Abstract
Background
Physical or psychological stress causes functional disorders in the upper gastrointestinal tract. This study aims to elucidate the ameliorating effect of exogenous acylated ghrelin or rikkunshito, a Kampo medicine which acts as a ghrelin enhancer, on gastric dysfunction during acute restraint stress in mice.
Methods
Fasted and postprandial motor function of the gastric antrum was wirelessly measured using a strain gauge force transducer and solid gastric emptying was detected in mice exposed to restraint stress. Plasma corticosterone and ghrelin levels were also measured. To clarify the role of ghrelin on gastrointestinal dysfunction in mice exposed to stress, exogenous acylated ghrelin or rikkunshito was administered, then the mice were subjected to restraint stress.
Key Results
Mice exposed to restraint stress for 60 min exhibited delayed gastric emptying and increased plasma corticosterone levels. Gastric motility was decreased in mice exposed to restraint stress in both fasting and postprandial states. Restraint stress did not cause any change in plasma acylated ghrelin levels, but it significantly increased the plasma des-acyl ghrelin levels. Administration of acylated ghrelin or rikkunshito improved the restraint stress-induced delayed gastric emptying and decreased antral motility. Ameliorating effects of rikkunshito on stress-induced gastric dysfunction were abolished by simultaneous administration of a ghrelin receptor antagonist.
Conclusions & Inferences
Plasma acylated/des-acyl ghrelin imbalance was observed in acute restraint stress. Supplementation of exogenous acylated ghrelin or enhancement of endogenous ghrelin signaling may be useful in the treatment of decreased gastric function caused by stress.
Keywords: acylated ghrelin, des-acyl ghrelin, dysmotility, gastric emptying, restraint stress, rikkunshito
Key Messages
Stress causes functional disorders in the upper gastrointestinal tract. This study aims to examine the effect of ghrelin signaling on gastric dysfunction in restraint-stressed mice.
Restraint stress caused delayed gastric emptying, decreased gastric motility, and plasma acylated/des-acyl ghrelin imbalance.
Supplementation of acylated ghrelin or rikkunshito may be useful in the treatment of decreased gastric function caused by stress.
Introduction
Physical or psychological stress responses cause psychiatric symptoms such as depression and may induce motility disorders in the upper gastrointestinal tract. Dysmotility of the stomach or duodenum, in particular, leads to early satiety, bloating, and loss of appetite.1 Abnormalities in gastrointestinal motility have also been reported in experimental stress models such as restraint2 and fatigue.3 However, the mechanism of gastrointestinal dysmotility under stressed conditions remains unclear.
Ghrelin is an orexigenic hormone mainly secreted from the stomach, and it plays an important role in the motility of the stomach and duodenum.4,5 Ghrelin is present in peripheral blood in two main forms: acylated and des-acyl. The former is an active form and the latter is a metabolite of acylated ghrelin formed by removal of the octanoyl group by an esterase or other enzyme. To enhance gastrointestinal motility, acylated ghrelin transmits signals to the brain via the growth hormone secretagogue receptor (GHS-R), which is expressed on vagal afferent nerve terminals in the stomach.6,7 The peak of plasma acylated ghrelin levels is reported to be strongly associated with spontaneous phase III-like contractions in rats.8 Furthermore, administration of the ghrelin receptor antagonist [D-Lys3]-GHRP-6 abolishes these phase III-like contractions in rodents.8,9 These findings suggest that endogenous ghrelin signaling seems to play a key role in the regulation of gastric motility in fasted rodents. In contrast, physiological role of des-acyl ghrelin for upper gastrointestinal motility is much less clear.10–13
Reports regarding plasma acylated ghrelin level in a stressed condition are inconsistent. It has been reported that exposure to water avoidance stress14 and chronic restraint stress2,3 increases the plasma acylated ghrelin levels. On the other hand, novel environment stress has been reported to decrease them.15 Although these mixed results indicate room for debate regarding the role of ghrelin in stress, compelling evidence from clinical16,17 and experimental studies18,19 suggests that the exogenous supplementation of acylated ghrelin may improve stress-induced gastric dysfunction. However, this issue has not yet been directly tested.
Kampo medicine is a traditional Japanese medicine prepared by combining prescribed amounts of specific raw botanical materials. Importantly, it is covered by the Japanese health insurance system. Several types of Kampo medicine have proven effective in the treatment of gastrointestinal tract disorders.20 Rikkunshito, one of Kampo medicines, is commonly prescribed in Japan to treat dyspeptic symptoms.21,22 In a double-blind controlled study, rikkunshito significantly improved upper gastrointestinal symptoms, such as nausea and anorexia, in patients with functional dyspepsia.23 Several studies using healthy human volunteers,24,25 patients with functional dyspepsia,26 and dog27 and rodent5,28,29 models of gastrointestinal disease have demonstrated that rikkunshito enhanced gastric emptying and food intake by increasing endogenous acylated ghrelin levels or by enhancing ghrelin signaling. In addition, 10-gingerol and atractyrodin, which are active ingredients in rikkunshito, were demonstrated to inhibit deacylation of endogenous acylated ghrelin30 and to increase ghrelin receptor sensitivity.29 However, there are few reports demonstrating the efficacy of rikkunshito on upper gastrointestinal dysfunction in a stressed condition.
The aim of this study was to determine whether supplementation of exogenous acylated ghrelin or potentiation of endogenous ghrelin signaling by rikkunshito has an ameliorating effect on stress-induced gastric dysfunction.
Materials and Methods
Animals
Male ICR mice aged 6–7 weeks (Charles River Laboratories, Yokohama, Japan) were used. Mice were housed in individual cages and maintained in a room with controlled temperature and humidity under a 12-h (07:00–19:00 h) light/dark cycle and free access to food and water. Before the experiments, mice were acclimated for at least 5 days to the environmental conditions. All experiments were performed between 09:00 and 18:00 h, and they were approved by experimental animal ethics committees of Tsumura & Co. (Tokyo, Japan; permit no. 10-054, 10-135, 11-139) and were conducted according to their guidelines. To avoid the influence of diurnal variations, blood and tissue samples were collected between 13:00 and 16:00 h.
