocity dependence of attachment rate (k 30 max) for certain model predictions in (a) and (b). (b) Force-velocity relationship for loads (forces) close to isometric (normalized force: 1.0) and for eccentric contractions (negative velocities). Inset: extended region for eccentric contraction. Purple symbols in (a) and (b): experimental data from [31] (circles), [191] (triangles), and [79] (squares). Green lines: model [110] with same attachment rate that would fit rise of tension in isometric contraction. Black lines: model [110] with attachment rate accounting well for the maximum power-output during shortening. Orange lines: model [110] with velocity dependent attachment rate for shortening (inset in (a)) and lengthening. In the latter case, the attachment rate constant increased from a maximum value of 67 s−1 in isometric contraction (inset (a)) to 335 s−1 at lengthening velocities ≥ 600 nm hs−1 s−1. Figures from Biophysical Journal [110] reprinted with permission from Elsevier/The Biophysical Society.
Another poorly understood issue is the pathogenesis of hereditary sarcomere myopathies which generally are due to single point mutations in myosin or regulatory proteins. The development of protein expression techniques for striated muscle myosin II [134–137] has enabled studies of the underlying functional deficit on the molecular level. However, the complex and multidimensional pathogenesis of the diseases [36–43] is the result of disturbed function on the whole muscle/heart level. This calls for new experimental approaches for studies of ensemble function on sarcomere or even super-sarcomere levels [43]. Whereas a reductionist approach will give important clues into the mechanisms of disease, a full understanding is likely to require studies on different levels of organization.
The challenges in understanding myopathies are very similar to those in understanding drug effects. Drugs with effects on muscle contraction are of interest for several reasons. For instance, there are compounds with activating potential [138, 139] that stimulate the actomyosin ATPase activity and enhance the contractility or even act as a kind of chemical chaperon that reactivates misfolded myosin molecules [140]. These substances represent a new generation of drugs, and improvements in their efficacy could provide new disease treatment strategies targeted against various hereditary myopathies, acute or chronical heart failure, and other forms of cardiovascular disease. Myosin inhibitors, on the other hand, could be useful for the treatment of myopathies caused by mutations in myosin that increase the active force while reducing the efficiency of muscle contractility [37, 141, 142].
More generally, any small chemical compound that effectively binds to the myosin motor domain and allosterically modulates the functional performance is of great interest in research on myosin and muscle [87, 100, 111, 143–157]. In contrast to point mutations, specific drug effects can be studied not only using expressed single molecules or disordered ensembles but also using muscle fiber preparations with maintained order of the myofilament lattice.
4. Different Experimental Systems
Below we consider why studies using different techniques and on different levels of hierarchical organization give results that are sometimes challenging to reconcile with each other (see also [158]). We also further consider model studies [1, 47, 52, 105, 107, 108, 112] because these have contributed to bridging the gap between different levels of hierarchical organization and integrated information from different experimental systems and from different scientific disciplines. Developments along these lines include studies [109, 117] attempting to integrate molecular structural models, biochemical schemes, and results from muscle mechanics. Furthermore, more recently, efforts have been made to achieve detailed fits of model predictions to a range of experimental data [31, 75, 103, 110, 111, 121, 122, 159].
Problems in integrating results of different types of studies are related to specific features and limitations that distinguish different experimental systems and approaches as outlined below.
4.1. Biochemical Solution Studies
Biochemical solution studies [4, 6, 50–54, 56–58, 63–65] have deciphered dominant parts of the kinetic scheme for the turnover of ATP by myosin and actin (Figure 2; reviewed in [16, 55, 57, 104]). Most of these studies have employed myosin subfragment 1 (S1) that contains the catalytic and actin binding sites of myosin and part of the lever arm (Figure 1(c)). Using this preparation, actomyosin states are generally probed under low ionic strength and unstrained conditions, corresponding to an elastic equilibrium position in muscle [107]. The lack of elastic strain is in contrast to the situation in muscle contraction where elastic strain is the basis for force-development and effects of force on actomyosin transition rate constants.
Whereas strain-dependent transitions cannot be probed in solution studies using S1, they can be studied in single molecule mechanics and in vitro motility assays where the myosin motor fragments are immobilized to surface substrates (Figure 4). Some aspects of strain-dependent transitions can also be investigated in solution using heavy meromyosin (HMM) motor fragments [99, 100, 160] because both of its motor domains can bind to actin filaments. This leads to strain between the heads although most likely different than that present in the ordered sarcomere lattice.
Figure 4.
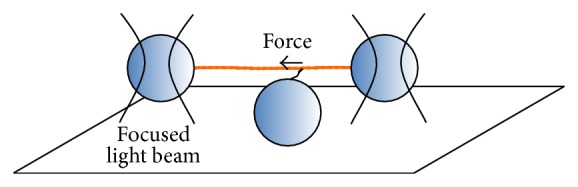
Optical trap system with actin filaments captured on two dielectric beads (optical traps) to which forces may be applied by a focused beam of near-infrared light. The actin filament held in the traps will interact with a single myosin motor on a third bead.
An interesting model, the so-called 3G model, was proposed in two influential papers [57, 58] based on evidence that myosin head binding to actin occurs in two steps. This led to the idea that each biochemical state (Figure 2) exists in three different structural states, with high, low, and very low affinity for actin. The 3G model furthermore assumes that the equilibrium between these states depends on the nucleotide occupancy of the active site. These ideas are relevant for the understanding of force-generation in muscle and have been incorporated into several more advanced statistical cross-bridge models (see below).
4.2. In Vitro Motility Assays and Mechanical Measurements from Small Ensembles and Single Molecules
In vitro motility assays may be viewed as extensions of biochemical solution studies with the key difference that the myosin motor fragments are immobilized to surfaces. Whereas the surface immobilization may affect protein function and complicate some aspects of data interpretation ([161, 162]) it ensures that strain-dependence of the actomyosin interaction is maintained. Therefore it also allows development of motion and forces. In vitro motility assay techniques [7] allow the observation of single actin filaments [21] when interacting with different numbers of myosin motor fragments and under different experimental conditions, for example, ATP concentration and ionic strength [163, 164]. This assay was later supplemented with a “laser trap” (“optical tweezers” technique [22–24, 68, 165, 166]). In this system, assumedly one myosin molecule attaches to an actin filament that is captured at the ends by beads “trapped” between two focused laser beams (Figure 4). Upon myosin-actin interactions, the displacement of actin filaments can be measured by tracking the position of the beads, showing that myosin II produces forces of 1–10 pN and maximum displacements of ~10 nm per interaction with actin [23, 94, 167–171]. In physiological conditions, however, the force and displacements produced by myosin and any other molecular motor are heavily influenced by the external load which dictates their functioning and mechanics.
The load dependence of the power stroke in single molecule studies has been investigated mostly in processive motors (e.g., myosin V) due to slow detachment kinetics and processivity, putting reduced demands on time resolution. The mechanics of myosin V has been studied when the motor was subjected to “pushing” and “pulling” forces, which corresponds to reduced and increased external load, respectively. The duration of attachments between the motor and actin filaments was decreased when the motors were pushed and increased when the motors were pulled [168, 172–174]. Furthermore, the attachment times were shortened with increasing ATP concentrations, suggesting that attachment was terminated when ATP binds to myosin following ADP release [168, 172–175]. Subsequent studies with myosins I and V and smooth muscle myosin II [94, 97, 98, 175, 176] suggest that increasing loads delay ADP release, resulting in a longer attachment time.
Single molecule mechanics studies using skeletal muscle myosin II [23, 169–171, 177, 178] are challenging due to high detachment rate and associated low duty ratio. Therefore, studying the effects of load of myosin II must occur during actomyosin attachments that are extremely short. A study performed with smooth muscle myosin, which has a longer average attachment time than striated muscle myosin, suggested that increasing loads increases this time [98]. Assuming that the total attachment/detachment cycle does not change during the actomyosin cycle, an increase in attachment time results in an increased duty ratio. The authors [98] also investigated the kinetics of the load dependence of attachment times and distinguished between two phases of attachment of myosin, consistent with structural studies showing two distinct myosin bond conformations: one conformation in the presence (phase 1) and the other in the absence (phase 2) of bound ADP. Increasing loads prolonged the duration of phase 1 but did not affect phase 2, suggesting that load dependence may be attributed to a transition between an actomyosin state with and without bound ADP (cf. k +5 in Figure 2). Later, using a laser trap system with improved time resolution [61], similar results were obtained using fast and slow myosin II from skeletal muscle.
The in vitro motility assays and related force-measuring techniques have answered a number of central questions with regard to striated muscle contraction suggesting that (i) only one myosin II head is necessary for production of motion and force [8, 179, 180], (ii) an unloaded displacement of 5–10 nm is produced by a myosin motor domain upon binding to an actin filament [23, 61, 67, 168, 171, 177] with the highest values in this range for two-headed myosin preparations and optimized orientations, (iii) a maximum force of about 10 pN is actively developed by a myosin motor domain [23, 170], (iv) there are target zones with sites, ~36 nm apart, along the actin filament to which an immobilized myosin II motor binds more readily [177, 181] than to other sites (see also [71, 74]), and (v) the displacement induced by a given strongly actin-attached motor domain occurs in two steps [61] where the second step is believed to be associated with the strain-dependent transition from the AM∗ADP to the AMADP state. Finally, recent developments [182] have allowed quite detailed probing of the force-dependence of several kinetic steps in the actomyosin turnover of ATP.
The importance of the surface-based assays is hard to overestimate. However, it is challenging to relate single molecule mechanics data to mechanics of muscle cells or myofibrils where very large ensembles of myosin motors interact simultaneously with the actin filaments (see below). Moreover, key aspects of muscle function such as the detailed shape of the force-velocity relationship and the apparent velocity dependence of the attachment rate constant have not been addressed because they result from interactions of a large ensemble of myosin motor domains with actin filaments in an ordered arrangement. In only few cases have the interaction between several (but not a large number of) motors and an actin filament been investigated using optical tweezers [183]. Furthermore, even if the interaction of a large number of myosin motors with actin filaments could be studied, it is difficult to assess cooperative phenomena properly. Such phenomena include that related to the role of the two myosin heads and their possible interaction with two actin filaments [160] or that due to an ordered arrangement of myosin motors in three dimensions around each actin filament.
Statistical and kinetic models of the type mentioned above (e.g., [1, 103, 108]) form an excellent basis for explaining results both from muscle cells, conventional in vitro motility assays and single molecule mechanical studies. However, there is risk of confusion when results from these different experimental systems are compared. This is exemplified by the myosin working stroke (power stroke) distance, as follows. First, we define this distance, h, as the average displacement of the actin filament actively produced when one myosin head binds to actin and completes its ATP turnover in the absence of counteracting load. The distance would be that measured in single molecule optical tweezers studies with low trap stiffness. It does not involve excursion of the myosin head elasticity into strains with negative forces (that resist sliding), that is, into the drag-stroke region [184]. This value for the working stroke would be identical to that: h = v f τ on(0) obtained from the in vitro sliding velocity (v f) and the myosin on time (τ on(0)) at zero strain (e.g., measured in solution) if it is assumed that precisely one myosin head at a time propels an actin filament. These conditions imply immediate execution of a power stroke (to reach its zero-strain elastic equilibrium) upon myosin head attachment to actin and subsequent detachment with time constant τ on(0) immediately followed, but not preceded, by attachment of a new myosin head and repetition of the cycle. Such ideal conditions are not fulfilled in reality. Therefore, the magnitude of the step length obtained from velocities measured in the vitro motility assay and optical tweezers studies differs by a factor up to ~2. This is clear by examining the condition with a very large ensemble of myosin heads that work together to propel the actin filament. This condition is fulfilled in muscle cells and approximately fulfilled in the in vitro motility assay if an actin filament is propelled over a surface with saturating density of myosin motor fragments. Under these conditions the elastic element of a large fraction of the myosin heads will be shortened to the extent that these cross-bridges resist sliding in the shortening direction (execute a drag-stroke). During steady-state unloaded shortening, the negative cross-bridge forces that counteract sliding are exactly balanced by the positive forces due to cross-bridges that undergo their power stroke. These force-levels are determined by the average strain of negatively (v f τ on−) and positively (〈h〉) strained cross-bridges, respectively, each factor multiplied by the cross-bridge stiffness. If the stiffness is Hookean the stiffness-values on the left and right sides of the equation cancel out and 〈h〉 = v f τ on−. This expression is deceivingly similar to that for h, given above. However, 〈h〉 is always smaller than h [103, 111, 184], generally 0.5h < 〈h〉 < h, consistent with τ on− < τ on(0) which, in turn, is consistent with τ on(x) = 1/k off(x) (see (1)-(2)).
These relationships can be further expanded by considering also h ∞ and τ on ∞, defined as the average sliding distance and time, respectively, over which a given myosin head stays attached to the actin filament while executing first positive and then negative force (executing working-stroke followed by drag-stroke) in a large ensemble. Naturally, it also applies that h ∞ = v f τ on ∞. Finally, it is readily shown (cf. [103]) that τ on(0) > τ on ∞ > τ on− and 2h > h ∞ > h > 〈h〉 > 0.5h, where the last inequality is approximate.
4.3. Muscle Fiber Mechanics and Statistical Models
In the field of muscle mechanics, mechanical and optical sensor systems are used to relate length changes of muscle sarcomeres to the stiffness and forces developed by muscle cells (muscle fibers). The experiments can be performed either on intact [19, 185, 186] or on skinned [187–189] muscle fibers. The intact muscle cells are dissected from a living muscle using micromechanical tools (scissors, forceps, needles, etc.) leaving the cell membrane intact. In contrast, the membrane of skinned muscle cell segments is removed, chemically or mechanically, allowing the myofilament environment to be controlled from the bath fluid.
Of central importance in muscle mechanics are studies relating the imposed steady load on a muscle cell to the resulting steady velocity of the length change or equivalently the force developed upon imposition of a ramp shaped length change of a given velocity [1, 32, 115, 187, 190–195]. Force-velocity relationships obtained in either of these ways have demonstrated, although indirectly, that increased load increases the duration of the myosin power stroke [1, 196–198]. The velocity in response to increasing loads is continuously reduced, approximately according to a rectangular hyperbola [190] (however see [191]) from its maximum value in unloaded shortening to zero during isometric contraction (without length change). At this point the derivative of steady velocity versus steady load is continuous [191] when load increases above isometric force to cause lengthening with constant velocity (negative shortening velocity; Figure 3(b)). When a stretch is performed at low speeds (less than 2 muscle lengths s−1; L 0 s−1), the increase in force during a length ramp has two components: (i) a fast phase, in which force increases substantially over a few nanometers per half-sarcomere and (ii) a slow phase, in which force increases a small amount or remains unchanged [79, 80, 86, 199–204]. The latter phase approximates the steady force during lengthening. The transition between these two phases occurs at a critical stretch amplitude of ~10 nm half-sarcomere, commonly associated with a critical strain of attached cross-bridges beyond which they are forcibly detached from actin [33, 34, 79, 80, 85, 86, 199–202, 204–206].
The mechanism behind the increase in force during stretch is still controversial. Several investigators have suggested that it is primarily due to an increased force per cross-bridge (increased strain) during stretch [33, 34, 80, 86, 207]. It has been made likely that this effect is caused by prepower stroke cross-bridges, in a state that precedes phosphate release [86, 201, 202, 208, 209]. Interestingly, in this connection, recent X-ray diffraction studies [124] suggested an increased fraction of non-stereo-specifically bound myosin heads during stretch, properties usually attributed to weakly bound prepower stroke cross-bridges. However, the idea of increased force-resistance being attributed to weakly bound myosin heads is not easy to reconcile with the above-mentioned critical strain of ~10 nm. Nevertheless, any model must account for the findings that the phosphate analogues vanadate and aluminium fluoride (AlF4), which are known to bias cross-bridges into a prepower stroke position, reduce isometric force of fibers treated with polyethylene glycol (which promotes myosin-actin interactions) considerably more than stretch forces [86, 208]. Similarly, the drugs butanedione monoxime (BDM) [207, 210] benzyl-toluene sulfonamide (BTS) [202] and blebbistatin [197], that are believed to inhibit main force-generating transitions, have similar effects. In this connection it is also of interest to mention that increased tonicity of the extracellular solution that causes volume shrinkage of intact muscle cells appreciably reduces the maximum isometric tension without affecting the maximum force during stretch [80, 204]. A similar result is seen at reduced temperature [211].
Many aspects of muscle mechanics have been strongly influenced by the pioneering work of AF Huxley from both a theoretical [1, 47] and experimental [19, 47, 212–215] perspective. Accordingly, muscle mechanical studies are often interpreted in terms of cross-bridge models that incorporate features of the Huxley and Simmons (1971; [47]) and the Huxley (1957; [1]) models. The latter model explains the basic steady-state properties of muscle (such as the force-velocity relationship) whereas the Huxley and Simmons (1971) model (Figure 5) accounts for the tension responses to rapid length changes imposed on a muscle cell. The combination of these two models accounts well for several aspects of muscle function [216].
Figure 5.
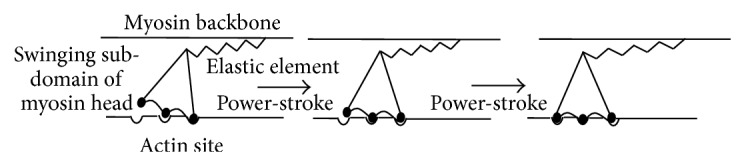
Model of the Huxley and Simmons type [47]. Force development is assumed to occur by thermally excited swing of a myosin head subdomain that stretches an independent elastic element. The swing is forward-biased (producing a power stroke) by progressively increased binding affinity between the myosin head and actin for each transition (to the right) that stretches the elastic element.
The Huxley and Simmons model was inspired by the swinging cross-bridge model proposed by H. E. Huxley [2] on basis of ultrastructural evidence. Interestingly, in similarity to later results based on the atomic structure of myosin [5] the model incorporates ideas with an increasing number of attachment points between actin and myosin that stabilize high-force states. However, the model also raises critical questions. First, an independent elastic structure has not been unequivocally identified in the actomyosin cycle. Bending of the entire light chain stabilized alpha helical lever arm [217] or structural changes in the neighboring regions in the converter domain [141, 218] have been implicated to represent the elastic element (see also [219]). However, this region has also been implicated as the main component that swings during the force-recovery after a length step [66, 220].
This so-called swinging lever arm model followed the swinging cross-bridge model upon accumulating evidence against large-scale orientation changes of the entire myosin motor domain during force-generation [30] (however, see [71]). A second problem with the Huxley and Simmons [47] model is related to the number of states and force-generating structural transitions required. In their original paper, two stable attached states were assumed where transition from the low-force to the high-force state was accompanied by ~10 nm extension of the elastic element. As already was pointed out by the authors, two states are insufficient to account for the high power-output of muscle as well as for the rate of the tension transients using a model with an independent elastic element. This issue has become increasingly challenging after emerging evidence that the stiffness of the elastic element is somewhere in the range 1.7–3.3 pN/nm [32, 67, 221], considerably higher than previously believed.
Whereas a cross-bridge stiffness of ~1.7 pN/nm seems to be consistent with two tension generating steps [114, 222], a larger number of structurally and mechanically distinct states are required for a cross-bridge stiffness of ~3 pN/nm [75, 121, 122, 222, 223]. There is limited evidence for such a large number of states with different stable positions of the lever arm. Possibly, the issue would be resolved if the lever arm swing is preceded by a “roll and lock” transition of the entire myosin head that also contributes to force-recovery after a length step ([72]; see above). However, for any model with a large number of states, validation is difficult because a wide range of experimental findings can be reproduced with several free parameters whether the model is correct or not. A final complication related to the Huxley and Simmons [47] model is that the rates of relevant biochemical transitions observed in solution studies are considerably lower than the rates required to account for the rapid tension transients. This complication is related to the incompletely understood relationship between the rapid tension transients in response to length steps and the Pi release step in the ATP turnover by actomyosin, that is, the biochemical transition being most closely associated with the force-generating structural change in the actomyosin cross-bridge (see below). This is suggested by comparison of solution studies and rapid perturbations of contraction in intact and skinned muscle cells including rapid length steps (see above) and sinusoidal oscillations [89, 224, 225], rapid changes in load [48, 122], temperature (temperature jumps; [209, 225–231]), hydrostatic pressure (pressure jump; [232]), and phosphate concentration (phosphate jump; [151, 233–235]). Moreover, in skinned fibers, insight into the force-generating step and its relationship to, for example, Pi release (see below), has been obtained by investigating the [Pi]-dependence of steady-state isometric tension and force-velocity data ([122, 192, 209, 236]).
An issue that has severely complicated the interpretation of a large number of muscle mechanical studies is the possibility of a nonlinear (non-Hookean) elasticity of the cross-bridges [67, 111, 237] and/or myofilaments [33, 238–242] or the presence of a time-invariant parallel-elastic element, possibly attributed to a fixed number of cross-bridges [243]. These issues (reviewed in [222]) have been considered further recently [244] but are not yet resolved making it challenging to interpret stiffness data in terms of the number of attached cross-bridges. This uncertainty is highlighted by experiments investigating the number of attached cross-bridges during shortening at different velocities [32, 85] and during slow stretch [33, 35, 120]. During shortening, stiffness measurements (after correction for presumed linear series elasticity) suggest that force and the number of attached cross-bridges are approximately proportional (at least at loads close to the isometric one) [32]. In contrast, an alternative approach for obtaining the number of attached cross-bridges based on the maximum tension response to very rapid stretches [206] suggests lack of such proportionality [85]. Furthermore, the assumption of linear filament elasticity suggests ([35] and later [120]) that the resistance to slow stretch of active muscle is mainly attributed to increased recruitment of cross-bridges. In contrast, Nocella et al. [33] found evidence for a nonlinear filament compliance suggesting the force-enhancement during stretch is mainly attributed to increased average cross-bridge strain (see also [34, 80]). Another type of studies that is not always easy to interpret is those based on time resolved low-angle X-ray scattering from contracting muscle cells. Whereas these studies have led to new important insights [217, 220, 238, 239, 245] there are conflicting views about the interpretation in some cases [246]. The interested reader is referred to other review-articles [247–249] for details.
Muscle fiber experiments have the advantage of maintained three-dimensional arrangement between the myofilaments in half-sarcomeres and preservation of accessory proteins that may affect contraction. On the other hand, the large number of protein components makes it necessary to use statistical models [1, 105] for interpretation of the experimental results and several different models are likely to account for a given data set. Furthermore, the interpretations of muscle mechanical and structural data (e.g., from low-angle X-ray diffraction) in terms of cross-bridge properties often rely on high degree of uniformity between half-sarcomeres along the length of a studied muscle fiber and over the muscle cross-section. In the absence of such order and uniformity, unpredictable emergent properties are possible. Model studies have suggested that the nonuniformities may cause residual force enhancement after stretch [250] and the suppression of oscillatory motion under certain conditions [43, 49]. Different types of nonuniformities between segments along muscle cells have also been observed experimentally [11, 19, 251–254] and found to play important physiological roles, for example, in speeding up relaxation after an isometric contraction [10] (see also [255, 256]) and contributing to aspects of the tension response to stretch [11, 12, 257].
The results of muscle fiber experiments may be affected, in unpredictable ways, by muscle fiber type, that is, by using fast or slow muscle (e.g., [61, 101, 258–260]) or due to mixtures of myosin isoforms in a given cell [251, 254] (see also [261, 262]). Furthermore, the level of activation and the presence of regulatory proteins ([151, 187, 263]) may affect the kinetics of the actomyosin interaction in different ways. Finally, a range of posttranslational modifications may affect function. This expanding field is not considered further here but it may be worth mentioning that the drug blebbistatin affected unloaded shortening velocity in skinned fibers differently in the presence and absence of phosphorylation of the regulatory myosin light chains [264].
4.4. Myofibril Mechanics
Myofibrils can be isolated both from skeletal and cardiac muscles and mounted for force-measurements and imposition of length perturbations (e.g., [207, 256, 265, 266]). Myofibrils are of particular interest to study because they are the smallest experimental units that maintain the three-dimensionally ordered myofilament lattice of striated muscle. The myofibrils are formed basically by sarcomeres arranged in series and with all major proteins intact (i.e., myosin, actin, troponin, tropomyosin, titin, and myosin binding protein C). Results from studies with myofibrils have been used to link studies on single molecules or proteins in solution with studies performed using muscle fibers. The length of myofibril segments to be studied can be virtually chosen by the investigators, and their diameter is substantially smaller (~1.0–1.5 μm) than that of muscle fibers (~10 μm). This is important, because it makes the diffusion time during activation of myofibrils very short, eliminating gradients of activation from the periphery to the core of the preparation. In contrast, the longer diffusion distances in muscle fibers can cause substantial gradients, not only in the activation level but also of ATP, ADP, and Pi concentrations, making interpretations at the actomyosin level complex.
The development of techniques for rapid solution exchanges during experiments with myofibrils enables exact determination of the rates of force development and relaxation during contraction, important indicators of the actomyosin interactions. Furthermore, the use of myofibril activation, in association with fast length changes imposed to the preparation, allows precise evaluation of the rate of force redevelopment (Ktr) following a shortening-stretching protocol [265] that was originally developed for application to muscle fibers [267]. The Ktr has been used effectively to define the kinetics of cross-bridges transiting between weakly bound and strongly bound states. The Ktr determined with high time resolution has been used not only for probing the steps of the actomyosin cycle, but also for comparison of myosin kinetics in muscles of different conditions, health, and disease (e.g., [268–270]). Finally, myofibril studies allow investigators to elucidate the detailed relation between force development, relaxation, and sarcomeres dynamics. Since myofibrils are formed by a single chain of sarcomeres, the force produced by the myofibril at both ends can only be produced and shared by these interconnected structures. Such structural geometry has been explored to infer the mechanical behavior of myofibrils upon activation and during/after loads that are imposed to the preparation [257, 266].
Recently, there have been studies using single sarcomeres [271] and isolated half-sarcomeres [272], preparations that by nature avoid sarcomere length nonuniformities, and thus open possibilities for investigations of contractile performance without confounding effects. The limitation of these preparations is their fragility—it is virtually impossible to activate single sarcomeres for more than 5-6 activation cycles.
4.5. Molecular Structure: X-Ray Crystallography and Cryo-Electron Microscopy
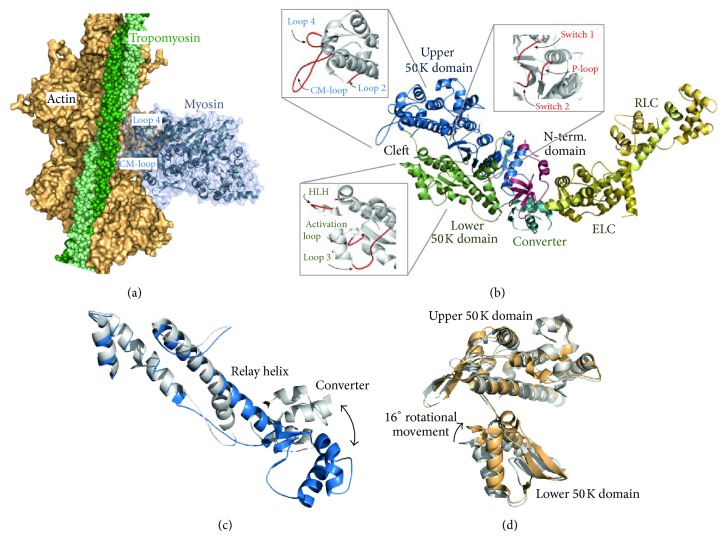
Structural insights (Figure 6(a)) into the acto-myosin interaction have been obtained by combining crystallographic data for the myosin motor domain with information derived by electron microscopy and small angle X-ray scattering studies from myosin-decorated actin filaments [27, 59, 273–275]. The X-ray structures of the myosin motor domains crystallized so far fall into three categories dependent on the structural state they represent in the ATPase cycle (Figure 2). The distinctions are made on basis of the relative position of the active site elements (switch 1 and switch 2 closed or open; Figure 6(b)), the lever arm orientation (up or down), and the conformation of the actin-binding cleft (open, closed, or partially closed). The switch elements act as nucleotide sensors responsible for communication between the nucleotide biding pocket and the actin binding sites. Their reversible switching between two conformations opens and closes the active site around the γ-phosphate enabling hydrolysis and the coupling of internal conformational changes to larger rearrangements and rigid body movements of subdomains in the myosin motor that eventually lead to force generation. When considering states based on X-ray scattering and cryo-electron microscopy it is important to emphasize that they only capture metastable structural states.
Figure 6.
Structures of the rigor actomyosin complex and the myosin motor domain (S1) at different nucleotide states. (a) High resolution structure of the nucleotide-free actin-myosin- tropomyosin complex as obtained by cryo-electron microscopy (ref. [291], PDB IDs 4a7n, 4a7l, 4a7h, and 4a7f). (b) Ribbon representation of the atomic structure of chicken skeletal muscle myosin S1 fragment (PDB: 2MYS). S1 comprises 843 amino acid residues of the myosin heavy chain and two light chains (RLC and ELC) bound to the C-terminal neck region of the molecule. The central core comprises a seven-stranded β-sheet surrounded by several α helices. Characteristic is the deep cleft in the molecule. The cleft extends from the active site (P-loop, switch 1, and switch 2) to the actin binding elements, which are located in the upper (blue) and lower 50 K (green) domains. The N-terminus is adjacent to the C-terminus forming a protruding SH3-like β-barrel structure (red). The long C-terminal helix (light green) contains two IQ motifs that bind the light chains (ELC and RLC) and acts as a lever arm and conveys together with the converter domain local conformational changes to large movements. Highlighted in red in the insets are the actin binding and nucleotide coordinating loop and switch elements. (c) Conformational rearrangements of the relay helix (unwinding and kinking) and the converter (rotational movement) during the recovery stroke. The recovery stroke drives the detached myosin from the postrigor state to the prepower stroke state. The structures depicted are PDB ID: 2JHR and PDB ID: 1G8X. (d) Structural model for the strong binding start-of-power stroke (ref. [145]). The myosin power stroke is initiated by a transition from a weak-to-strong actin binding state. A rotational movement of the lower 50 K domain from the prepower stroke state (light grey, PDB ID: 2JJ9) enables a rigor-like strong binding geometry of the myosin at the actin interface (shown in brown ribbon representation) without changing the position of the converter domain. The structures were prepared with the PyMOL Molecular Graphics System, Version 1.7.2, Schrödinger, LCC.
The majority of the myosin structures crystallized with ADP.Pi analogs represent the prepower stroke state after ATP hydrolysis with weak affinity of the myosin cross-bridge for actin [157, 274, 276–279]. The cleft in most of these structures is partially closed. Further, switch 1 and switch 2 adopt closed conformations and the lever arm is in the up position. The second group of structures comprises states of the myosin motor domain assigned as postrigor [280–283]. These are thought to represent the prehydrolysis state (cf. Figure 2) of the myosin from which the recovery stroke takes place, transferring the motor to a catalytically competent prepower stroke state. In the postrigor states, the cleft is open, switch1 is closed, switch 2 is open, and the lever arm is down. A third group of structures, defined as rigor-like, have been obtained for myosin V and myosin VI [284–286]. According to the functional properties of these high duty ratio myosins, the crystallized states are thought to represent high actin affinity binding at a time after the power stroke has occurred. Characteristic for the majority of these nucleotide-free structures is a closed-cleft conformation and the lever arm down. Of relevance here, the rigor-like structure has also been obtained for muscle and nonmuscle myosin II [287, 288]. Despite the small differences seen in the extent and location of the cleft closure between the different rigor-like structures, it becomes apparent that cleft closure, although enthalpically unfavorable [289], is essential for facilitating the release of the hydrolysis products.
There is no crystal structure of the myosin motor domain bound to actin, but the rigor-like structures all exhibit features of an actin-bound state and high resolution cryo-electron microscopy support this view [290, 291]. Other limitations of available structural data are the lack of crystal structures showing states between the prepower stroke states and the rigor-like state.
In view of the limited availability of structural data, determining the sequence of events by which the myosin cross-bridge generates force has been made possible only by the combined analysis of structural information and biochemical data from solution kinetics together with model building including molecular dynamic simulations. In the absence of ATP, myosin forms a high affinity complex with actin (Figure 6(a)). In this strongly bound rigor state, the active site elements, switch 1 and switch 2, are thought to adopt an open conformation with the lever arm in a down position (Figure 2) [59]. The state subsequently turns into a low affinity state as Mg2+-ATP irreversibly binds to the myosin active site [292].