Chemicals
Rat acylated ghrelin (Peptide Institute, Osaka, Japan) and ghrelin receptor antagonist, [D-Lys3]-GHRP-6 (Bachem, Bubendorf, Switzerland), were dissolved in sterilized physiological saline (Otsuka Pharmaceutical, Tokyo, Japan) before use. Rikkunshito (Tsumura & Co.), as described in the revised 16th edition of the Japanese Pharmacopoeia, was used as a powdered extract manufactured by spray drying of the hot-water extract of a mixture of eight varieties of crude drugs: Atractylodis lanceae rhizoma, Ginseng radix, Pinelliae tuber, Hoelen, Zizyphi fructus, Aurantii nobilis pericarpium, Glycyrrhizae radix, and Zingiberis rhizoma, and suspended in distilled water.
Induction of restraint stress
Mice were placed in 50-mL centrifuge tubes perforated for ventilation to load stress, as previously reported.31 The tube was large enough to restrain a mouse, allowing it to move its limbs and head, but not to move back and forth.
Measurement of solid gastric emptying
Gastric emptying was measured through a slight modification in the method by Zheng et al.2 Briefly, mice were fasted for 24 h with free access to water, after which they received preweighed standard chow (MF; Oriental Yeast, Tokyo, Japan) for 20 min, followed by restraint stress loading. The mice were decapitated after 60 min of restraint, the pylorus and cardia were clamped, and the stomach was removed (Fig. S1A). The gastric content was collected in a preweighed centrifuge tube with distilled water, centrifuged, dried overnight at 45 °C, and weighed to obtain the dry weight of the gastric content. We calculated the amount of food intake as the difference between the preprandial and postprandial food weight. Gastric emptying was calculated according to the following formula: gastric emptying (%) = (1 − dried weight of gastric content/amount of food intake) × 100.
Fixation of strain gauge force transducer
Mice were fasted overnight and anesthetized by intraperitoneal injection of pentobarbital sodium (Kyoritsu Seiyaku, Tokyo, Japan). After a midline laparotomy, a single-channel strain gauge force transducer (5 × 3 mm, F-04IT; Star Medical, Tokyo, Japan) with sensitivity of 40–60 μV/g with a bridge excitation voltage of 2 V was sutured to the serosal surface of the antrum in a direction that made it possible to measure circular muscle contractions. A transmitter (IMT-10T; Star Medical) was implanted subcutaneously on the backs of the mice to enable wireless measurement of contractions in the antrum, and the abdominal cavity was then closed. After surgery, mice were housed individually and given food and water ad libitum. Mice were allowed to recover for 1 week before the experiment (Fig. S1B).
Analysis of gastric motility
After 24 h of fasting, antral contractions were measured in conscious, freely moving mice (Fig. S1B). The electrical signal was wirelessly transmitted to the receiver (IMT-10RA; Star Medical). Recording and analysis were performed with the recommended software package (Eight Star; Star Medical). After monitoring interdigestive contractions for >2 h, mice were loaded with restraint stress for 60 min; the control group was lightly raised by the tail. In measurements of postprandial gastric motility, mice received standard chow for 20 min followed by 60 min of restraint stress. The effects of restraint stress on gastric motility were evaluated by changes in the motility index (MI). Motility index was defined as mean of area under contractility recording curve per minute. Baseline shifts were corrected by adjusting the waveform display before analysis. The percentage change in MI in fasting conditions was calculated as the ratio of MI during 60 min of restraint to that before restraint. The percentage change in MI for postprandial conditions was calculated as a ratio of MI during 60 min of restraint to that during eating for 20 min.
Measurement of hormone and enzyme levels
To examine the changes in plasma corticosterone and ghrelin levels and gastric ghrelin O-acyltransferase (GOAT) content during restraint stress, mice were exposed to restraint stress, and blood and stomach samples were collected under isoflurane anesthesia from different animal groups at each time point (Fig. S1C). Blood was collected from the abdominal vena cava as previously reported28 in a tube containing EDTA-2Na (Dojindo Laboratories, Kumamoto, Japan) and aprotinin (Wako Pure Chemical Industries, Osaka, Japan), and it was immediately centrifuged at 4 °C. Plasma was acidified with 1 M HCl (1/10 volume) and stored at −80 °C until measurement. Stomach samples were homogenized in phosphate buffered saline containing 1/100 volume of protease inhibitor solution. The homogenate was frozen at −20 °C overnight, freeze thawed twice to destroy a cell membrane, and centrifuged. The supernatant was stored at −80 °C until measurement. Corticosterone levels were measured with non-acidified plasma samples using an AssayMax Corticosterone enzyme-linked immunosorbent assay (ELISA) Kit (AssayPro, St. Charles, MO, USA). Ghrelin levels were measured using the Active- and Des-acyl-Ghrelin ELISA Kit (Mitsubishi Chemical Medience, Tokyo, Japan). GOAT contents were measured using the Mouse GOAT (MBOAT4) ELISA Kit (Cusabio Biotech, Wuhan, China) and corrected by amount of total protein in the stomach.
Measurement of plasma carboxylesterase (CES) activity
To examine the changes in plasma CES (EC 3.1.1.1) activity, fasted mice were given food for 20 min and then exposed to restraint stress. Blood was collected under isoflurane anesthesia from different animal groups at each time point (Fig. S1C). The activity of CES was determined by measuring the hydrolysis of α-naphthyl acetate. After preincubation of the sample solutions for 20 min with 0.01 U CES (Sigma-Aldrich, St. Louis, MO, USA) in 100-mM phosphate buffer (pH 7.0), 10 μL of 20-mM α-naphthyl acetate was added. Absorbance was measured at 321 nm every 15 s for 10 min. The rate of the absorbance increase (Δ321 nm/s/mL) was calculated as the amount of enzyme hydrolytic activity.