The Mg2+-ATP binding induces a closing of switch 1, which drives the formation of several new interactions such as a salt-bridge between switch 1 and switch 2 that assists in stabilizing the β-phosphate and enables the coordination of the Mg2+-ion and proper positioning of the surrounding water molecules for ATP hydrolysis. Kinetic studies with myosin mutants in which the formation of the salt bridge is disrupted are not capable of hydrolysis, emphasizing the critical role of the switch 1/switch 2 interaction [293–296]. At the same time, the active site rearrangements induced by Mg2+-ATP binding are coupled to the distortion of the seven-stranded β-sheet forcing the upper 50 kDa subdomain to undergo a large movement, which reduces the contact area and weakens the affinity to actin. This enables cleft opening and the full dissociation of the actomyosin complex [297]. The dissociated state is the hydrolysis competent state of myosin. According to current data, the hydrolysis reaction requires the closing of switch 2 [298, 299], which is coupled to larger rearrangements of the relay helix and the converter [300–302]. The 6 Å shift of switch 2—as seen between the postrigor and prepower stroke state structures (Figure 6(c))—causes a partial unwinding of the relay helix and a kink. Since the tip of the relay-helix is connected via hydrogen bonds and hydrophobic interactions with the converter, the relatively small switch 2 movement is amplified via the relay helix to a 65° rotation of the converter and a swinging of the lever arm from the initial down to the up position. This structural transition is known as the recovery-stroke [275, 282, 303–309].
The up position of the lever arm is the starting point of the force producing working stroke or power stroke, which requires rebinding of myosin to actin (Figure 2). Otherwise Pi is released from myosin without actin-binding, following a lever arm swing that represents the reversal of the power stroke and that is futile with regard to force-production [62].
Characterization of the actin binding elements by mutational analysis assumes that actin binding occurs sequentially through the contribution of at least six flexible myosin loops (Figure 6(b), close-up views) that modulate, in a nucleotide-dependent manner, the interaction strength and coupling to actin [296, 310–315] (see also [316]). According to solution kinetics, binding of myosin heads to the actin filament occurs in two distinct ways, weak and strong [57, 58, 317], that cannot entirely be explained by the current set of structures. The rigor-like structures allow predictions of how cleft closure induced by actin binding accelerates product release [284, 287]. However, what cannot be deduced from the rigor-like structural state are details of the conformational changes that initiate the power stroke and that accompany the transition from an initial weakly bound acto-myosin-ADP.Pi state to the actin-myosin rigor complex (Figure 2). A priori, there are several possibilities [62], including the presence of a start-of-power stroke state in which the myosin motor domain is strongly bound to actin and the lever arm is in an up position. Structure-based modeling of this putative state [318] suggests that the power stroke is not a reversal of the recovery-stroke, because the tight actin-binding constrains the relative motion of the upper and lower 50 K domain [59]. Rather, the power stroke is thought to be realized in at least two steps, involving a transition from the prepower stroke state to the proposed start-of-power stroke state. This could be accomplished by a rotational movement of the lower 50 K domain (Figure 6(d)), which subsequently closes the cleft thereby putting a torsional strain on the β4-strand of the central β-sheet via the W-helix forcing the molecule to subsequently straighten the relay helix, which in turn drives power stroke. The exact position of the active site switch elements in this transition state and their mutual interplay in the following states with respect to additional coupled rearrangements of the relay-helix and core β-strand cannot be accurately predicted from the current structures and models. With the help of kinetic studies, some speculations about the series of switch movements coupled to the power stroke can be made [319, 320]. However, additional structural and biochemical work is necessary to resolve the exact communication pathway that links actin binding to force production.
5. Poorly Understood Molecular Mechanisms in relation to Contractile Properties
An explanation for poorly understood phenomena in muscle contraction (Section 3) requires better understanding of the incompletely understood molecular mechanisms considered below.
5.1. Attachment of Myosin Head to Actin, Phosphate Release, and the Main Force-Generating Transition
There is currently rather incomplete understanding of the biochemical, mechanical, and structural events associated with myosin head attachment to actin and subsequent force-production.
Whereas we here focus on the force-generating transition it is of relevance to describe some uncertainties about the rate-limiting step for the ATP turnover cycle that has been placed somewhere between the ATP-hydrolysis and Pi release step (Figure 2) [16, 55, 65, 108, 321, 322]. The issue is important for explaining the increased apparent attachment rate during shortening against intermediate loads compared to isometric contraction (see above). For instance, if the attachment rate is limited by the rate of the hydrolysis process rather than by the attachment step or Pi release one may foresee higher apparent attachment rate during shortening. This is due to completion of the hydrolysis step during the time that actin target sites (with 36 nm separation) move past myosin heads that are incorrectly oriented for binding. This means that any sterically feasible cross-bridge attachment is faster under these conditions than during isometric contraction. The situation is similar if the rate-limiting step is between a so-called refractory and nonrefractory M.ADP.Pi state. This was the case in the model of Eisenberg et al. [108] and it is the basis for the capability of this model to account for the fast repriming of the power stroke by rapid reattachment of cross-bridges from a nonrefractory M.ADP.Pi state into a low-force state that is competent to undergo a force-generating transition upon a length step [118].
With regard to the relationship between the force-generating transition and Pi release, several issues are controversial. First, the major component of the fast tension recovery in response to length steps [47, 212, 225] is an order of magnitude faster than the tension responses to sudden changes in Pi concentration [233] hydrostatic pressure [232], temperature ([228–230] reviewed in [209]), and the force-generating process detected in spectroscopic studies [323]. Furthermore, the rate of tension recovery after length steps depended on the phosphate concentration after stretches but not after rapid releases. Thus, clearly the physical basis of the tension response to length steps and the other perturbations is not identical and the relationship between the length perturbation responses and phosphate release is complex. The idea of different molecular basis for the tension response to length jumps and temperature jumps is consistent with different structural changes according to X-ray diffraction patterns of skinned muscle fibers [217] but the relationship is complex. Thus, the tension response to temperature jumps seems to correspond to a slow phase of the tension relaxation in response to rapid length steps [225, 229, 230] and the overall rate of the tension response to length steps increases with temperature. The observed complexities (see also [230]) add to concerns [114, 158] that rather large number of states found necessary to account for the length-step response [122, 152] are not readily associated with states observed in biochemical and structural studies.
In order to elucidate the apparent incommensurability between results from different perturbation studies one may consider the characteristics of the observed tension responses in some detail. First, the dominant rate observed in the tension response to steps in pressure and temperature is rather similar [209] and the response to jumps in Pi concentration has a similar rate. Accordingly, temperature jumps have been claimed to affect an endothermic force-generating transition [209] in series with a rapid Pi-binding equilibrium. Whereas most available data suggest that the force-generating transition occurs prior to the Pi release (see Table 1) there has been appreciable controversy about the exact temporal relationship (Table 1) and the possibility has also been considered that the Pi release is more or less loosely coupled to the force-generating transition [75, 121, 122]. Furthermore, whether force-generation occurs before or after phosphate release, there is controversy about the exact number of substeps and their rates [122, 151, 209, 229, 230, 236, 321, 323, 324]. If Pi release occurs before the force-generating transition (Table 1), then it seems that Pi release must be rate-limiting for force-generation because direct measurements of Pi release in solution [65] yield a similar rate as that attributed to tension generation following temperature jumps, phosphate jumps, and so forth. A slow Pi release has also been favored on basis of kinetic modeling [324], but in this case, the Pi release was believed to occur after the force-generating transition. Under such conditions, an AM.ADP.Pi state would be the main force-generating state and phosphate release would be rate-limiting for cross-bridge detachment. This is consistent with findings that an AM.ADP.Pi state is the dominant biochemical species in contracting myofibrils [325] and with spectroscopic studies of relay-helix motion in Dictyostelium myosin II catalytic domain [323]. The latter motion precedes a slower Pi release. However, numerous other findings suggest that Pi release is fast [122, 209, 236] and that an AM.ADP state (AM∗ADP in Figure 2) rather than and AM.ADP.Pi state dominates during steady-state contraction [89, 100, 326, 327].
Table 1.
Conflicting evidence from experimental studies and theoretical considerations related to temporal relationship between Pi release and force-generating step in actomyosin cross-bridge cycle in muscle.
| Force-gen. before Pi release | Pi release before force-gen. | Loose coupling |
|---|---|---|
| Kawai and Halvorson [89] Pi release fast |
Davis and Epstein [230]. L-jumps and T-jumps in skinned muscle fibers | Caremani et al. [122]. Load-clamp expts. in skinned muscle fibers at varied [Pi] |
|
| ||
| Dantzig et al. [233] based on Pi jump experiments in skinned muscle fibers Pi release fast |
Davis and Rodgers [229] | |
|
| ||
| Tesi et al. [235] Pi-jumps and tension versus [Pi] in myofibrils Pi release fast |
Spudich [16] | |
|
| ||
| Smith and Sleep [324] based on comparison of kinetic models Pi release slow |
Sweeney and Houdusse [60] from reviewing structural data from several studies | |
|
| ||
| Ranatunga [209] from reviewing own work and work by others Pi release fast |
Conibear et al. [359] | |
|
| ||
| Caremani et al. [236]. Contraction of skinned fibres at different [Pi] Pi release fast |
||
|
| ||
| Muretta et al. [323]. Spectroscopy applied to Dictyostelium myosin II Pi release slow |
||
Difficulties to reconcile results from experimental systems with different ionic strength, strain dependence, and so forth may contribute to the different views about the temporal relationship between Pi release and force-generation. The importance of strain, for instance, is reflected in 500-fold faster 18O exchange (reflecting Pi-exchange) in isometric contraction of skinned fibers than in acto-S1 in solution [328]. Moreover, Pi release was inhibited during ramp stretches in cardiac muscle [329] and, finally, the Pi-concentration affected the tension recovery after rapid stretches but not after rapid releases ([225]; see also [330]).
Another possibility is that the conflicting interpretations are due to models that do not capture certain critical features of cross-bridge operation. Furthermore, the lack of generality and stringency in definition of terms such as “main force-generating step” and “power stroke” contribute to the problems. These terms are used differently between researchers and between subfields such as muscle mechanics, single molecule mechanics, and actomyosin structural biology and biochemistry. The ambiguity is reflected in the discussion of the power stroke distance in Section 4.2 (see also [67, 114, 184, 222]).
A direct identity of the force-generating transition associated with Pi release and length jumps was assumed in some early model studies [52, 107, 331] before the broad availability of data from perturbation studies other than length steps. The simplification was also used in recent models [111, 114] where the relationship between Pi release and force-generation was not in focus. In these cases, with key model states and their free energies illustrated in Figure 7, it is of interest to note that the model had high explanatory power accounting for both length-jump responses and a range of steady-state properties, for example, the force-velocity relationship, both in the presence and in absence of a drug affecting the strain-dependent transition from the AM∗ADP to the AM.ADP state [100, 110]. Later developments of the same model [111] also accounted for the effects of varying concentrations of ATP and ADP. Whereas temperature jump and Pi-effects were not considered it was found that some temperature effects could be accounted for by increasing the free energy between the AM.ADP.Pi and AM∗ADP states [111]. These models could, however, not account for the high power-output of muscle during steady-state shortening or the high steady-state resistance to lengthening without assuming velocity dependent attachment rates (Section 3). Furthermore, due to the very fast detachment from pretension AM.ADP.Pi states, suggested by single molecule studies [182] and the associated weak actin affinity of these states, it seems unlikely that prepower stroke AM.ADP.Pi states can account for the high resistance to slow stretch. Thus, under slow stretches, the rupture force of a majority of the force-resistant cross-bridges seems to be high and with appreciable elastic strain [33, 81], seemingly incompatible with properties of AM.ADP.Pi states. However, future models must reconcile this finding with results (see above), based on effects of nucleotide analogs and drugs [197, 202, 208, 210], varied temperature, and altered Pi-concentration [209], suggesting that prepower stroke cross-bridges in the AM.ADP.Pi state contribute appreciably to the stretch response.
Figure 7.
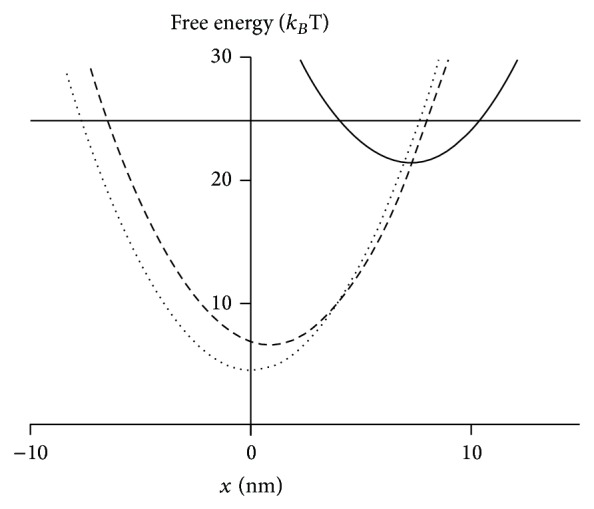
Free energy of main cross-bridge states in Figure 2 as function of the strain parameter x. Straight full line: detached states M.ATP and M.ADP.Pi lumped together into one state. Curved full line: AM.ADP.Pi. Dashed line: AM∗ADP. Dotted line: AM, AM.ADP, and AM.ATP states lumped together. The parameter x = 0 when force is zero in the AM, AM.ADP, and AM.ATP states.
A further problem of current models for force-generation is, as pointed out above, that they assume an independent elastic element and require that the force-generating transition occurs in a large number of steps. An interesting alternative possibility, similar to that originally proposed in [107] is to assume an elastic element that is not independent from the swinging component and that is strained by a subnanometer structural change (in contrast to ~10 nm in the Huxley and Simmons model). This may be termed an Eyring like model [104, 110] where a local chemical change causes a transition into the new state, followed by ~10 nm relaxation of the elastic element into the minimum free energy of the new state. One model for how this could occur is schematically illustrated in Figure 8. Here, the localized structural changes strain the elastic element. The latter is here attributed to bending of the lever arm but the bending could also occur in the converter domain [141, 218]. The localized structural changes, on the other hand, involve thermal fluctuations of structural domains (e.g., related to relay helix, converter domain, central beta-sheet, and the loops around the nucleotide binding site), fluctuations that may precede Pi release [323]. A difference from the original Huxley and Simmons model [47] is the very small amplitude of the structural changes that lead to the high force-state in Figure 8, a fact that substantially reduces the energy barrier to be overcome. This Eyring mechanism [104] is in contrast to a more Kramers-like process, that is where a large scale diffusional straining of an elastic element against a load (as in the Huxley and Simmons [47] model) precedes the chemical change.
Figure 8.
Tentative model. The elastic element is represented by bending of the lever arm being an integral part of the myosin head. This elastic element is stretched by a small amplitude structural change in the catalytic domain (from left to right). The schematic illustration is for isometric contraction. The position of the lever arm at elastic equilibrium of the main force-generating state (right) is illustrated by the lever arm drawn in dashed lines. The force-generating transition from left to right is orthogonal to the phosphate release step (vertical).
Something that further hampers our understanding of the force-generating transition is the fact that the atomic structural correlates of Pi release and force-generation in response to different perturbations are not well-defined. First, a question about the Pi release mechanism, including the exact time point in the ATPase cycle (does Pi release precede the power stroke or does the power stroke precede Pi release?), cannot be readily answered with the present structural models (see Section 4.5). On the other hand, structural information and computational analysis of the hydrolysis reaction postulate different equally feasible escape routes [62]. However, as touched upon above, a recent study based on solution kinetics and time-resolved fluorescence resonance energy transfer (FRET) experiments revealed that actin binding straightens the relay helix before phosphate dissociation assuming that the power stroke occurs before Pi-release [323]. Structural details of the Pi release state of myosin are needed to understand how actin triggers product release and how active site switch elements rearrange to facilitate the Pi release.
The difficulty of crystallizing the actin-bound state of myosin has hampered detailed insights into the mechanism of force production. One possible way to overcome this problem would be the production of stable dimeric or trimeric actin oligomers. This minimal number of actin subunits could form a functional and crystallizable rigor complex for detailed analysis. In this way it would be possible to resolve, both the exact actin-myosin binding interface, the structural state and interactions between actin and the second myosin head [332], resolving the functional role of the latter. Optical trap experiments with native myosin II have shown that the degree of flexibility of the heads is sufficient to allow attachment to at least three subsequent binding sites on one actin filament [181].
In order to account for some of the apparently conflicting evidence it is interesting to consider ideas that each biochemical state exists in different mechanical/structural states in rapid equilibrium with each other [57, 58, 215, 333] (cf. Figures 5 and 8). More recently, these ideas have gained additional support [334] and been incorporated into rather complete cross-bridge models [75, 109, 117, 121, 122]. In the latter types of model each biochemical state in Figure 2 would be composed of several substates that differ mechanically and structurally by different extension of their elastic element, different degree of completion of the lever-arm swing and different affinity between actin and myosin (cf. Figures 5 and 8).
In terms of such a model, the tension response to length steps is due to very rapid transitions between mechanical/structural states (similar to those in Figure 5) without transitions between biochemical states (horizontal transitions in Figure 8).
The tension response to other perturbations, for example, temperature and phosphate jumps, is due to slower chemical reactions, for example, an isomerization prior to phosphate release that may or may not be strain-dependent.
Whereas models of this type account well for several experimental phenomena it will be important to limit the number of states to an absolute minimum and firmly define the properties of these states, including strain-dependence of interstate transitions, on basis of a range of different experiments. A further challenge will be to relate the structural course of events as defined by X-ray crystallography to the events seen in response to rapid perturbation experiments such as length-jumps, Pi jumps, and temperature jumps in muscle fibers. Possibly, mechanical experiments on different levels of hierarchical organization from single molecules over small ordered ensembles and myofibrils to muscle cells can help to bridge the gap if they are combined with spectroscopic techniques, for example, single-molecule FRET.
5.2. Number of Actin Sites within Reach for a Given Myosin Head
Several models of muscle contraction assume that only one binding site on the actin filament is within reach for binding of a given myosin head. This approximation allows simulation of most aspects of muscle contraction without severe limitations [110]. However, some additional phenomena may be accounted for if more binding sites are available. For steric reasons, such as the helical arrangement of the actin binding sites on the actin filament, it is likely that only groups of few (3–5) neighboring sites at 5.5 nm intersite distance are reached by myosin heads belonging to a given crown on the thick filament. This idea was supported by laser trap studies showing target zones for myosin binding corresponding to the helical repeat of 36–38 nm of the actin filament. The presence of neighboring sites on the same filament allows extension of the above models such as rapid reattachment to the neighboring actin binding site of a myosin head forcibly detached into an M∗ADP or MADPPi state during shortening or stretch [75, 121, 122]. Such behavior may form the basis for an apparent velocity dependence of the cross-bridge attachment rate.
5.3. Role of Two Myosin Heads
The role of the second head in the dimeric myosin II molecule remains enigmatic, for example, whether the two heads are independent or cooperative in their interaction with actin and whether there is an alternating stepping behavior, where the heads subsequently bind to actin in a processive manner, thus enhancing force-output [160, 335–339].
Some studies have indicated that interhead cooperativity between the two heads of each myosin II molecule may not be important and the two heads are often viewed as independent force generators ([68, 171, 179]; see further above). However, there are a range of experimental results suggesting different forms of cooperativity of the two heads [74, 100, 124, 160, 168, 338, 340, 341]. One possibility is negative cooperativity between the two heads, that is, binding of one head to actin inhibits binding of the other [342] or one head prevents dissociation of the other head. Negative cooperativity of the latter type would enable maintenance of tension without energy consumption.
On the other hand, positive cooperativity has also been proposed [110, 160, 168, 341]. This may take different forms but one possibility is that binding of one head promotes binding of the other in a way that sequential actions of the two heads are promoted [110, 160]. Such effects may play a role in explaining the apparently faster attachment rate of cross-bridges during shortening and during lengthening. For instance, a mechanism with attachment of the second head was suggested [35], based on muscle X-ray diffraction data (see also [120]), to account for the effective resistance to lengthening. A similar model was proposed more recently [124], but with additional details, suggesting that a large fraction of the actomyosin cross-bridges during stretch are non-stereo-specifically bound (see also above). On the other hand, the idea that the appreciable resistance to stretch of active muscle is attributed mainly to increased number of attached myosin heads could not be corroborated in another recent study [33].
Modeling based on data showing two-headed attachment of fast myosin II [100] hinted [110] that sequential actions of the two heads of myosin II may become important in shortening against intermediate loads [60, 110] (intermediate velocities) when power-output is maximal. The rapid repriming of the working stroke [203] mentioned above has also been explained on this basis. Whereas recent in vitro motility assay tests [111] failed to corroborate the idea of sequential head actions they could neither falsify the hypothesis with certainty. Thus the predicted changes that were looked for were small and it is possible that the loss of cellular order in the in vitro motility assay was the reason for the failure to detect cooperativity [160]. Furthermore, effective interhead cooperativity may also require binding of the two myosin heads to neighboring actin filaments at interfilament distances similar to that in muscle [160] or there may be other forms of interhead cooperativity where the presence of two heads facilitates attachment of one of them but where sequential force-producing actions are not occurring [168, 340, 341].
5.4. Role of Structural Changes in Actin Filaments
The active role of myosin in force-production by actomyosin is universally accepted, but the actin filaments are generally [6], except for their allosteric activation of phosphate release from myosin, viewed as rope-like passive interaction partners. However, the actin filament structure is highly dynamic and altered during the force-generating process [21, 129, 132, 343, 344]. It is therefore reasonable to assume that structural changes in the actin filament are important for effective generation of force and power. Some authors have even proposed a dominant role of the actin filaments, for example, in providing gross structural changes that cause translation of actin over myosin or an asymmetric potential for biased diffusion of the myosin head. However, there is firm evidence for more facilitating and modulatory roles of the actin filaments. Thus, several studies [21, 127–133, 345, 346] suggest that myosin binding to actin or tension on the actin filament causes structural changes that propagate along the actin filament.
6. Understanding of Muscle Function Requires the Combination of Top-Down Disassembly and Bottom-Up Assembly of the Contractile Machinery
In addressing incompletely understood molecular mechanisms of muscle contraction, it will be critical to integrate results from experimental systems on different levels of hierarchical organization. It will also be essential to use a stringent joint terminology so that key concepts, such as “power stroke” have a similar meaning among muscle physiologists, biochemists, single molecule biophysicists, and structural biologists. Such an integrated approach should lead to models with a minimal number of states that integrate structural, biochemical, and mechanical information from fibers and single molecules, thereby laying a solid foundation for insight into poorly understood phenomena in normal muscle contraction as well as into effects of drugs and myopathy mutations.
Naturally, any cooperative interactions between myosin motors are lost in single molecule studies but detailed nonambiguous information about the strain-dependence of transition rates [61, 98, 182] is more readily derived using single molecules than ensembles. Such studies are now also possible using expressed myosin II from human striated muscle, both normal and with myopathy mutations [135]. On the other hand, the force-velocity relationship of striated muscle is a property of an ordered ensemble that cannot be fully characterized using single myosin motors. However, other challenges plague studies on muscle cells and myofibrils. Thus, there may be uncertainties about interpretation of mechanical and structural data from muscle fiber and myofibril mechanics due to incompletely characterized elastic components, emerging properties in the large ensemble of motors [347] that are not readily extrapolated back to actomyosin interactions or related effects of sarcomere nonuniformities. Clearly, it would be of great interest with new types of experiments to bridge the gap between the ordered system and single molecule studies. One may consider two complementary approaches. First, top-down disassembly, or rather a combined top-down disassembly and bottom-up assembly, of the contractile systems may be achieved by starting with skinned fibers or myofibrils [66, 348, 349] and removing more and more components, possibly followed by reconstitution. The other approach is pure bottom-up assembly of ordered contractile systems from single molecules. Simple versions of such experimental systems, for example, using different number of myosin motors randomly adsorbed to surfaces [183, 350] or incorporated into synthetic myosin filaments on a pedestal [67, 171], would allow studies of force-velocity relationships of ordered and disordered ensembles of myosin motors with one or two heads [179] interacting with actin filaments. Next, it may be of relevance to add other protein components one molecule after the other such as troponin and tropomyosin and protein C.
On more advanced level one may consider the use of DNA-origami scaffolds (cf. [351]) and engineered attachment points of the motors [135] to produce well-defined ordered arrays. Furthermore, if the scaffold could be produced inside a hollow nanowire [352] it may even be possible to build up in vitro assay systems with maintained 3D order similar to that in the myofilament lattice. Finally, it is of interest to use extensively engineered myosins [353–355] to investigate specific properties.
Interesting questions that may be possible to answer by combining single molecule studies and modelling with new experimental systems featuring few to many protein components in different ordered arrangements would be the following.
Overall, are there emerging properties when going from single molecules to ordered ensembles or are the ensemble properties fully characterized on basis of single molecule components?
What is the role of the two heads in single molecules and in ordered ensembles of different size?
What is the role of 3D order, for example, the possibility of a given two-headed myosin motor to interact with two or more actin filaments at similar inter-filament distances as in a muscle cell?
What are the roles of different accessory proteins?
What is the importance of variations between half-sarcomeres in overlap between the thick and thin filaments and other variability in contractile properties over the fiber cross-section and along the length of a myofibril?
What are the mechanisms determining the force-velocity relationship, for example, the role of two-myosin heads, structural changes in actin filaments, 3D order, and single myosin heads rapidly “jumping” from one actin site to the next when they are part of an ensemble. These issues may be accessible by studying force-velocity relationships of ordered ensembles of different sizes, using one-headed and two-headed myosin fragments and myosin motors with critical mutations, for example, affecting attachment properties or Pi release.
Can ensemble effects of myopathy mutations and drugs be predicted from single molecule studies and solution biochemistry?
Acknowledgments
Alf Månsson is grateful to the Faculty of Health and Life Sciences of the Linnaeus University for funding and to the Wenner-Green Foundation for funding a sabbatical in the lab of Dilson Rassier at McGill University during which period the present study was initiated. Dilson Rassier acknowledges support from NSERC of Canada and Georgios Tsiavaliaris is grateful to the German Research Foundation and the State of Lower Saxony for funding.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Huxley A. F. Muscle structure and theories of contraction. Progress in biophysics and biophysical chemistry. 1957;7:255–318. [PubMed] [Google Scholar]
- 2.Huxley H. E. The mechanism of muscular contraction. Science. 1969;164(3886):1356–1366. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 3.Huxley A. F. Muscular contraction. The Journal of Physiology. 1974;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 5.Rayment I., Holden H. M., Whittaker M., et al. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 6.Geeves M. A., Holmes K. C. The molecular mechanism of muscle contraction. Advances in Protein Chemistry. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 7.Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz P. R. H., Kraus J. E. M., Larroux C., et al. Independent evolution of striated muscles in cnidarians and bilaterians. Nature. 2012;487(7406):231–234. doi: 10.1038/nature11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edman K. A. P., Flitney F. W. Laser diffraction studies of sarcomere dynamics during ‘isometric’ relaxation in isolated muscle fibres of the frog. Journal of Physiology. 1982;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edman K. A. P. Residual force enhancement after stretch in striated muscle. A consequence of increased myofilament overlap? Journal of Physiology. 2012;590(6):1339–1345. doi: 10.1113/jphysiol.2011.222729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rassier D. E. Residual force enhancement in skeletal muscles: one sarcomere after the other. Journal of Muscle Research and Cell Motility. 2012;33(3-4):155–165. doi: 10.1007/s10974-012-9308-7. [DOI] [PubMed] [Google Scholar]
- 13.Batters C., Veigel C., Homsher E., Sellers J. R. To understand muscle you must take it apart. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00090.Article 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huxley H. E. A personal view of muscle and motility mechanisms. Annual Review of Physiology. 1996;58:1–19. doi: 10.1146/annurev.ph.58.030196.000245. [DOI] [PubMed] [Google Scholar]
- 15.Schiaffino S., Reggiani C. Fiber types in Mammalian skeletal muscles. Physiological Reviews. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 16.Spudich J. A. The myosin swinging cross-bridge model. Nature Reviews Molecular Cell Biology. 2001;2(5):387–392. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- 17.Huxley A. F., Niedergerke R. Structural changes in muscle during contraction: interference microscopy of living muscle fibres. Nature. 1954;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 18.Huxley H., Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- 19.Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. Journal of Physiology. 1966;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spudich J. A., Kron S. J., Sheetz M. P. Movement of myosin-coated beads on oriented filaments reconstituted from purified actin. Nature. 1985;315(6020):584–586. doi: 10.1038/315584a0. [DOI] [PubMed] [Google Scholar]
- 21.Yanagida T., Nakase M., Nishiyama K., Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- 22.Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- 23.Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 24.Block S. M., Goldsteint L. S. B., Schnapp B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature. 1990;348(6299):348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 25.Funatsu T., Harada Y., Tokunaga M., Saito K., Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995;374(6522):555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- 26.Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- 27.Rayment I., Rypniewski W. R., Schmidt-Bäse K., et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 28.De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 29.Kubalek E. W., Uyeda T. Q. P., Spudich J. A. A dictyostelium myosin II lacking a proximal 58-kDa portion of the tail is functional in vitro and in vivo. Molecular Biology of the Cell. 1992;3(12):1455–1462. doi: 10.1091/mbc.3.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke R. The mechanism of muscle contraction. CRC Critical Reviews in Biochemistry. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- 31.Edman K. A. P., Månsson A., Caputo C. The biphasic force-velocity relationship in frog muscle fibres and its evaluation in terms of cross-bridge function. The Journal of Physiology. 1997;503, part 1:141–156. doi: 10.1111/j.1469-7793.1997.141bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piazzesi G., Reconditi M., Linari M., et al. Skeletal muscle performance determined by modulation of number of Myosin motors rather than motor force or stroke size. Cell. 2007;131(4):784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 33.Nocella M., Bagni M. A., Cecchi G., Colombini B. Mechanism of force enhancement during stretching of skeletal muscle fibres investigated by high time-resolved stiffness measurements. Journal of Muscle Research and Cell Motility. 2013;34(1):71–81. doi: 10.1007/s10974-012-9335-4. [DOI] [PubMed] [Google Scholar]
- 34.Piazzesi G., Francini F., Linari M., Lombardi V. Tension transients during steady lengthening of tetanized muscle fibres of the frog. The Journal of Physiology. 1992;445:659–711. doi: 10.1113/jphysiol.1992.sp018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunello E., Reconditi M., Elangovan R., et al. Skeletal muscle resists stretch by rapid binding of the second motor domain of myosin to actin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20114–20119. doi: 10.1073/pnas.0707626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey P. A., Leinwand L. A. The cell biology of disease: cellular mechanisms of cardiomyopathy. The Journal of Cell Biology. 2011;194(3):355–365. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spudich J. A. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophysical Journal. 2014;106(6):1236–1249. doi: 10.1016/j.bpj.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teekakirikul P., Padera R. F., Seidman J. G., Seidman C. E. Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics. The Journal of Cell Biology. 2012;199(3):417–421. doi: 10.1083/jcb.201207033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laing N. G. Congenital myopathies. Current Opinion in Neurology. 2007;20(5):583–589. doi: 10.1097/WCO.0b013e3282ef6e69. [DOI] [PubMed] [Google Scholar]