Total RNA extraction for reverse transcription-polymerase chain reaction (PCR)
After an overnight fast, mice were given food for 20 min and then exposed to restraint stress. Stomach was collected under isoflurane anesthesia from different animal groups at each time point (Fig. S1C). Stomach samples were homogenized and total RNA was extracted according to the protocol from the RNeasy Universal Tissue Kit (Qiagen, Valencia, CA, USA). Total RNA from each sample was diluted to 100 ng/μL and incubated at 70 °C for 5 min and then cooled on ice. An aliquot of 1000 ng of total RNA was reverse transcribed using the TaqMan Reverse Transcription Reagent kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Quantitative PCR was performed with the Prism 7900HT Sequence Detection System (Applied Biosystems) using the TaqMan Gene Expression Master Mix (Applied Biosystems). To compensate for the differences in the amount of total RNA added to each reaction, mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an endogenous control as expressed by the Δ threshold cycle (ΔCt) value: ΔCt = 2(−|A−B|), where A is the number of cycles that reached the endogenous control gene threshold and B is the number of cycles that reached the target gene threshold. The set of oligonucleotide primers and fluorescent probes for TaqMan real-time PCR were manufactured by Applied Biosystems (Gapdh: Mm99999915_g1, GOAT: Mm01200389_m1).
Comparison of clearance of plasma des-acyl ghrelin
After an overnight fast, mice were administered des-acyl ghrelin (0.3 nmol/mouse) intraperitoneally and immediately exposed to restraint stress. After 15 or 60 min, blood was taken from the abdominal vena cava under isoflurane anesthesia from different animal groups at each time point (Fig. S1D). The experiment was carried out similarly in non-stressed (control) mice. Plasma des-acyl ghrelin levels were measured as described above.
Effect of acylated ghrelin and rikkunshito administration on gastric emptying and motility
To examine the effect of acylated ghrelin and rikkunshito on gastric emptying and motility of the mice exposed to restraint stress, acylated ghrelin (1 or 3 nmol/mouse),15 [D-Lys3]-GHRP-6 (0.2 μmol/mouse),15 which is a ghrelin receptor antagonist, or saline were intraperitoneally administered to mice. The acylated ghrelin dose that we used has been proven to inhibit the decrease in food intake observed in stress models.15 Furthermore, we used a dose of [D-Lys3]-GHRP6, which has no demonstrable effect on basal food intake, but blocks the improvement in food intake caused by rikkunshito administration.15 Immediately after, rikkunshito (100, 250 mg/kg) or distilled water was orally administered, followed by restraint stress loading. Gastric emptying and gastric motility after 60 min of restraint were measured as above (Fig. S1A and B). The effect of drugs on gastric motility was evaluated as the percentage change in MI between MI during 20 min eating and MI 20 min after drug administration.
Effect of rikkunshito administration on plasma ghrelin levels in mice exposed to acute restraint stress
To examine the effect of rikkunshito on plasma acylated and des-acyl ghrelin levels of the mice exposed to restraint stress, rikkunshito (250 mg/kg) or distilled water was orally administered, which was then followed by restraint stress loading. After 30 min, blood was taken from the abdominal vena cava under isoflurane anesthesia, and plasma acylated and des-acyl ghrelin levels were measured as described above.
Statistical analyses
Student's t-test was used to assess differences between control and stress group. Dunnett's test was used for multigroup comparisons among the vehicle-treated stress group and treatment groups, while the Tukey–Kramer test was used for multiple comparisons among the treatment groups. Data are expressed as mean ± SEM, and p < 0.05 was considered statistically significant.
Results
Effect of acute restraint stress on plasma corticosterone levels and gastric emptying
Plasma levels of corticosterone were significantly higher in restraint stress mice after 60 min of restraint stress than in the control mice in fasting state (Table1). In addition, solid gastric emptying during that time was significantly lower in the restraint stress mice than in the control mice (Table1).
Table 1.
Effect of acute restraint stress on plasma corticosterone levels and gastric emptying
| Plasma corticosterone (ng/mL) | Gastric emptying (%) | |
|---|---|---|
| Control | 814.0 ± 115.2 | 71.7 ± 3.1 |
| Restraint | 1417.3 ± 126.0** | 59.6 ± 3.9* |
Plasma corticosterone levels in fasted mice and solid gastric emptying were measured after 60 min of restraint; n = 7–8;
p < 0.05,
p < 0.01 vs control.
Effect of acute restraint stress on gastric motility
The strain gauge force transducer measurements showed a typical group of contractions, called phase III-like contractions, in the antrum of the fasted mice (Fig.1A). As shown in Fig.1A, the frequency of phase III-like contractions was considerably decreased in the restraint stress mice. As shown in Fig.1B, the phase III-like contractions observed in both groups during fasting disappeared after 20 min of feeding and contractions transitioned to a postprandial pattern. In the control mice, postprandial contractions were observed even after the chow was removed, but postprandial gastric antral contractions were considerably decreased in the restraint stress mice.
Figure 1.
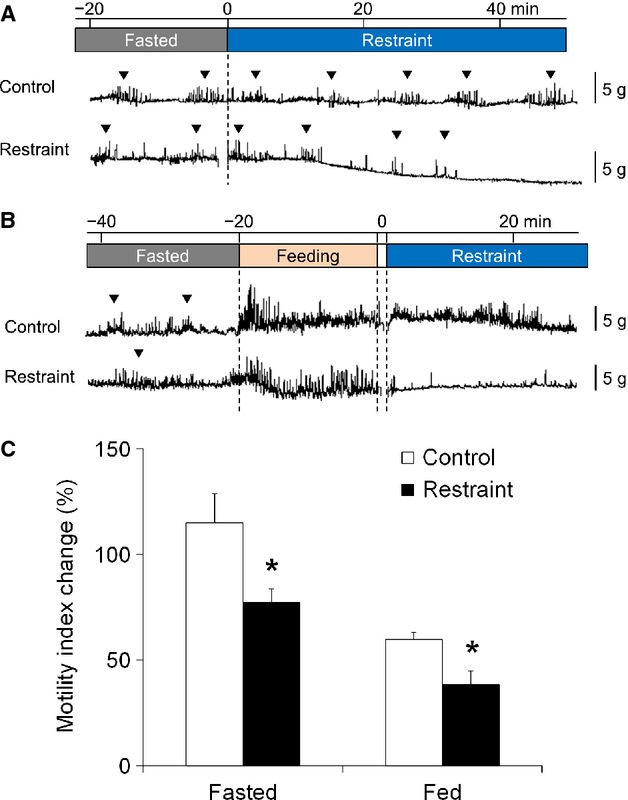
Effect of acute restraint stress on gastric motility. (A) Gastric motility in the fasting state. The phase III-like contractions (▽) were attenuated by restraint stress. (B) Gastric motility in the postprandial state. The phase III-like contractions (▽) were abolished by feeding and restraint stress attenuated postprandial gastric antral contractions. (C) Percentage change in the motility index after restraint stress loading. The motility index was significantly decreased by restraint stress in both fasting and postprandial states; n = 5–6; *p < 0.05 vs control.
The percentage changes in MI were significantly lower in the restraint stress group than in the control group in both fasting and fed states (Fig.1C).
Plasma corticosterone and ghrelin levels in mice exposed to acute restraint stress in the postprandial state
Blood was collected in fed conditions and the effects of restraint stress on postprandial plasma levels of corticosterone and ghrelin were examined. Corticosterone levels decreased slowly in the control group (at 0 min, 224.6 ± 23.4; at 60 min, 74.2 ± 14.4 ng/mL; p = 0.002), but markedly increased in the restraint stress mice after 15 min of exposure stress; the difference with respect to the control group was significant at 15, 30, and 60 min (Fig.2A).
Figure 2.
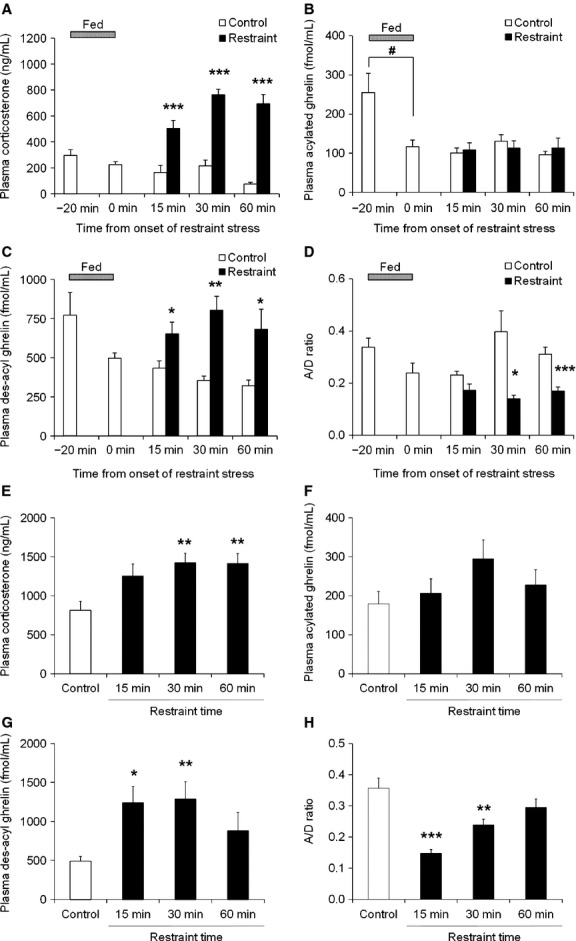
Plasma corticosterone and ghrelin levels in restraint stress mice in the postprandial or the fasting state. After overnight fasting, mice were exposed to restraint stress following 20 min of either feeding or continued fasting. Blood was collected under isoflurane anesthesia. (A–D) Plasma corticosterone, acylated ghrelin, des-acyl ghrelin levels, and the ratio of plasma acylated to des-acyl ghrelin levels (A/D ratio) in the postprandial state. (E–H) Plasma corticosterone, acylated ghrelin, des-acyl ghrelin levels, and the A/D ratio in the fasting state. n = 7–8/group/time point; *p < 0.05, **p < 0.01, ***p < 0.001 vs control. #p < 0.05 vs “−20 min” group.
Plasma acylated ghrelin levels markedly decreased after 20 min of feeding, but no changes were observed until 60 min afterward regardless of exposure to restraint stress (Fig.2B). In contrast, plasma des-acyl ghrelin levels decreased slowly in the control group (at 0 min, 496.9 ± 33.5; at 60 min, 320.7 ± 36.2 fmol/mL; p = 0.006), but significantly increased in the restraint stress group and reached a maximum after 30 min of exposure to stress (Fig.2C). In addition, the ratio of plasma acylated ghrelin levels to des-acyl ghrelin levels (A/D ratio) significantly decreased after exposure to stress (Fig.2D).
In fasting conditions, 30 and 60 min of exposure to stress caused plasma corticosterone levels to increase significantly compared with the control group (Fig.2E). In addition, plasma acylated ghrelin levels tended to increase after 30 min of exposure to restraint stress, while des-acyl ghrelin levels significantly increased after 15 and 30 min of exposure to stress (Fig.2F and G). The A/D ratio significantly decreased after exposure to stress (Fig.2H).
Effect of acute restraint stress on plasma CES activity
We compared plasma CES (one of the deacylation enzymes) activity between the control and restraint groups. No change in enzyme activity was observed under restraint stress (Fig.3).
Figure 3.
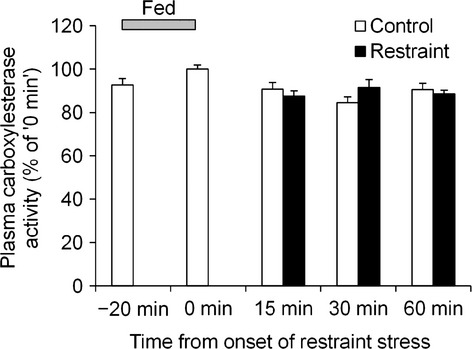
Effect of acute restraint stress on plasma carboxylesterase activity. After an overnight fast, mice were given food for 20 min and then exposed to restraint stress. Blood was collected under isoflurane anesthesia. n = 7–8/group/time point.
Effect of acute restraint stress on gastric content and mRNA expression of GOAT
We examined whether gastric protein content and mRNA expression of GOAT, an enzyme on which octanoylate ghrelin exerts biological activities, changed with restraint stress. There was no difference in either protein content or mRNA expression between the control and restraint groups (Fig.4A and B).
Figure 4.