- 40.Tajsharghi H., Oldfors A. Myosinopathies: pathology and mechanisms. Acta Neuropathologica. 2013;125(1):3–18. doi: 10.1007/s00401-012-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Memo M., Marston S. Skeletal muscle myopathy mutations at the actin tropomyosin interface that cause gain-or loss-of-function. Journal of Muscle Research and Cell Motility. 2013;34(3-4):165–169. doi: 10.1007/s10974-013-9344-y. [DOI] [PubMed] [Google Scholar]
- 42.Ochala J., Gokhin D. S., Pénisson-Besnier I., et al. Congenital myopathy-causing tropomyosin mutations induce thin filament dysfunction via distinct physiological mechanisms. Human Molecular Genetics. 2012;21(20):4473–4485. doi: 10.1093/hmg/dds289.dds289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Månsson A. Hypothesis and theory: mechanical instabilities and non-uniformities in hereditary sarcomere myopathies. Frontiers in Physiology. 2014;5, article 350 doi: 10.3389/fphys.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon A. M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiological Reviews. 2000;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 45.Lehrer S. S. The 3-state model of muscle regulation revisited: is a fourth state involved? Journal of Muscle Research and Cell Motility. 2011;32(3):203–208. doi: 10.1007/s10974-011-9263-8. [DOI] [PubMed] [Google Scholar]
- 46.Sellers J. R. Myosins. 2nd. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- 47.Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 48.Edman K. A. P., Curtin N. A. Synchronous oscillations of length and stiffness during loaded shortening of frog muscle fibres. Journal of Physiology. 2001;534(2):553–563. doi: 10.1111/j.1469-7793.2001.t01-2-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duke T. A. J. Molecular model of muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):2770–2775. doi: 10.1073/pnas.96.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenberg E., Moos C. The adenosine triphosphatase activity of acto-heavy meromyosin. A kinetic analysis of actin activation. Biochemistry. 1968;7(4):1486–1489. doi: 10.1021/bi00844a035. [DOI] [PubMed] [Google Scholar]
- 51.Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. Journal of Biological Chemistry. 1970;245(9):2451–2456. [PubMed] [Google Scholar]
- 52.Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annual Review of Physiology. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- 53.Chock S. P., Chock P. B., Eisenberg E. The mechanism of the skeletal muscle myosin ATPase. II. Relationship between the fluorescence enhancement induced by ATP and the initial Pi burst. The Journal of Biological Chemistry. 1979;254(9):3236–3243. [PubMed] [Google Scholar]
- 54.Stein L. A., Chock P. B., Eisenberg E. The rate-limiting step in the actomyosin adenosinetriphosphatase cycle. Biochemistry. 1984;23(7):1555–1563. doi: 10.1021/bi00302a033. [DOI] [PubMed] [Google Scholar]
- 55.Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Critical Reviews in Biochemistry. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y.-Z., Taylor E. W. Kinetic mechanism of myofibril ATPase. Biophysical Journal. 1994;66(5):1542–1553. doi: 10.1016/S0006-3495(94)80945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geeves M. A., Goody R. S., Gutfreund H. Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. Journal of Muscle Research and Cell Motility. 1984;5(4):351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- 58.Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochemical Journal. 1991;274(1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holmes K. C., Schröder R. R., Sweeney H. L., Houdusse A. The structure of the rigor complex and its implications for the power stroke. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359(1452):1819–1828. doi: 10.1098/rstb.2004.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney H. L., Houdusse A. Structural and functional insights into the myosin motor mechanism. Annual Review of Biophysics. 2010;39(1):539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 61.Capitanio M., Canepari M., Cacciafesta P., et al. Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(1):87–92. doi: 10.1073/pnas.0506830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Málnási-Csizmadia A., Kovács M. Emerging complex pathways of the actomyosin powerstroke. Trends in Biochemical Sciences. 2010;35(12):684–690. doi: 10.1016/j.tibs.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagshaw C. R., Trentham D. R. The characterization of myosin product complexes and of product release steps during the magnesium ion dependent adenosine triphosphatase reaction. Biochemical Journal. 1974;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Highsmith S. The effects of temperature and salts on myosin subfragment 1 and F actin association. Archives of Biochemistry and Biophysics. 1977;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- 65.White H. D., Belknap B., Webb M. R. Kinetics of nucleoside triphosphate cleavage and phosphate release steps by associated rabbit skeletal actomyosin, measured using a novel fluorescent probe for phosphate. Biochemistry. 1997;36(39):11828–11836. doi: 10.1021/bi970540h. [DOI] [PubMed] [Google Scholar]
- 66.Irving M., St Clair-Allen T., Sabido-David C., et al. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995;375(6533):688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- 67.Kaya M., Higuchi H. Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science. 2010;329(5992):686–689. doi: 10.1126/science.1191484. [DOI] [PubMed] [Google Scholar]
- 68.Molloy J. E., Burns J. E., Kendrick-Jones J., Tregear R. T., White D. C. S. Movement and force produced by a single myosin head. Nature. 1995;378(6553):209–212. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 69.Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geeves M. A., Holmes K. C. Structural mechanism of muscle contraction. Annual Review of Biochemistry. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 71.Taylor K. A., Schmitz H., Reedy M. C., et al. Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell. 1999;99(4):421–431. doi: 10.1016/S0092-8674(00)81528-7. [DOI] [PubMed] [Google Scholar]
- 72.Ferenczi M. A., Bershitsky S. Y., Koubassova N., et al. The “roll and lock” mechanism of force generation in muscle. Structure. 2005;13(1):131–141. doi: 10.1016/j.str.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Baumann B. A. J., Liang H., Sale K., Hambly B. D., Fajer P. G. Myosin regulatory domain orientation in skeletal muscle fibers: application of novel electron paramagnetic resonance spectral decomposition and molecular modeling methods. Biophysical Journal. 2004;86(5):3030–3041. doi: 10.1016/S0006-3495(04)74352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu S., Liu J., Reedy M. C., et al. Electron tomography of cryofixed, isometrically contracting insect flight muscle reveals novel actin-myosin interactions. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012643.e12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith D. A., Geeves M. A., Sleep J., Mijailovich S. M. Towards a unified theory of muscle contraction. I: foundations. Annals of Biomedical Engineering. 2008;36(10):1624–1640. doi: 10.1007/s10439-008-9536-6. [DOI] [PubMed] [Google Scholar]
- 76.Abbott B. C., Aubert X. M., Hill A. V. The absorption of work when a muscle is stretched. The Journal of Physiology. 1950;111(1-2):41p–42p. [PubMed] [Google Scholar]
- 77.Ashikaga H., van der Spoel T. I. G., Coppola B. A., Omens J. H. Transmural myocardial mechanics during isovolumic contraction. JACC: Cardiovascular Imaging. 2009;2(2):202–211. doi: 10.1016/j.jcmg.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Curtin N. A., Davies R. E. Very high tension with very little ATP breakdown by active skeletal muscle. Journal of Mechanochemistry and Cell Motility. 1975;3(2):147–154. [PubMed] [Google Scholar]
- 79.Lombardi V., Piazzesi G. The contractile response during steady lengthening of stimulated frog muscle fibres. Journal of Physiology. 1990;431:141–171. doi: 10.1113/jphysiol.1990.sp018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mansson A. The tension response to stretch of intact skeletal muscle fibres of the frog at varied tonicity of the extracellular medium. Journal of Muscle Research and Cell Motility. 1994;15(2):145–157. doi: 10.1007/BF00130425. [DOI] [PubMed] [Google Scholar]
- 81.Colombini B., Nocella M., Benelli G., Cecchi G., Griffiths P. J., Bagni M. A. Reversal of the myosin power stroke induced by fast stretching of intact skeletal muscle fibers. Biophysical Journal. 2009;97(11):2922–2929. doi: 10.1016/j.bpj.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colombini B., Nocella M., Benelli G., Cecchi G., Bagni M. A. Crossbridge properties during force enhancement by slow stretching in single intact frog muscle fibres. The Journal of Physiology. 2007;585(2):607–615. doi: 10.1113/jphysiol.2007.141440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishizaka T., Miyata H., Yoshikawa H., Ishiwata S., Kinosita K., Jr. Unbinding force of a single motor molecule of muscle measured using optical tweezers. Nature. 1995;377(6546):251–254. doi: 10.1038/377251a0. [DOI] [PubMed] [Google Scholar]
- 84.Guo B., Guilford W. H. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):9844–9849. doi: 10.1073/pnas.0601255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bagni M. A., Cecchi G., Colombini B. Crossbridge properties investigated by fast ramp stretching of activated frog muscle fibres. Journal of Physiology. 2005;565, part 1:261–268. doi: 10.1113/jphysiol.2005.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Getz E. B., Cooke R., Lehman S. L. Phase transition in force during ramp stretches of skeletal muscle. Biophysical Journal. 1998;75(6):2971–2983. doi: 10.1016/S0006-3495(98)77738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minozzo F. C., Hilbert L., Rassier D. E. Pre-power-stroke cross-bridges contribute to force transients during imposed shortening in isolated muscle fibers. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029356.e29356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sleep J. A., Hutton R. L. Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment. Biochemistry. 1980;19(7):1276–1283. doi: 10.1021/bi00548a002. [DOI] [PubMed] [Google Scholar]
- 89.Kawai M., Halvorson H. R. Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas muscle. Biophysical Journal. 1991;59(2):329–342. doi: 10.1016/S0006-3495(91)82227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dantzig J. A., Hibberd M. G., Trentham D. R., Goldman Y. E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. The Journal of Physiology. 1991;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyitrai M., Geeves M. A. Adenosine diphosphate and strain sensitivity in myosin motors. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359(1452):1867–1877. doi: 10.1098/rstb.2004.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jontes J. D., Wilson-Kubalek E. M., Milligan R. A. A 32° tail swing in brush border myosin I on ADP release. Nature. 1995;378(6558):751–753. doi: 10.1038/378751a0. [DOI] [PubMed] [Google Scholar]
- 93.Whittaker M., Wilson-Kubalek E. M., Smith J. E., Faust L., Milligant R. A., Sweeney H. L. A 35-A movement of smooth muscle myosin on ADP release. Nature. 1995;378(6558):748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]
- 94.Veigel C., Coluccio L. M., Jontes J. D., Sparrow J. C., Milligan R. A., Molloy J. E. The motor protein myosin-I produces its working stroke in two steps. Nature. 1999;398(6727):530–533. doi: 10.1038/19104. [DOI] [PubMed] [Google Scholar]
- 95.Gollub J., Cremo C. R., Cooke R. ADP release produces a rotation of the neck region of smooth myosin but not skeletal myosin. Nature Structural Biology. 1996;3(9):796–802. doi: 10.1038/nsb0996-796. [DOI] [PubMed] [Google Scholar]
- 96.Rosenfeld S. S., Sweeney H. L. A model of myosin V processivity. The Journal of Biological Chemistry. 2004;279(38):40100–40111. doi: 10.1074/jbc.M402583200. [DOI] [PubMed] [Google Scholar]
- 97.Veigel C., Wang F., Bartoo M. L., Sellers J. R., Molloy J. E. The gated gait of the processive molecular motor, myosin V. Nature Cell Biology. 2002;4(1):59–65. doi: 10.1038/ncb732. [DOI] [PubMed] [Google Scholar]
- 98.Veigel C., Molloy J. E., Schmitz S., Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nature Cell Biology. 2003;5(11):980–986. doi: 10.1038/ncb1060. [DOI] [PubMed] [Google Scholar]
- 99.Kovács M., Thirumurugan K., Knight P. J., Sellers J. R. Load-dependent mechanism of nonmuscle myosin 2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(24):9994–9999. doi: 10.1073/pnas.0701181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albet-Torres N., Bloemink M. J., Barman T., et al. Drug effect unveils inter-head cooperativity and strain-dependent ADP release in fast skeletal actomyosin. The Journal of Biological Chemistry. 2009;284(34):22926–22937. doi: 10.1074/jbc.M109.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nyitrai M., Rossi R., Adamek N., Pellegrino M. A., Bottinelli R., Geeves M. A. What limits the velocity of fast-skeletal muscle contraction in mammals? Journal of Molecular Biology. 2006;355(3):432–442. doi: 10.1016/j.jmb.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 102.Uyeda T. Q. P., Kron S. J., Spudich J. A. Myosin step size: estimation from slow sliding movement of actin over low densities of heavy meromyosin. Journal of Molecular Biology. 1990;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- 103.Walcott S., Warshaw D. M., Debold E. P. Mechanical coupling between myosin molecules causes differences between ensemble and single-molecule measurements. Biophysical Journal. 2012;103(3):501–510. doi: 10.1016/j.bpj.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sunderland, Mass, USA: Sinauer Associates; 2001. [Google Scholar]
- 105.Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle .Part I. Progress in Biophysics and Molecular Biology. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- 106.Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle part II. Progress in Biophysics & Molecular Biology. 1976;29:105–159. doi: 10.1016/0079-6107(76)90021-3. [DOI] [PubMed] [Google Scholar]
- 107.Eisenberg E., Hill T. L. A cross-bridge model of muscle contraction. Progress in Biophysics and Molecular Biology. 1978;33(1):55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- 108.Eisenberg E., Hill T. L., Chen Y. Cross-bridge model of muscle contraction. Quantitative analysis. Biophysical Journal. 1980;29(2):195–227. doi: 10.1016/S0006-3495(80)85126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith D. A., Geeves M. A. Strain-dependent cross-bridge cycle for muscle. Biophysical Journal. 1995;69(2):524–537. doi: 10.1016/S0006-3495(95)79926-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Månsson A. Actomyosin-ADP states, interhead cooperativity, and the force-velocity relation of skeletal muscle. Biophysical Journal. 2010;98(7):1237–1246. doi: 10.1016/j.bpj.2009.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Persson M., Bengtsson E., ten Siethoff L., Månsson A. Nonlinear cross-bridge elasticity and post-power-stroke events in fast skeletal muscle actomyosin. Biophysical Journal. 2013;105(8):1871–1881. doi: 10.1016/j.bpj.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jülicher F., Prost J. Cooperative molecular motors. Physical Review Letters. 1995;75(13):2618–2621. doi: 10.1103/PhysRevLett.75.2618. [DOI] [PubMed] [Google Scholar]
- 113.Vilfan A., Frey E. Oscillations in molecular motor assemblies. Journal of Physics Condensed Matter. 2005;17(47):S3901–S3911. doi: 10.1088/0953-8984/17/47/018. [DOI] [PubMed] [Google Scholar]
- 114.Offer G., Ranatunga K. W. A cross-bridge cycle with two tension-generating steps simulates skeletal muscle mechanics. Biophysical Journal. 2013;105(4):928–940. doi: 10.1016/j.bpj.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seow C. Y. Hill's equation of muscle performance and its hidden insight on molecular mechanisms. The Journal of General Physiology. 2013;142(6):561–573. doi: 10.1085/jgp.201311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barclay C. J. Estimation of cross-bridge stiffness from maximum thermodynamic efficiency. Journal of Muscle Research and Cell Motility. 1998;19(8):855–864. doi: 10.1023/A:1005409708838. [DOI] [PubMed] [Google Scholar]
- 117.Smith D. A., Geeves M. A. Strain-dependent cross-bridge cycle for muscle II. Steady-state behavior. Biophysical Journal. 1995;69(2):538–552. doi: 10.1016/S0006-3495(95)79927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y.-D., Brenner B. On the regeneration of the actin-myosin power stroke in contracting muscle. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(11):5148–5152. doi: 10.1073/pnas.90.11.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huxley A. F., Tideswell S. Rapid regeneration of power stroke in contracting muscle by attachment of second myosin head. Journal of Muscle Research and Cell Motility. 1997;18(1):111–114. doi: 10.1023/A:1018641218961. [DOI] [PubMed] [Google Scholar]
- 120.Fusi L., Reconditi M., Linari M., et al. The mechanism of the resistance to stretch of isometrically contracting single muscle fibres. The Journal of Physiology. 2010;588(3):495–510. doi: 10.1113/jphysiol.2009.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith D. A., Mijailovich S. M. Toward a unified theory of muscle contraction. II. Predictions with the mean-field approximation. Annals of Biomedical Engineering. 2008;36(8):1353–1371. doi: 10.1007/s10439-008-9514-z. [DOI] [PubMed] [Google Scholar]