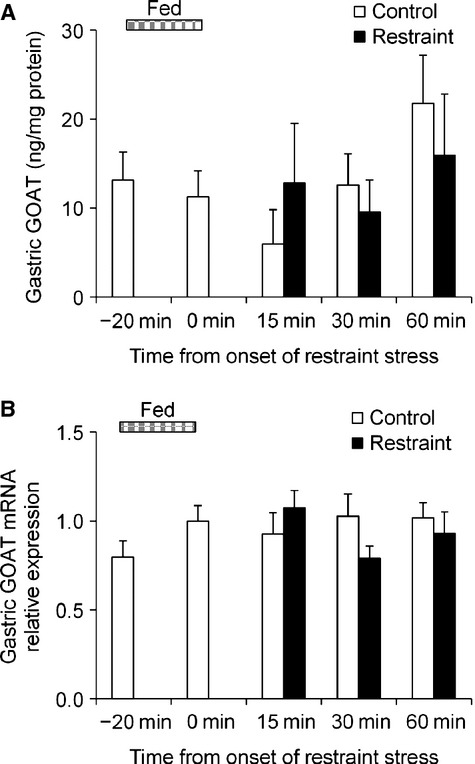
Effect of acute restraint stress on gastric content and mRNA expression of ghrelin O-acyltransferase (GOAT). After an overnight fast, mice were given food for 20 min and then exposed to restraint stress. Stomach was collected under isoflurane anesthesia. (A) Content of GOAT. (B) GOAT mRNA expression. n = 8/group/time point.
Effect of acute restraint stress on clearance of plasma des-acyl ghrelin
When 0.3 nmol of des-acyl ghrelin was intraperitoneally administered to the mice, plasma des-acyl ghrelin levels increased after 15 min and decreased after 60 min in both the control and the restraint groups (Fig.5). There was no difference in plasma des-acyl ghrelin levels between the control and restraint groups.
Figure 5.
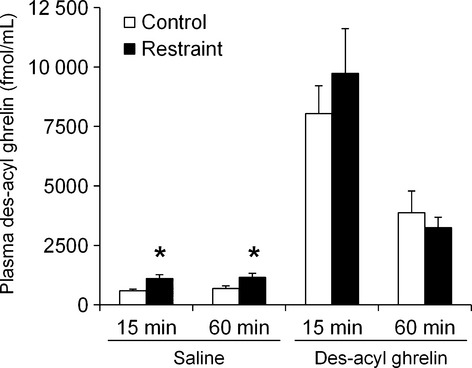
Effect of acute restraint stress on clearance of plasma des-acyl ghrelin. After an overnight fast, mice were administered des-acyl ghrelin (0.3 nmol/mouse) intraperitoneally and immediately exposed to restraint stress. After 15 or 60 min, blood was taken from the abdominal vena cava under isoflurane anesthesia. n = 7–8/group/time point; *p < 0.05 vs control.
Effects of acylated ghrelin or rikkunshito administration on delayed gastric emptying induced by acute restraint stress
The effects of exogenous and endogenous acylated ghrelin supplementation on delayed gastric emptying induced by restraint stress were examined. Gastric emptying was significantly delayed after 60 min of exposure to stress. Administration of acylated ghrelin significantly improved delayed gastric emptying at a dose of 3 nmol/mouse (p = 0.01; Fig.6A). Administration of rikkunshito (100, 250 mg/kg) significantly improved delayed gastric emptying in a dose-dependent manner (Fig.6B). The improvement of delayed gastric emptying effected by rikkunshito (250 mg/kg) was reversed by coadministration of the ghrelin receptor antagonist [D-Lys3]-GHRP-6 (Fig.6C).
Figure 6.
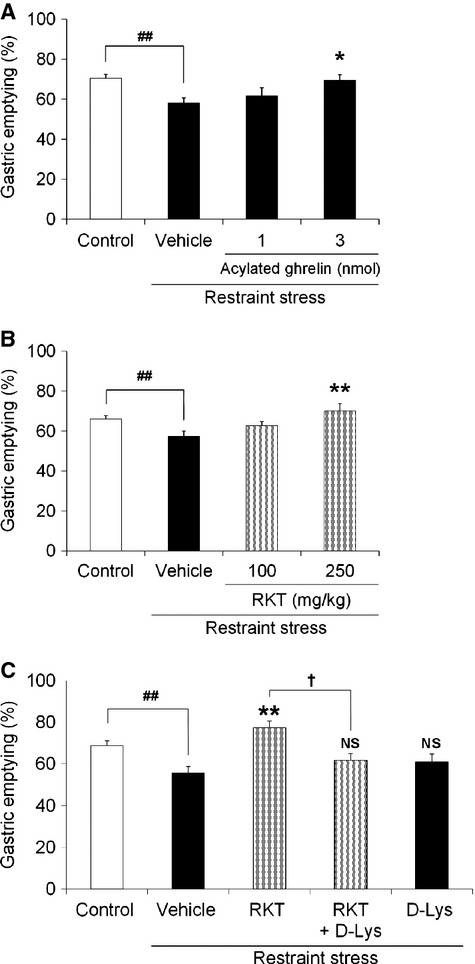
Effects of acylated ghrelin or rikkunshito administration on gastric emptying in restraint stress mice. After fasting, mice were given food for 20 min and exposed to restraint stress. Gastric emptying was measured after 60 min of restraint. (A) Effects of acylated ghrelin (1, 3 nmol/mouse, i.p.) administration. (B) Effects of rikkunshito (100, 250 mg/kg, p.o.) administration. (C) Effects of coadministration of rikkunshito (250 mg/kg, p.o.) and a ghrelin receptor antagonist [D-Lys3]-GHRP-6 (0.2 μmol/mouse, i.p.). RKT: rikkunshito, D-Lys: [D-Lys3]-GHRP-6. n = 5–13; ##p < 0.01 vs control. *p < 0.05, **p < 0.01, NS, not significant vs vehicle-treated restraint stress mice. †p < 0.05 vs rikkunshito-treated restraint stress mice.
Effects of acylated ghrelin or rikkunshito administration on decreased postprandial gastric motility induced by acute restraint stress
The effects of acylated ghrelin supplementation on decreased postprandial gastric motility induced by restraint stress were examined. A gastric motility pattern is shown in Fig.7A. Although postprandial gastric antral contractions after 20 min of feeding were attenuated upon exposure to restraint stress, improvement of postprandial contractions was observed after administration of acylated ghrelin (3 nmol/mouse) or rikkunshito (250 mg/kg). The enhancing effect of rikkunshito (250 mg/kg) on gastric motility was not seen when coadministered with the ghrelin receptor antagonist [D-Lys3]-GHRP-6. The percentage changes in MI after drug administration are shown in Fig.7B. These results were similar to those from the gastric emptying measurement experiment as shown in Fig.6C.
Figure 7.
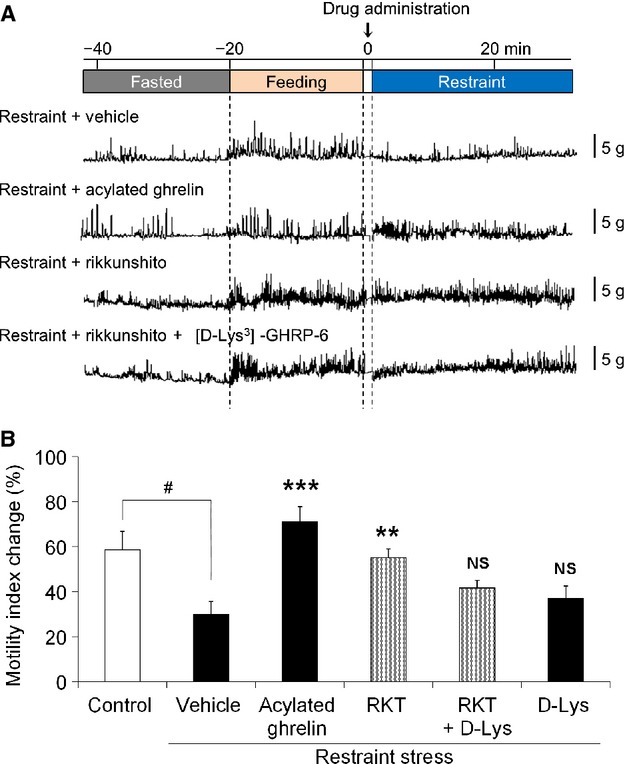
Effects of acylated ghrelin or rikkunshito administration on gastric motility in restraint stress mice. After fasting, mice were given food for 20 min. Acylated ghrelin (3 nmol/mouse, i.p.), rikkunshito (250 mg/kg, p.o.), or a ghrelin receptor antagonist [D-Lys3]-GHRP-6 (0.2 μmol/mouse, i.p.) were administered and mice were then exposed to restraint stress. Gastric motility was measured in conscious, freely moving mice using a wireless strain gauge force transducer. (A) Effects of drugs on gastric motility decrease induced by restraint stress. (B) Effects of drugs on the percentage change in the motility index. RKT: rikkunshito, D-Lys: [D-Lys3]-GHRP-6. n = 4–8. #p < 0.05 vs control. **p < 0.01, ***p < 0.001, NS, not significant vs vehicle (p.o., i.p.)-treated restraint stress mice.
Effect of rikkunshito administration on plasma ghrelin levels in mice exposed to acute restraint stress
To examine whether the effect of rikkunshito on delayed gastric emptying and decreased gastric motility was mediated by the increasing plasma acylated ghrelin levels, we measured plasma ghrelin levels in rikkunshito-administered restraint-stressed mice. Rikkunshito administration affected neither plasma acylated nor des-acyl ghrelin levels 30 min after the onset of restraint stress when the increase in plasma des-acyl ghrelin levels was maximal (acylated ghrelin: vehicle-treated, 92.6 ± 17.0; rikkunshito-treated, 91.8 ± 11.9 fmol/mL; p = 0.97; des-acyl ghrelin: vehicle-treated, 675.1 ± 127.7; rikkunshito-treated, 626.5 ± 74.2 fmol/mL; p = 0.75).
Discussion
In this study, we found that restraint stress caused a significant elevation of plasma des-acyl ghrelin levels in mice, although it did not affect plasma acylated ghrelin levels. In addition, administration of exogenous acylated ghrelin or rikkunshito, a ghrelin signal enhancer, improved delayed gastric emptying and decreased gastric antral contractions induced by restraint stress.
In our study, restraint stress significantly decreased phase III-like contractions in the fasting state and postprandial gastric contractions, and decreased gastric emptying. Previous study has already reported the similar results.2 These results suggest that stress not only decreases stomach peristalsis, but also decreases the movements that mix stomach contents. We also observed a decrease from baseline over time in the fasting state. This indicates that excessive gastric relaxation occurs together with decreased peristalsis. Enhanced corticotropin-releasing factor (CRF) synthesis in stress-related regions of the brain is observed in experimental stress models,32 and the activation of CRF receptors 1 or 2 results in decreased gastrointestinal function.33–35 These findings suggest that CRF is associated with stress-induced gastrointestinal dysmotility. Furthermore, the decrease in both feeding and plasma ghrelin levels caused by psychological stress can be reversed by CRF1 receptor antagonist administration.15 We hypothesize that abnormal ghrelin dynamics downstream of CRF are involved in the mechanism underlying dysmotility after acute stress. A previous study using water avoidance stress showed that total blood levels of ghrelin increased after 1 h of exposure to stress, but it failed to differentiate between acylated and des-acyl ghrelin.14 Another research group reported that there was no change in plasma acylated or des-acyl ghrelin levels in the initial phase (until 24 h) in a rat model of chronic fatigue. Nevertheless, it did not provide any information regarding blood ghrelin levels in the very early phase (up to 4 h) of stress exposure.3 Therefore, there is a lack of definitive information about the changes in ghrelin dynamics during the acute period of stress loading. This study therefore aimed to examine the acute changes in peripheral ghrelin levels together with gastric motor function after exposure to acute restraint stress.
In mice exposed to restraint stress after 20 min of feeding, there was no apparent change in acylated ghrelin levels, whereas there was a remarkable increase in des-acyl ghrelin levels in conjunction with decrease in the A/D ratio. As ghrelin blood levels are known to be greatly affected by feeding,36 plasma ghrelin levels in fasted mice were also examined in the same manner. Although plasma acylated ghrelin levels tended to increase in fasted mice exposed to restraint stress, this increase was not statistically significant. In contrast, there was a significant increase in des-acyl ghrelin levels, similar to those in the fed condition, indicating that the observed dysregulation of ghrelin dynamics was most likely related to the restraint stress itself, rather than the experimental protocol.