- 122.Caremani M., Melli L., Dolfi M., Lombardi V., Linari M. The working stroke of the myosin II motor in muscle is not tightly coupled to release of orthophosphate from its active site. The Journal of Physiology. 2013;591, part 20:5187–5205. doi: 10.1113/jphysiol.2013.257410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piazzesi G., Lombardi V. A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophysical Journal. 1995;68(5):1966–1979. doi: 10.1016/S0006-3495(95)80374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferenczi M. A., Bershitsky S. Y., Koubassova N. A., et al. Why muscle is an efficient shock absorber. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0085739.e85739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kozuka J., Yokota H., Arai Y., Ishii Y., Yanagida T. Dynamic polymorphism of single actin molecules in the actin filament. Nature Chemical Biology. 2006;2(2):83–86. doi: 10.1038/nchembio763. [DOI] [PubMed] [Google Scholar]
- 126.Prochniewicz E., Katayama E., Yanagida T., Thomas D. D. Cooperativity in F-actin: chemical modifications of actin monomers affect the functional interactions of myosin with unmodified monomers in the same actin filament. Biophysical Journal. 1993;65(1):113–123. doi: 10.1016/S0006-3495(93)81057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galkin V. E., Orlova A., Egelman E. H. Actin filaments as tension sensors. Current Biology. 2012;22(3):R96–R101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galkin V. E., Orlova A., Schröder G. F., Egelman E. H. Structural polymorphism in F-actin. Nature Structural and Molecular Biology. 2010;17(11):1318–1323. doi: 10.1038/nsmb.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orlova A., Egelman E. H. A conformational change in the actin subunit can change the flexibility of the actin filament. Journal of Molecular Biology. 1993;232(2):334–341. doi: 10.1006/jmbi.1993.1393. [DOI] [PubMed] [Google Scholar]
- 130.Uyeda T. Q. P., Iwadate Y., Umeki N., Nagasaki A., Yumura S. Stretching actin filaments within cells enhances their affinity for the myosin ii motor domain. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0026200.e26200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tokuraku K., Kurogi R., Toya R., Uyeda T. Q. P. Novel mode of cooperative binding between myosin and Mg2+ -actin filaments in the presence of low concentrations of ATP. Journal of Molecular Biology. 2009;386(1):149–162. doi: 10.1016/j.jmb.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 132.Vikhorev P. G., Vikhoreva N. N., Månsson A. Bending flexibility of actin filaments during motor-induced sliding. Biophysical Journal. 2008;95(12):5809–5819. doi: 10.1529/biophysj.108.140335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prochniewicz E., Walseth T. F., Thomas D. D. Structural dynamics of actin during active interaction with myosin: different effects of weakly and strongly bound myosin heads. Biochemistry. 2004;43(33):10642–10652. doi: 10.1021/bi049914e. [DOI] [PubMed] [Google Scholar]
- 134.Miller G., Maycock J., White E., Peckham M., Calaghan S. Heterologous expression of wild-type and mutant β-cardiac myosin changes the contractile kinetics of cultured mouse myotubes. Journal of Physiology. 2003;548(1):167–174. doi: 10.1113/jphysiol.2002.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sommese R. F., Sung J., Nag S., et al. Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):12607–12612. doi: 10.1073/pnas.1309493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bloemink M., Deacon J., Langer S., et al. The hypertrophic cardiomyopathy myosin mutation R453C alters ATP binding and hydrolysis of human cardiac β-myosin. Journal of Biological Chemistry. 2014;289(8):5158–5167. doi: 10.1074/jbc.M113.511204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Resnicow D. I., Deacon J. C., Warrick H. M., Spudich J. A., Leinwand L. A. Functional diversity among a family of human skeletal muscle myosin motors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(3):1053–1058. doi: 10.1073/pnas.0913527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malik F. I., Hartman J. J., Elias K. A., et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331(6023):1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan B. P., Muci A., Lu P.-P., et al. Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac myosin. ACS Medicinal Chemistry Letters. 2010;1(9):472–477. doi: 10.1021/ml100138q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Radke M. B., Taft M. H., Stapel B., Hilfiker-Kleiner D., Preller M., Manstein D. J. Small molecule-mediated refolding and activation of myosin motor function. eLife. 2014;2014(3) doi: 10.7554/eLife.01603.e01603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kohler J., Winkler G., Schulte I., et al. Mutation of the myosin converter domain alters cross-bridge elasticity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3557–3562. doi: 10.1073/pnas.062415899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferrantini C., Belus A., Piroddi N., Scellini B., Tesi C., Poggesi C. Mechanical and energetic consequences of HCM-causing mutations. Journal of Cardiovascular Translational Research. 2009;2(4):441–451. doi: 10.1007/s12265-009-9131-8. [DOI] [PubMed] [Google Scholar]
- 143.Bond L. M., Tumbarello D. A., Kendrick-Jones J., Buss F. Small-molecule inhibitors of myosin proteins. Future Medicinal Chemistry. 2013;5(1):41–52. doi: 10.4155/fmc.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heissler S. M., Selvadurai J., Bond L. M., et al. Kinetic properties and small-molecule inhibition of human myosin-6. FEBS Letters. 2012;586(19):3208–3214. doi: 10.1016/j.febslet.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Preller M., Chinthalapudi K., Martin R., Knölker H.-J., Manstein D. J. Inhibition of myosin ATPase activity by halogenated pseudilins: a structure-activity study. Journal of Medicinal Chemistry. 2011;54(11):3675–3685. doi: 10.1021/jm200259f. [DOI] [PubMed] [Google Scholar]
- 146.Fedorov R., Böhl M., Tsiavaliaris G., et al. The mechanism of pentabromopseudilin inhibition of myosin motor activity. Nature Structural & Molecular Biology. 2009;16(1):80–88. doi: 10.1038/nsmb.1542. [DOI] [PubMed] [Google Scholar]
- 147.Chinthalapudi K., Taft M. H., Martin R., et al. Mechanism and specificity of pentachloropseudilin-mediated inhibition of myosin motor activity. The Journal of Biological Chemistry. 2011;286(34):29700–29708. doi: 10.1074/jbc.M111.239210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramachandran I., Terry M., Ferrari M. B. Skeletal muscle myosin cross-bridge cycling is necessary for myofibrillogenesis. Cell Motility and the Cytoskeleton. 2003;55(1):61–72. doi: 10.1002/cm.10113. [DOI] [PubMed] [Google Scholar]
- 149.Kovács M., Tóth J., Hetényi C., Málnási-Csizmadia A., Seller J. R. Mechanism of blebbistatin inhibition of myosin II. The Journal of Biological Chemistry. 2004;279(34):35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 150.Straight A. F., Cheung A., Limouze J., et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299(5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 151.Regnier M., Morris C., Homsher E. Regulation of the cross-bridge transition from a weakly to strongly bound state in skinned rabbit muscle fibers. American Journal of Physiology. 1995;269(6, part 1):C1532–C1539. doi: 10.1152/ajpcell.1995.269.6.C1532. [DOI] [PubMed] [Google Scholar]
- 152.Linari M., Piazzesi G., Lombardi V. The effect of myofilament compliance on kinetics of force generation by myosin motors in muscle. Biophysical Journal. 2009;96(2):583–592. doi: 10.1016/j.bpj.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McKillop D. F. A., Fortune N. S., Ranatunga K. W., Geeves M. A. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. Journal of Muscle Research and Cell Motility. 1994;15(3):309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- 154.Bagni M. A., Cecchi G., Colomo F., Garzella P. Effects of 2,3-butanedione monoxime on the crossbridge kinetics in frog single muscle fibres. Journal of Muscle Research and Cell Motility. 1992;13(5):516–522. doi: 10.1007/BF01737994. [DOI] [PubMed] [Google Scholar]
- 155.Sun Y.-B., Lou F., Edman K. A. P. The effects of 2,3-butanedione monoxime (BDM) on the force-velocity relation in single muscle fibres of the frog. Acta Physiologica Scandinavica. 1995;153(4):325–334. doi: 10.1111/j.1748-1716.1995.tb09870.x. [DOI] [PubMed] [Google Scholar]
- 156.Webb M., Jackson D. R., Stewart T. J., et al. The myosin duty ratio tunes the calcium sensitivity and cooperative activation of the thin filament. Biochemistry. 2013;52(37):6437–6444. doi: 10.1021/bi400262h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Allingham J. S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nature Structural & Molecular Biology. 2005;12(4):378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 158.Månsson A. Encyclopedia of Biophysics. 2013. ATP-driven mechanical work performed by molecular motors; pp. 135–141. [Google Scholar]
- 159.Chin L., Yue P., Feng J. J., Seow C. Y. Mathematical simulation of muscle cross-bridge cycle and force-velocity relationship. Biophysical Journal. 2006;91(10):3653–3663. doi: 10.1529/biophysj.106.092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conibear P. B., Geeves M. A. Cooperativity between the two heads of rabbit skeletal muscle heavy meromyosin in binding to actin. Biophysical Journal. 1998;75(2):926–937. doi: 10.1016/S0006-3495(98)77581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Månsson A. Translational actomyosin research: fundamental insights and applications hand in hand. Journal of Muscle Research and Cell Motility. 2012;33(3-4):219–233. doi: 10.1007/s10974-012-9298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mansson A., Balaz M., Albet-Torres N., Rosengren K. J. In vitro assays of molecular motors—impact of motor-surface interactions. Frontiers in Bioscience. 2008;13(15):5732–5754. doi: 10.2741/3112. [DOI] [PubMed] [Google Scholar]
- 163.Homsher E., Wang F., Sellers J. R. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. American Journal of Physiology: Cell Physiology. 1992;262(3, part 1):C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- 164.Chizhov I., Hartmann F. K., Hundt N., Tsiavaliaris G. Global fit analysis of myosin-5b motility reveals thermodynamics of Mg2+-sensitive acto-myosin-ADP states. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064797.e64797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ashkin A. Forces of a single-beam gradient laser trap on a dielectric sphere in the ray optics regime. Methods in Cell Biology. 1998;55:1–27. doi: 10.1016/s0091-679x(08)60399-4. [DOI] [PubMed] [Google Scholar]
- 166.Ashkin A. Applications of laser radiation pressure. Science. 1980;210(4474):1081–1088. doi: 10.1126/science.210.4474.1081. [DOI] [PubMed] [Google Scholar]
- 167.Simmons R. M., Finer J. T., Chu S., Spudich J. A. Quantitative measurements of force and displacement using an optical trap. Biophysical Journal. 1996;70(4):1813–1822. doi: 10.1016/S0006-3495(96)79746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tyska M. J., Dupuis D. E., Guilford W. H., et al. Two heads of myosin are better than one for generating force and motion. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4402–4407. doi: 10.1073/pnas.96.8.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Veigel C., Bartoo M. L., White D. C. S., Sparrow J. C., Molloy J. E. The stiffness of rabbit skeletal actomyosin cross-bridges determined with an optical tweezers transducer. Biophysical Journal. 1998;75(3):1424–1438. doi: 10.1016/S0006-3495(98)74061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takagi Y., Homsher E. E., Goldman Y. E., Shuman H. Force generation in single conventional actomyosin complexes under high dynamic load. Biophysical Journal. 2006;90(4):1295–1307. doi: 10.1529/biophysj.105.068429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tanaka H., Ishijima A., Honda M., Saito K., Yanagida T. Orientation dependence of displacements by a single one-headed myosin relative to the actin filament. Biophysical Journal. 1998;75(4):1886–1894. doi: 10.1016/S0006-3495(98)77629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guilford W. H., Dupuis D. E., Kennedy G., Wu J., Patlak J. B., Warshaw D. M. Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophysical Journal. 1997;72(3):1006–1021. doi: 10.1016/S0006-3495(97)78753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rief M., Rock R. S., Mehta A. D., Mooseker M. S., Cheney R. E., Spudich J. A. Myosin-V stepping kinetics: a molecular model for processivity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(17):9482–9486. doi: 10.1073/pnas.97.17.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Visscher K., Schnltzer M. J., Block S. M. Single kinesin molecules studied with a molecular force clamp. Nature. 1999;400(6740):184–189. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 175.Veigel C., Schmitz S., Wang F., Sellers J. R. Load-dependent kinetics of myosin-V can explain its high processivity. Nature Cell Biology. 2005;7(9):861–869. doi: 10.1038/ncb1287. [DOI] [PubMed] [Google Scholar]
- 176.de la Cruz E. M., Wells A. L., Rosenfeld S. S., Ostap E. M., Sweeney H. L. The kinetic mechanism of myosin V. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):13726–13731. doi: 10.1073/pnas.96.24.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Molloy J. E., Burns J. E., Sparrow J. C., et al. Single-molecule mechanics of heavy meromyosin and S1 interacting with rabbit or Drosophila actins using optical tweezers. Biophysical Journal. 1995;68(4):298S–305S. [PMC free article] [PubMed] [Google Scholar]
- 178.Molloy J. E., Kendrick-Jones J., Veigel C., Tregear R. T. An unexpectedly large working stroke from chymotryptic fragments of myosin II. FEBS Letters. 2000;480(2-3):293–297. doi: 10.1016/S0014-5793(00)01937-2. [DOI] [PubMed] [Google Scholar]
- 179.Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- 180.Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- 181.Steffen W., Smith D., Simmons R., Sleep J. Mapping the actin filament with myosin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):14949–14954. doi: 10.1073/pnas.261560698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Capitanio M., Canepari M., Maffei M., et al. Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke. Nature Methods. 2012;9(10):1013–1019. doi: 10.1038/nmeth.2152. [DOI] [PubMed] [Google Scholar]
- 183.Debold E. P., Patlak J. B., Warshaw D. M. Slip sliding away: load-dependence of velocity generated by skeletal muscle myosin molecules in the laser trap. Biophysical Journal. 2005;89(5):L34–L36. doi: 10.1529/biophysj.105.072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pate E., White H., Cooke R. Determination of the myosin step size from mechanical and kinetic data. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2451–2455. doi: 10.1073/pnas.90.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ramsey R. W., Street S. F. The isometric length-tension diagram of isolated skeletal muscle fibers of the frog. Journal of Cellular and Comparative Physiology. 1940;15(1):11–34. doi: 10.1002/jcp.1030150103. [DOI] [Google Scholar]
- 186.Lannergren J., Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. Journal of Physiology. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. The Journal of Physiology. 1971;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Natori R. The role of myofibrils, sarcoplasm and sarcolemma. Jikeikai Medical Journal. 1954;1:177–192. [Google Scholar]
- 189.Szent-Gyorgyi A. Free-energy relations and contraction of actomyosin. The Biological Bulletin. 1949;96(2):140–161. [PubMed] [Google Scholar]
- 190.Hill A. V. The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society B. 1938;126:136–195. [Google Scholar]
- 191.Edman K. A. P. Double-hyperbolic force-velocity relation in frog muscle fibres. Journal of Physiology. 1988;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophysical Journal. 1985;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophysical Journal. 1979;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporara. The Journal of Physiology. 1984;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cecchi G., Colomo F., Lombardi V. Force-velocity relation in normal and nitrate-treated frog single muscle fibres during rise of tension in an isometric tetanus. Journal of Physiology. 1978;285:257–273. doi: 10.1113/jphysiol.1978.sp012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reconditi M., Linari M., Lucii L., et al. The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature. 2004;428(6982):578–581. doi: 10.1038/nature02380. [DOI] [PubMed] [Google Scholar]
- 197.Minozzo F. C., Rassier D. E. Effects of blebbistatin and Ca2+ concentration on force produced during stretch of skeletal muscle fibers. American Journal of Physiology: Cell Physiology. 2010;299(5):C1127–C1135. doi: 10.1152/ajpcell.00073.2010. [DOI] [PubMed] [Google Scholar]
- 198.Minozzo F. C., Rassier D. E. The effects of Ca2+ and MgADP on force development during and after muscle length changes. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068866.e68866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edman K. A. P., Elzinga G., Noble M. I. M. Critical sarcomere extension required to recruit a decaying component of extra force during stretch in tetanic contractions of frog skeletal muscle fibers. The Journal of General Physiology. 1981;78(4):365–382. doi: 10.1085/jgp.78.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Flitney F. W., Hirst D. G. Cross-bridge detachment and sarcomere “give” during stretch of active frog's muscle. The Journal of Physiology. 1978;276:449–465. doi: 10.1113/jphysiol.1978.sp012246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pinniger G. J., Ranatunga K. W., Offer G. W. Crossbridge and non-crossbridge contributions to tension in lengthening rat muscle: Force-induced reversal of the power stroke. Journal of Physiology. 2006;573, part 3:627–643. doi: 10.1113/jphysiol.2005.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Roots H., Offer G. W., Ranatunga K. W. Comparison of the tension responses to ramp shortening and lengthening in intact mammalian muscle fibres: crossbridge and non-crossbridge contributions. Journal of Muscle Research and Cell Motility. 2007;28(2-3):123–139. doi: 10.1007/s10974-007-9110-0. [DOI] [PubMed] [Google Scholar]
- 203.Lombardi V., Piazzesi G., Linari M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature. 1992;355(6361):638–641. doi: 10.1038/355638a0. [DOI] [PubMed] [Google Scholar]