Several recent studies have examined the pharmacological action of des-acyl ghrelin.37 In a few reports, administration of des-acyl ghrelin resulted in a decrease in either food intake or gastric motility in fasted mice, and transgenic mice with overexpression of des-acyl ghrelin have delayed gastric emptying compared with wild-type mice.10,11 It is now believed that a decreased A/D ratio as well as the interaction between acylated ghrelin and des-acyl ghrelin influence feeding behavior and gastrointestinal motility.13,28,38–40 Previous studies have suggested that a decreased A/D ratio may play a role in decreased food intake following the administration of lipopolysaccharides38 or cisplatin28 in rats. Moreover, other studies have shown that coadministration of des-acyl ghrelin with acylated ghrelin abolishes the effects of promotion of feeding behavior or intestinal motility.13,39,40 From these results, it seems likely that des-acyl ghrelin may block the action of acylated ghrelin. However, conflicting results indicating that des-acyl ghrelin administration alone failed to affect food intake have been obtained.37 The pathophysiological role of des-acyl ghrelin in the regulation of gastrointestinal motility remains a controversial issue, and further studies are required to clarify this topic.
To identify the mechanism underlying the increase in plasma des-acyl ghrelin after exposure to restraint stress, we studied the effects of restraint stress on the activity of the metabolizing enzymes of acylated ghrelin. Because des-acyl ghrelin is a metabolite of acylated ghrelin, it seemed reasonable to assume that peripheral deacylation enzyme activity was affected by restraint stress. Therefore, we compared plasma CES activity between the control and restraint groups; however, we failed to find any significant differences between them. Another possibility was the downregulation of GOAT activity (a mediator of des-acyl ghrelin octanoylation) in the stomach mucosa. Although this possibility was considered unlikely because there was no definite change in the gastric mucosal content of either the GOAT protein or its mRNA levels, it cannot be completely excluded because GOAT activity was not directly measured. Alternatively, decreased des-acyl ghrelin clearance from circulating blood may contribute to the increased des-acyl ghrelin levels in this restraint stress model. Unfortunately, we failed to find any significant differences in the clearance rates of des-acyl ghrelin from the circulating blood between the control and restraint groups.
Although most of the des-acyl ghrelin in the blood is generally believed to be produced by the degradation of plasma acylated ghrelin by peripheral esterases,41–43 it is possible that both acylated and des-acyl ghrelin were secreted from the stomach into the blood. In support of this, a recent study by Sakata et al.,44 which used isolated gastric mucosal cells, suggested that ghrelin-producing X/A-like cells secrete des-acyl ghrelin as well. In a future study, it should be determined whether des-acyl ghrelin secretion from the stomach is enhanced in the restraint stress model.
It was hypothesized that acylated ghrelin supplementation could play a role in improving stress-induced gastric dysmotility. To test this hypothesis, we examined the effects of administration of exogenous acylated ghrelin on delayed gastric emptying induced by exposure to restraint stress and found that delayed gastric emptying was improved by ghrelin supplementation. Similar results were obtained after the administration of rikkunshito. Rikkunshito has been reported to increase plasma acylated ghrelin levels5,28 and inhibit the deacylation of acylated ghrelin.30 However, the administration of rikkunshito did not affect plasma acylated or des-acyl ghrelin levels in our restraint model. Interestingly, these effects of rikkunshito were almost completely abolished by coadministration of a GHS-R antagonist, suggesting that rikkunshito may improve stress-induced delayed gastric emptying and gastric dysmotility via the potentiation of endogenous ghrelin signaling.29 We believe that the administration of a pharmacological dose of acylated ghrelin or rikkunshito caused an overwhelming dominance of ghrelin signaling, thereby ameliorating both gastric dysmotility and delayed gastric emptying. This suggests that administration of acylated ghrelin or rikkunshito may be effective to improve symptoms in functional dyspepsia, the onset of which is known to be influenced by stress.
In conclusion, administration of a pharmacological dose of exogenous acylated ghrelin or rikkunshito led to improvements in delayed gastric emptying and decreased gastric antral motility through supplementation of acylated ghrelin or promotion of its signal transmission in mice exposed to acute restraint stress. Thus, ghrelin supplementation or ghrelin signal enhancement may be an effective, novel approach to treat stress-induced gastric dysmotility.
Funding
This work was supported in part by the Research Funding for Longevity Sciences (23–25) from the National Center for Geriatrics and Gerontology (NCGG), Japan, a Grant-in-Aid for research (22590676) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, a grant for Interdisciplinary Project for Psychosomatological Research in Hokkaido University, and a grant from Tsumura & Co (Ibaraki, Japan). The authors have no competing interests.
Disclosure
H. Takeda received grant support from Tsumura & Co. M. Nahata, Y. Saegusa, C. Sadakane, C. Yamada, and T. Hattori are employed by Tsumura & Co. K. Nakagawa, N. Okubo, S. Ohnishi, and N. Sakamoto have nothing to declare.
Author Contribution
MN performed the research, analyzed the data, and drafted the manuscript; YS, CS, and CY performed the research and analyzed the data. KN, NO, and SO analyzed and reviewed the data; TH analyzed and reviewed the data and supervised; NS reviewed the data and supervised; HT designed the research study and drafted the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
The experimental protocol.