- 204.Mansson A. Changes in force and stiffness during stretch of skeletal muscle fibers, effects of hypertonicity. Biophysical Journal. 1989;56(2):429–433. doi: 10.1016/S0006-3495(89)82689-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Edman K. A. P., Elzinga G., Noble M. I. M. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. Journal of Physiology. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Colombini B., Bagni M. A., Romano G., Cecchi G. Characterization of actomyosin bond properties in intact skeletal muscle by force spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(22):9284–9289. doi: 10.1073/pnas.0611070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rassier D. E. Pre-power stroke cross bridges contribute to force during stretch of skeletal muscle myofibrils. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1651):2577–2586. doi: 10.1098/rspb.2008.0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chinn M., Getz E. B., Cooke R., Lehman S. L. Force enhancement by PEG during ramp stretches of skeletal muscle. Journal of Muscle Research and Cell Motility. 2003;24(8):571–578. doi: 10.1023/B:JURE.0000009846.05582.89. [DOI] [PubMed] [Google Scholar]
- 209.Ranatunga K. W. Force and power generating mechanism(s) in active muscle as revealed from temperature perturbation studies. Journal of Physiology. 2010;588(19):3657–3670. doi: 10.1113/jphysiol.2010.194001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rassier D. E., Herzog W. Active force inhibition and stretch-induced force enhancement in frog muscle treated with BDM. Journal of Applied Physiology. 2004;97(4):1395–1400. doi: 10.1152/japplphysiol.00377.2004. [DOI] [PubMed] [Google Scholar]
- 211.Piazzesi G., Reconditi M., Koubassova N., et al. Temperature dependence of the force-generating process in single fibres from frog skeletal muscle. The Journal of Physiology. 2003;549(1):93–106. doi: 10.1113/jphysiol.2002.038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. Journal of Physiology. 1977;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. The Journal of Physiology. 1981;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ford L. E., Huxley A. F., Simmons R. M. Tension transients during steady shortening of frog muscle fibres. Journal of Physiology. 1985;361:131–150. doi: 10.1113/jphysiol.1985.sp015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. Journal of Physiology. 1986;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barclay C. J., Woledge R. C., Curtin N. A. Inferring crossbridge properties from skeletal muscle energetics. Progress in Biophysics and Molecular Biology. 2010;102(1):53–71. doi: 10.1016/j.pbiomolbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 217.Bershitsky S. Y., Tsaturyan A. K., Bershitskaya O. N., et al. Muscle force is generated by myosin heads stereospecifically attached to action. Nature. 1997;388(6638):186–190. doi: 10.1038/40651. [DOI] [PubMed] [Google Scholar]
- 218.Seebohm B., Matinmehr F., Köhler J., et al. Cardiomyopathy mutations reveal variable region of myosin converter as major element of cross-bridge compliance. Biophysical Journal. 2009;97(3):806–824. doi: 10.1016/j.bpj.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pant K., Watt J., Greenberg M., Jones M., Szczesna-Cordary D., Moore J. R. Removal of the cardiac myosin regulatory light chain increases isometric force production. The FASEB Journal. 2009;23(10):3571–3580. doi: 10.1096/fj.08-126672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dobbie I., Linari M., Piazzesi G., et al. Elastic bending and active tilting of myosin heads during muscle contraction. Nature. 1998;396(6709):383–387. doi: 10.1038/24647. [DOI] [PubMed] [Google Scholar]
- 221.Lewalle A., Steffen W., Stevenson O., Ouyang Z., Sleep J. Single-molecule measurement of the stiffness of the rigor myosin head. Biophysical Journal. 2008;94(6):2160–2169. doi: 10.1529/biophysj.107.119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Offer G., Ranatunga K. W. Crossbridge and filament compliance in muscle: implications for tension generation and lever arm swing. Journal of Muscle Research and Cell Motility. 2010;31(4):245–265. doi: 10.1007/s10974-010-9232-7. [DOI] [PubMed] [Google Scholar]
- 223.Highsmith S. Electrostatic contributions to the binding of myosin and myosin-MgADP to F-actin in solution. Biochemistry. 1990;29(47):10690–10694. doi: 10.1021/bi00499a017. [DOI] [PubMed] [Google Scholar]
- 224.Zhao Y., Kawai M. The hydrophobic interaction between actin and myosin underlies the mechanism of force generation by cross-bridges. Biophysical Journal. 1995;68(4, supplement):p. 332s. [Google Scholar]
- 225.Ranatunga K. W., Coupland M. E., Mutungi G. An asymmetry in the phosphate dependence of tension transients induced by length perturbation in mammalian (rabbit psoas) muscle fibres. The Journal of Physiology. 2002;542(3):899–910. doi: 10.1113/jphysiol.2002.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Goldman Y. E., McCray J. A., Ranatunga K. W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. The Journal of Physiology. 1987;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Davis J. S., Harrington W. F. Force generation by muscle fibers in rigor: a laser temperature-jump study. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(4):975–979. doi: 10.1073/pnas.84.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ranatunga K. W. Endothermic force generation in fast and slow mammalian (rabbit) muscle fibers. Biophysical Journal. 1996;71(4):1905–1913. doi: 10.1016/S0006-3495(96)79389-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Davis J. S., Rodgers M. E. Force generation and temperature-jump and length-jump tension transients in muscle fibers. Biophysical Journal. 1995;68(5):2032–2040. doi: 10.1016/S0006-3495(95)80380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Davis J. S., Epstein N. D. Mechanistic role of movement and strain sensitivity in muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6140–6145. doi: 10.1073/pnas.0812487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bershitsky S. Y., Tsaturyan A. K. Effect of joule temperature jump on tension and stiffness of skinned rabbit muscle fibers. Biophysical Journal. 1989;56(5):809–816. doi: 10.1016/S0006-3495(89)82727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fortune N. S., Geeves M. A., Ranatunga K. W. Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7323–7327. doi: 10.1073/pnas.88.16.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. The Journal of Physiology. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Homsher E., Lacktis J., Regnier M. Strain-dependent modulation of phosphate transients in rabbit skeletal muscle fibers. Biophysical Journal. 1997;72(4):1780–1791. doi: 10.1016/S0006-3495(97)78824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tesi C., Colomo F., Nencini S., Piroddi N., Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophysical Journal. 2000;78(6):3081–3092. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Caremani M., Dantzig J., Goldman Y. E., Lombardi V., Linari M. Effect of inorganic phosphate on the force and number of myosin cross-bridges during the isometric contraction of permeabilized muscle fibers from rabbit psoas. Biophysical Journal. 2008;95(12):5798–5808. doi: 10.1529/biophysj.108.130435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.van der Heide U. A., Ketelaars M., Treijtel B. W., de Beer E. L., Blangé T. Strain dependence of the elastic properties of force-producing cross- bridges in rigor skeletal muscle. Biophysical Journal. 1997;72(2, part 1):814–821. doi: 10.1016/S0006-3495(97)78715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophysical Journal. 1994;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophysical Journal. 1994;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Higuchi H., Yanagida T., Goldman Y. E. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophysical Journal. 1995;69(3):1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Månsson A. Significant impact on muscle mechanics of small nonlinearities in myofilament elasticity. Biophysical Journal. 2010;99(6):1869–1875. doi: 10.1016/j.bpj.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Edman K. A. P. Non-linear myofilament elasticity in frog intact muscle fibres. Journal of Experimental Biology. 2009;212(8):1115–1119. doi: 10.1242/jeb.020982. [DOI] [PubMed] [Google Scholar]
- 243.Colombina B., Nocella M., Bagni M. A., Griffiths P. J., Cecchi G. Is the cross-bridge stiffness proportional to tension during muscle fiber activation? Biophysical Journal. 2010;98(11):2582–2590. doi: 10.1016/j.bpj.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Piazzesi G., Dolfi M., Brunello E., et al. The myofilament elasticity and its effect on kinetics of force generation by the myosin motor. Archives of Biochemistry and Biophysics. 2014;552-553:108–116. doi: 10.1016/j.abb.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 245.Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. J. Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications. Journal of Molecular Biology. 1983;169(2):469–506. doi: 10.1016/S0022-2836(83)80062-X. [DOI] [PubMed] [Google Scholar]
- 246.Knupp C., Offer G., Ranatunga K. W., Squire J. M. Probing muscle myosin motor action: x-ray (m3 and m6) interference measurements report motor domain not lever arm movement. Journal of Molecular Biology. 2009;390(2):168–181. doi: 10.1016/j.jmb.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 247.Huxley H. E. Recent X-ray diffraction studies of muscle contraction and their implications. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359(1452):1879–1882. doi: 10.1098/rstb.2004.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Squire J. M., Knupp C. X-ray diffraction studies of muscle and the crossbridge cycle. Advances in Protein Chemistry. 2005;71:195–255. doi: 10.1016/S0065-3233(04)71006-2. [DOI] [PubMed] [Google Scholar]
- 249.Bershitsky S. Y., Ferenczi M. A., Koubassova N. A., Tsaturyan A. K. Insight into the actin-myosin motor from x-ray diffraction on muscle. Frontiers in Bioscience. 2009;14(8):3188–3213. doi: 10.2735/3444. [DOI] [PubMed] [Google Scholar]
- 250.Campbell S. G., Hatfield P. C., Campbell K. S. A mathematical model of muscle containing heterogeneous half-sarcomeres exhibits residual force enhancement. PLoS Computational Biology. 2011;7(9) doi: 10.1371/journal.pcbi.1002156.e1002156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Edman K. A. P., Reggiani C., Schiaffino S., Te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. The Journal of Physiology. 1988;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Edman K. A. P., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. Journal of Physiology. 1984;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cleworth D., Edman K. A. P. Laser diffraction studies on single skeletal muscle fibers. Science. 1969;163(3864):296–298. doi: 10.1126/science.163.3864.296. [DOI] [PubMed] [Google Scholar]
- 254.Rosser B. W. C., Bandman E. Heterogeneity of protein expression within muscle fibers. Journal of Animal Science. 2003;81:E94–E101. [Google Scholar]
- 255.Poggesi C., Tesi C., Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflügers Archiv. 2005;449(6):505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 256.Stehle R., Krüger M., Pfitzer G. Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid Ca2+ changes. Biophysical Journal. 2002;83(4):2152–2161. doi: 10.1016/S0006-3495(02)73975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Telley I. A., Stehle R., Ranatunga K. W., Pfitzer G., Stüssi E., Denoth J. Dynamic behaviour of half-sarcomeres during and after stretch in activated rabbit psoas myofibrils: sarcomere asymmetry but no “sarcomere popping”. The Journal of Physiology. 2006;573(1):173–185. doi: 10.1113/jphysiol.2006.105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Weiss S., Rossi R., Pellegrino M.-A., Bottinelli R., Geeves M. A. Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. Journal of Biological Chemistry. 2001;276(49):45902–45908. doi: 10.1074/jbc.M107434200. [DOI] [PubMed] [Google Scholar]
- 259.Hook P., Larsson L. Actomyosin interactions in a novel single muscle fiber in vitro motility assay. Journal of Muscle Research and Cell Motility. 2000;21(4):357–365. doi: 10.1023/a:1005614212575. [DOI] [PubMed] [Google Scholar]
- 260.Canepari M., Rossi R., Pellegrino M. A., Reggiani C., Bottinelli R. Speeds of actin translocation in vitro by myosins extracted from single rat muscle fibres of different types. Experimental Physiology. 1999;84(4):803–806. doi: 10.1017/S0958067099003504. [DOI] [PubMed] [Google Scholar]
- 261.Sata M., Sugiura S., Yamashita H., Momomura S.-I., Serizawa T. Dynamic interaction between cardiac myosin isoforms modifies velocity of actomyosin sliding in vitro. Circulation Research. 1993;73(4):696–704. doi: 10.1161/01.RES.73.4.696. [DOI] [PubMed] [Google Scholar]
- 262.Harris D. E., Warshaw D. M. Smooth and skeletal muscle actin are mechanically indistinguishable in the in vitro motility assay. Circulation Research. 1993;72(1):219–224. doi: 10.1161/01.RES.72.1.219. [DOI] [PubMed] [Google Scholar]
- 263.Homsher E., Lee D. M., Morris C., Pavlov D., Tobacman L. S. Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. The Journal of Physiology. 2000;524(1):233–243. doi: 10.1111/j.1469-7793.2000.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Stewart M., Franks-Skiba K., Cooke R. Myosin regulatory light chain phosphorylation inhibits shortening velocities of skeletal muscle fibers in the presence of the myosin inhibitor blebbistatin. Journal of Muscle Research and Cell Motility. 2009;30(1-2):17–27. doi: 10.1007/s10974-008-9162-9. [DOI] [PubMed] [Google Scholar]
- 265.Tesi C., Colomo F., Piroddi N., Poggesi C. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. Journal of Physiology. 2002;541(1):187–199. doi: 10.1113/jphysiol.2001.013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pun C., Syed A., Rassier D. E. History-dependent properties of skeletal muscle myofibrils contracting along the ascending limb of the force-length relationship. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1680):475–484. doi: 10.1098/rspb.2009.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Belus A., Piroddi N., Scellini B., et al. The familial hypertrophic cardiomyopathy-associated myosin mutation R403Q accelerates tension generation and relaxation of human cardiac myofibrils. The Journal of Physiology. 2008;586(15):3639–3644. doi: 10.1113/jphysiol.2008.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kurosaka S., Leu N. A., Pavlov I., et al. Arginylation regulates myofibrils to maintain heart function and prevent dilated cardiomyopathy. Journal of Molecular and Cellular Cardiology. 2012;53(3):333–341. doi: 10.1016/j.yjmcc.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ribeiro P. A. B., Ribeiro J. P., Minozzo F. C., et al. Contractility of myofibrils from the heart and diaphragm muscles measured with atomic force cantilevers: effects of heart-specific deletion of arginyl-tRNA-protein transferase. International Journal of Cardiology. 2013;168(4):3564–3571. doi: 10.1016/j.ijcard.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 271.Rassier D. E., Pavlov I. Force produced by isolated sarcomeres and half-sarcomeres after an imposed stretch. The American Journal of Physiology—Cell Physiology. 2012;302(1):C240–C248. doi: 10.1152/ajpcell.00208.2011. [DOI] [PubMed] [Google Scholar]
- 272.Minozzo F. C., Baroni B. M., Correa J. A., Vaz M. A., Rassier D. E. Force produced after stretch in sarcomeres and half-sarcomeres isolated from skeletal muscles. Scientific Reports. 2013;3, article 2320 doi: 10.1038/srep02320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schroder R. R., Manstein D. J., Jahn W., et al. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993;364(6433):171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- 274.Lorenz M., Holmes K. C. The actin-myosin interface. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12529–12534. doi: 10.1073/pnas.1003604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fischer S., Windshügel B., Horak D., Holmes K. C., Smith J. C. Structural mechanism of the recovery stroke in the myosin molecular motor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(19):6873–6878. doi: 10.1073/pnas.0408784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smith C. A., Rayment I. X-ray structure of the magnesium(II)-pyrophosphate complex of the truncated head of Dictyostelium discoideum myosin to 2.7 A resolution. Biochemistry. 1995;34(28):8973–8981. doi: 10.1021/bi00028a005. [DOI] [PubMed] [Google Scholar]
- 277.Dominguez R., Freyzon Y., Trybus K. M., Cohen C. Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell. 1998;94(5):559–571. doi: 10.1016/S0092-8674(00)81598-6. [DOI] [PubMed] [Google Scholar]
- 278.Gourinath S., Himmel D. M., Brown J. H., Reshetnikova L., Szent-Györgyi A. G., Cohen C. Crystal structure of scallop Myosin s1 in the pre-power stroke state to 2.6 Å resolution: flexibility and function in the head. Structure. 2003;11(12):1621–1627. doi: 10.1016/j.str.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 279.Houdusse A., Szent-Györgyi A. G., Cohen C. Three conformational states of scallop myosin S1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11238–11243. doi: 10.1073/pnas.200376897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Bauer C. B., Holden H. M., Thoden J. B., Smith R., Rayment I. X-ray structures of the Apo and MgATP-bound states of Dictyostelium discoideum myosin motor domain. The Journal of Biological Chemistry. 2000;275(49):38494–38499. doi: 10.1074/jbc.M005585200. [DOI] [PubMed] [Google Scholar]
- 281.Gulick A. M., Bauer C. B., Thoden J. B., Rayment I. X-ray structures of the MgADP, MgATPγS, and MgAMPPNP complexes of the Dictyostelium discoideum myosin motor domain. Biochemistry. 1997;36(39):11619–11628. doi: 10.1021/bi9712596. [DOI] [PubMed] [Google Scholar]
- 282.Ménétrey J., Llinas P., Cicolari J., et al. The post-rigor structure of myosin VI and implications for the recovery stroke. The EMBO Journal. 2008;27(1):244–252. doi: 10.1038/sj.emboj.7601937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Winkelmann D. A., Baker T. S., Rayment I. Three-dimensional structure of myosin subfragment-1 from electron microscopy of sectioned crystals. The Journal of Cell Biology. 1991;114(4):701–713. doi: 10.1083/jcb.114.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Coureux P.-D., Sweeney H. L., Houdusse A. Three myosin V structures delineate essential features of chemo-mechanical transduction. The EMBO Journal. 2004;23(23):4527–4537. doi: 10.1038/sj.emboj.7600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Menetrey J., Bahloul A., Wells A. L., et al. The structure of the myosin VI motor reveals the mechanism of directionality reversal. Nature. 2005;435(7043):779–785. doi: 10.1038/nature03592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Coureux P.-D., Wells A. L., Ménétrey J., et al. A structural state of the myosin V motor without bound nucleotide. Nature. 2003;425(6956):419–423. doi: 10.1038/nature01927. [DOI] [PubMed] [Google Scholar]
- 287.Reubold T. F., Eschenburg S., Becker A., Kull F. J., Manstein D. J. A structural model for actin-induced nucleotide release in myosin. Nature Structural Biology. 2003;10(10):826–830. doi: 10.1038/nsb987. [DOI] [PubMed] [Google Scholar]
- 288.Yang Y., Gourinath S., Kovács M., et al. Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor. Structure. 2007;15(5):553–564. doi: 10.1016/j.str.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 289.Takács B., O'Neall-Hennessey E., Hetényi C., Kardos J., Szent-Györgyi A. G., Kovács M. Myosin cleft closure determines the energetics of the actomyosin interaction. The FASEB Journal. 2011;25(1):111–121. doi: 10.1096/fj.10-164871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Holmes K. C., Angert I., Kull F. J., Jahn W., Schröder R. R. Elechron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425(6956):423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 291.Behrmann E., Müller M., Penczek P. A., Mannherz H. G., Manstein D. J., Raunser S. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150(2):327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Geeves M. A., Jeffries T. E., Millar N. C. ATP-induced dissociation of rabbit skeletal actomyosin subfragment 1. Characterization of an isomerization of the ternary Acto-S1-ATP complex. Biochemistry. 1986;25(26):8454–8458. doi: 10.1021/bi00374a020. [DOI] [PubMed] [Google Scholar]
- 293.Furch M., Fujita-Becker S., Geeves M. A., Holmes K. C., Manstein D. J. Role of the salt-bridge between switch-1 and switch-2 of Dictyostelium myosin. Journal of Molecular Biology. 1999;290(3):797–809. doi: 10.1006/jmbi.1999.2921. [DOI] [PubMed] [Google Scholar]
- 294.Onishi H., Morales M. F., Kojima S.-I., Katoh K., Fujiwara K. Functional transitions in myosin: role of highly conserved Gly and Glu residues in the active site. Biochemistry. 1997;36(13):3767–3772. doi: 10.1021/bi9630772. [DOI] [PubMed] [Google Scholar]
- 295.Sasaki N., Sutoh K. Structure-mutation analysis of the ATPase site of Dictyostelium discoideum myosin II. Advances in Biophysics. 1998;35:1–24. doi: 10.1016/S0065-227X(98)80002-6. [DOI] [PubMed] [Google Scholar]
- 296.Sasaki N., Shimada T., Sutoh K. Mutational analysis of the switch II loop of Dictyostelium myosin II. Journal of Biological Chemistry. 1998;273(32):20334–20340. doi: 10.1074/jbc.273.32.20334. [DOI] [PubMed] [Google Scholar]
- 297.Conibear P. B., Bagshaw C. R., Fajer P. G., Kovács M., Málnási-Csizmadia A. Myosin cleft movement and its coupling to actomyosin dissociation. Nature Structural Biology. 2003;10(10):831–835. doi: 10.1038/nsb986. [DOI] [PubMed] [Google Scholar]
- 298.Onishi H., Mochizuki N., Morales M. F. On the myosin catalysis of ATP hydrolysis. Biochemistry. 2004;43(13):3757–3763. doi: 10.1021/bi040002m. [DOI] [PubMed] [Google Scholar]
- 299.Ohki T., Mikhailenko S. V., Morales M. F., Onishi H., Mochizuki N. Transmission of force and displacement within the myosin molecule. Biochemistry. 2004;43(43):13707–13714. doi: 10.1021/bi048954f. [DOI] [PubMed] [Google Scholar]
- 300.Fisher A. J., Smith C. A., Thoden J. B., et al. X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP·BeFx and MgADP·AlF4- Biochemistry. 1995;34(28):8960–8972. doi: 10.1021/bi00028a004. [DOI] [PubMed] [Google Scholar]
- 301.Fisher A. J., Smith C. A., Thoden J., et al. Structural studies of myosin:nucleotide complexes: a revised model for the molecular basis of muscle contraction. Biophysical Journal. 1995;68(4, supplement):19S–28S. [PMC free article] [PubMed] [Google Scholar]
- 302.Tsiavaliaris G., Fujita-Becker S., Batra R., et al. Mutations in the relay loop region result in dominant-negative inhibition of myosin II function in dictyostelium. EMBO Reports. 2002;3(11):1099–1105. doi: 10.1093/embo-reports/kvf214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mesentean S., Koppole S., Smith J. C., Fischer S. The principal motions involved in the coupling mechanism of the recovery stroke of the myosin motor. Journal of Molecular Biology. 2007;367(2):591–602. doi: 10.1016/j.jmb.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 304.Málnási-Csizmadia A., Tóth J., Pearson D. S., et al. Selective perturbation of the myosin recovery stroke by point mutations at the base of the lever arm affects ATP hydrolysis and phosphate release. The Journal of Biological Chemistry. 2007;282(24):17658–17664. doi: 10.1074/jbc.M701447200. [DOI] [PubMed] [Google Scholar]
- 305.Koppole S., Smith J. C., Fischer S. The structural coupling between ATPase activation and recovery stroke in the myosin II motor. Structure. 2007;15(7):825–837. doi: 10.1016/j.str.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 306.Koppole S., Smith J. C., Fischer S. Simulations of the myosin II motor reveal a nucleotide-state sensing element that controls the recovery stroke. Journal of Molecular Biology. 2006;361(3):604–616. doi: 10.1016/j.jmb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 307.Gyimesi M., Kintses B., Bodor A., et al. The mechanism of the reverse recovery step, phosphate release, and actin activation of Dictyostelium myosin II. The Journal of Biological Chemistry. 2008;283(13):8153–8163. doi: 10.1074/jbc.M708863200. [DOI] [PubMed] [Google Scholar]
- 308.Baumketner A. The mechanism of the converter domain rotation in the recovery stroke of myosin motor protein. Proteins. 2012;80(12):2701–2710. doi: 10.1002/prot.24155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Baumketner A., Nesmelov Y. Early stages of the recovery stroke in myosin II studied by molecular dynamics simulations. Protein Science. 2011;20(12):2013–2022. doi: 10.1002/pro.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yengo C. M., Sweeney H. L. Functional role of loop 2 in myosin V. Biochemistry. 2004;43(9):2605–2612. doi: 10.1021/bi035510v. [DOI] [PubMed] [Google Scholar]
- 311.Lieto-Trivedi A., Dash S., Coluccio L. M. Myosin surface loop 4 modulates inhibition of actomyosin 1b atpase activity by tropomyosin. Biochemistry. 2007;46(10):2779–2786. doi: 10.1021/bi602439f. [DOI] [PubMed] [Google Scholar]
- 312.Funabara D., Osawa R., Ueda M., Kanoh S., Hartshorne D. J., Watabe S. Myosin loop 2 is involved in the formation of a trimeric complex of twitchin, actin, and myosin. The Journal of Biological Chemistry. 2009;284(27):18015–18020. doi: 10.1074/jbc.M109.016485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Clobes A. M., Guilford W. H. Loop 2 of myosin is a force-dependent inhibitor of the rigor bond. Journal of Muscle Research and Cell Motility. 2014;35(2):143–152. doi: 10.1007/s10974-014-9375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Várkuti B. H., Yang Z., Kintses B., et al. A novel actin binding site of myosin required for effective muscle contraction. Nature Structural & Molecular Biology. 2012;19(3):299–306. doi: 10.1038/nsmb.2216. [DOI] [PubMed] [Google Scholar]
- 315.Onishi H., Mikhailenko S. V., Morales M. F. Toward understanding actin activation of myosin ATPase: the role of myosin surface loops. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(16):6136–6141. doi: 10.1073/pnas.0601595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Díaz Baños F. G., Bordas J., Lowy J., Svensson A. Small segmental rearrangements in the myosin head can explain force generation in muscle. Biophysical Journal. 1996;71(2):576–589. doi: 10.1016/S0006-3495(96)79292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Thomas D. D., Prochniewicz E., Roopnarine O. Changes in actin and myosin structural dynamics due to their weak and strong interactions. Results and Problems in Cell Differentiation. 2002;36:7–19. doi: 10.1007/978-3-540-46558-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Preller M., Holmes K. C. The myosin start-of-power stroke state and how actin binding drives the power stroke. Cytoskeleton. 2013;70(10):651–660. doi: 10.1002/cm.21125. [DOI] [PubMed] [Google Scholar]
- 319.Kintses B., Gyimesi M., Pearson D. S., et al. Reversible movement of switch 1 loop of myosin determines actin interaction. The EMBO Journal. 2007;26(1):265–274. doi: 10.1038/sj.emboj.7601482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zeng W., Conibear P. B., Dickens J. L., et al. Dynamics of actomyosin interactions in relation to the cross-bridge cycle. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359(1452):1843–1855. doi: 10.1098/rstb.2004.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sleep J., Irving M., Burton K. The ATP hydrolysis and phosphate release steps control the time course of force development in rabbit skeletal muscle. The Journal of Physiology. 2005;563, part 3:671–687. doi: 10.1113/jphysiol.2004.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.He Z.-H., Chillingworth R. K., Brune M., et al. ATPase kinetics on activation of rabbit and frog permeabilized isometric muscle fibres: a real time phosphate assay. The Journal of Physiology. 1997;501(1):125–148. doi: 10.1111/j.1469-7793.1997.125bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Muretta J. M., Petersen K. J., Thomas D. D. Direct real-time detection of the actin-activated power stroke within the myosin catalytic domain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(18):7211–7216. doi: 10.1073/pnas.1222257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Smith D. A., Sleep J. Mechanokinetics of rapid tension recovery in muscle: the myosin working stroke is followed by a slower release of phosphate. Biophysical Journal. 2004;87(1):442–456. doi: 10.1529/biophysj.103.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lionne C., Brune M., Webb M. R., Travers F., Barman T. Time resolved measurements show that phosphate release is the rate limiting step on myofibrillar ATPases. FEBS Letters. 1995;364(1):59–62. doi: 10.1016/0014-5793(95)00356-E. [DOI] [PubMed] [Google Scholar]
- 326.Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Elangovan R., Capitanio M., Melli L., Pavone F. S., Lombardi V., Piazzesi G. An integrated in vitro and in situ study of kinetics of myosin II from frog skeletal muscle. The Journal of Physiology. 2012;590(5):1227–1242. doi: 10.1113/jphysiol.2011.222984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Bowater R., Sleep J. Demembranated muscle fibers catalyze a more rapid exchange between phosphate and adenosine triphosphate than actomyosin subfragment. Biochemistry. 1988;27(14):5314–5323. doi: 10.1021/bi00414a055. [DOI] [PubMed] [Google Scholar]
- 329.Mansfield C., West T. G., Curtin N. A., Ferenczi M. A. Stretch of contracting cardiac muscle abruptly decreases the rate of phosphate release at high and low calcium. The Journal of Biological Chemistry. 2012;287(31):25696–25705. doi: 10.1074/jbc.M112.373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Takagi Y., Shuman H., Goldman Y. E. Coupling between phosphate release and force generation in muscle actomyosin. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359(1452):1913–1920. doi: 10.1098/rstb.2004.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. Journal of Muscle Research and Cell Motility. 1989;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- 332.Liu J., Wendt T., Taylor D., Taylor K. Refined model of the 10S conformation of smooth muscle myosin by cryo-electron microscopy 3D image reconstruction. Journal of Molecular Biology. 2003;329(5):963–972. doi: 10.1016/S0022-2836(03)00516-3. [DOI] [PubMed] [Google Scholar]
- 333.Shriver J. W. Energy transduction in myosin. Trends in Biochemical Sciences. 1984;9(7):322–328. doi: 10.1016/0968-0004(84)90303-7. [DOI] [Google Scholar]
- 334.Xu S., White H. D., Offer G. W., Yu L. C. Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophysical Journal. 2009;96(9):3673–3681. doi: 10.1016/j.bpj.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Norstrom M. F., Smithback P. A., Rock R. S. Unconventional processive mechanics of non-muscle myosin IIB. The Journal of Biological Chemistry. 2010;285(34):26326–26334. doi: 10.1074/jbc.M110.123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hackney D. D., Clark P. K. Catalytic consequences of oligometric organization: Kinetic evidence for “tethered” acto-heavy meromyosin at low ATP concentrations. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(17):5345–5349. doi: 10.1073/pnas.81.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ishiwata S., Shimamoto Y., Sasaki D., Suzuki M. Molecular synchronization in actomyosin motors—from single molecule to muscle fiber via nanomuscle. Advances in Experimental Medicine and Biology. 2005;565:25–36. doi: 10.1007/0-387-24990-7_3. [DOI] [PubMed] [Google Scholar]
- 338.Ito K., Liu X., Katayama E., Uyeda T. Q. P. Cooperativity between two heads of Dictyostelium myosin II in in vitro motility and ATP hydrolysis. Biophysical Journal. 1999;76(2):985–992. doi: 10.1016/S0006-3495(99)77262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Molloy J. E. Muscle contraction: actin filaments enter the fray. Biophysical Journal. 2005;89(1):1–2. doi: 10.1529/biophysj.105.059501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lidke D. S., Thomas D. D. Coordination of the two heads of myosin during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14801–14806. doi: 10.1073/pnas.232161999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kad N. M., Rovner A. S., Fagnant P. M., et al. A mutant heterodimeric myosin with one inactive head generates maximal displacement. The Journal of Cell Biology. 2003;162(3):481–488. doi: 10.1083/jcb.200304023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Vale R. D., Milligan R. A. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288(5463):88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 343.Oosawa F., Maeda Y., Fujime S., Ishiwata S., Yanagida T., Taniguchi M. Dynamic characteristics of F-actin and thin filaments in vivo and in vitro. Journal of Mechanochemistry & Cell Motility. 1977;4(1):63–78. [PubMed] [Google Scholar]
- 344.Tsaturyan A. K., Koubassova N., Ferenczi M. A., Narayanan T., Roessle M., Bershitsky S. Y. Strong binding of myosin heads stretches and twists the actin helix. Biophysical Journal. 2005;88(3):1902–1910. doi: 10.1529/biophysj.104.050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Egelman E. H. New angles on actin dynamics. Structure. 1997;5(9):1135–1137. doi: 10.1016/S0969-2126(97)00264-5. [DOI] [PubMed] [Google Scholar]
- 346.Schutt C. E., Lindberg U. Actin as the generator of tension during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):319–323. doi: 10.1073/pnas.89.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tanner B. C. W., Daniel T. L., Regnier M. Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Computational Biology. 2007;3(7, article e115) doi: 10.1371/journal.pcbi.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Bai F., Wang L., Kawai M. A study of tropomyosin's role in cardiac function and disease using thin-filament reconstituted myocardium. Journal of Muscle Research and Cell Motility. 2013;34(3-4):295–310. doi: 10.1007/s10974-013-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Cornachione A. S., Rassier D. E. A non-cross-bridge, static tension is present in permeabilized skeletal muscle fibers after active force inhibition or actin extraction. American Journal of Physiology: Cell Physiology. 2012;302(3):C566–C574. doi: 10.1152/ajpcell.00355.2011. [DOI] [PubMed] [Google Scholar]
- 350.Baker J. E., Brosseau C., Joel P. B., Warshaw D. M. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophysical Journal. 2002;82(4):2134–2147. doi: 10.1016/S0006-3495(02)75560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Elting M. W., Spudich J. A. Future challenges in single-molecule fluorescence and laser trap approaches to studies of molecular motors. Developmental Cell. 2012;23(6):1084–1091. doi: 10.1016/j.devcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lard M., Ten Siethoff L., Mansson A., Linke H. Molecular motor transport through hollow nanowires. Nano Letters. 2014;14(6):3041–3046. doi: 10.1021/nl404714b. [DOI] [PubMed] [Google Scholar]
- 353.Tsiavaliaris G., Fujita-Becker S., Manstein D. J. Molecular engineering of a backwards-moving myosin motor. Nature. 2004;427(6974):558–561. doi: 10.1038/nature02303. [DOI] [PubMed] [Google Scholar]
- 354.Schindler T. D., Chen L., Lebel P., Nakamura M., Bryant Z. Engineering myosins for long-range transport on actin filaments. Nature Nanotechnology. 2014;9(1):33–38. doi: 10.1038/nnano.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Amrute-Nayak M., Diensthuber R. P., Steffen W., et al. Targeted optimization of a protein nanomachine for operation in biohybrid devices. Angewandte Chemie International Edition. 2010;49(2):312–316. doi: 10.1002/anie.200905200. [DOI] [PubMed] [Google Scholar]
- 356.Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods in Enzymology. 1982;85:55–71. doi: 10.1016/0076-6879(82)85009-X. [DOI] [PubMed] [Google Scholar]
- 357.Kron S. J., Toyoshima Y. Y., Uyeda T. Q. P., Spudich J. A. Assays for actin sliding movement over myosin-coated surfaces. Methods in Enzymology. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-P. [DOI] [PubMed] [Google Scholar]
- 358.Kliche W., Fujita-Becker S., Kollmar M., Manstein D. J., Kull F. J. Structure of a genetically engineered molecular motor. The EMBO Journal. 2001;20(1-2):40–46. doi: 10.1093/emboj/20.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Conibear P. B., Málnási-Csizmadia A., Bagshaw C. R. The effect of F-actin on the relay helix position of myosin II, as revealed by tryptophan fluorescence, and its implications for mechanochemical coupling. Biochemistry. 2004;43(49):15404–15417. doi: 10.1021/bi048338j. [DOI] [PubMed] [Google Scholar]