References
- 1.Tack J, Lee KJ. Pathophysiology and treatment of functional dyspepsia. J Clin Gastroenterol. 2005;39:S211–6. doi: 10.1097/01.mcg.0000156109.97999.d1. [DOI] [PubMed] [Google Scholar]
- 2.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1358–65. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]
- 3.Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Oshitani N, et al. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 2008;82:862–8. doi: 10.1016/j.lfs.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, Kojima S, Fujimiya M, Inui A. Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry. 2009;65:748–59. doi: 10.1016/j.biopsych.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 7.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–40. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675–80. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 9.Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil. 2009;21:78–84. doi: 10.1111/j.1365-2982.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 10.Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55:135. [PMC free article] [PubMed] [Google Scholar]
- 13.Inhoff T, Monnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–68. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville delaCourC, Hakanson R, Lindstrom E. Acute psychological stress raises plasma ghrelin in the rat. Regul Pept. 2006;134:114–7. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Saegusa Y, Takeda H, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Nahata M, Hattori T, et al. Decreased plasma ghrelin contributes to anorexia following novelty stress. Am J Physiol Endocrinol Metab. 2011;301:E685–96. doi: 10.1152/ajpendo.00121.2011. [DOI] [PubMed] [Google Scholar]
- 16.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–53. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 17.Falken Y, Webb DL, Abraham-Nordling M, Kressner U, Hellstrom PM, Naslund E. Intravenous ghrelin accelerates postoperative gastric emptying and time to first bowel movement in humans. Neurogastroenterol Motil. 2013;25:474–80. doi: 10.1111/nmo.12098. [DOI] [PubMed] [Google Scholar]
- 18.Qiu WC, Wang ZG, Wang WG, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J Gastroenterol. 2008;14:1419–24. doi: 10.3748/wjg.14.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Murphy NP, Stengel A, Goebel-Stengel M, St Pierre DH, Maidment NT, Tache Y. Ghrelin prevents levodopa-induced inhibition of gastric emptying and increases circulating levodopa in fasted rats. Neurogastroenterol Motil. 2012;24:e235–45. doi: 10.1111/j.1365-2982.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, Inadomi JM, Hibi T. Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:688–96. doi: 10.1111/j.1365-2982.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka T, Tamagawa Y, Hayashida S, Kaneda Y, Kodama N, Tsuji S. Rikkunshi-to attenuates adverse gastrointestinal symptoms induced by fluvoxamine. Biopsychosoc Med. 2007;1:21. doi: 10.1186/1751-0759-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusunoki H, Haruma K, Hata J, Ishii M, Kamada T, Yamashita N, Honda K, Inoue K. Efficacy of Rikkunshito, a traditional Japanese medicine (Kampo), in treating functional dyspepsia. Intern Med. 2010;49:2195–202. doi: 10.2169/internalmedicine.49.3803. [DOI] [PubMed] [Google Scholar]
- 23.Hattori T, Fujitsuka N, Asakawa A, Inui A. A new strategy using Rikkunshito (Liu-Jun-Zi-Tang), a Japanese traditional medicine, to treat gastrointestinal disease. In: Satoh H, editor. Basics of Evidences-Based Herbal Medicine. Kerala: Research Signpost; 2010. pp. 149–60. [Google Scholar]
- 24.Matsumura T, Arai M, Yonemitsu Y, Maruoka D, Tanaka T, Suzuki T, Yoshikawa M, Imazeki F, et al. The traditional Japanese medicine Rikkunshito increases the plasma level of ghrelin in humans and mice. J Gastroenterol. 2010;45:300–7. doi: 10.1007/s00535-009-0166-z. [DOI] [PubMed] [Google Scholar]
- 25.Shiratori M, Shoji T, Kanazawa M, Hongo M, Fukudo S. Effect of rikkunshito on gastric sensorimotor function under distention. Neurogastroenterol Motil. 2011;23:323–9. doi: 10.1111/j.1365-2982.2010.01648.x. e155–326. [DOI] [PubMed] [Google Scholar]
- 26.Arai M, Matsumura T, Tsuchiya N, Sadakane C, Inami R, Suzuki T, Yoshikawa M, Imazeki F, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology. 2012;59:62–6. doi: 10.5754/hge11246. [DOI] [PubMed] [Google Scholar]
- 27.Yanai M, Mochiki E, Ogawa A, Morita H, Toyomasu Y, Ogata K, Tabe Y, Ando H, et al. Intragastric administration of rikkunshito stimulates upper gastrointestinal motility and gastric emptying in conscious dogs. J Gastroenterol. 2013;48:611–9. doi: 10.1007/s00535-012-0687-8. [DOI] [PubMed] [Google Scholar]
- 28.Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, Nagai K, Asaka M, Kasuga M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–13. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 29.Fujitsuka N, Asakawa A, Uezono Y, Minami K, Yamaguchi T, Niijima A, Yada T, Maejima Y, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. doi: 10.1038/tp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadakane C, Muto S, Nakagawa K, Ohnishi S, Saegusa Y, Nahata M, Hattori T, Asaka M, et al. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem Biophys Res Commun. 2011;412:506–11. doi: 10.1016/j.bbrc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–34. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–10. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 33.Maillot C, Million M, Wei JY, Gauthier A, Tache Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–79. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 34.Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R427–32. doi: 10.1152/ajpregu.00499.2004. [DOI] [PubMed] [Google Scholar]
- 35.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 37.Inhoff T, Wiedenmann B, Klapp BF, Monnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake? Peptides. 2009;30:991–4. doi: 10.1016/j.peptides.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Stengel A, Goebel M, Wang L, Reeve JR, Jr, Tache Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–96. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda K, Miura T, Kaiya H, Maruyama K, Shimakura S, Uchiyama M, Kangawa K, Shioda S. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27:2321–5. doi: 10.1016/j.peptides.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 40.Hirayama H, Shiina T, Shima T, Kuramoto H, Takewaki T, B FurnessJ, Shimizu Y. Contrasting effects of ghrelin and des-acyl ghrelin on the lumbo-sacral defecation center and regulation of colorectal motility in rats. Neurogastroenterol Motil. 2010;22:1124–31. doi: 10.1111/j.1365-2982.2010.01553.x. [DOI] [PubMed] [Google Scholar]
- 41.De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- 42.Shanado Y, Kometani M, Uchiyama H, Koizumi S, Teno N. Lysophospholipase I identified as a ghrelin deacylation enzyme in rat stomach. Biochem Biophys Res Commun. 2004;325:1487–94. doi: 10.1016/j.bbrc.2004.10.193. [DOI] [PubMed] [Google Scholar]
- 43.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Tache Y, Reeve JR. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–8. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakata I, Park WM, Walker AK, Piper PK, Chuang JC, Osborne-Lawrence S, Zigman JM. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab. 2012;302:E1300–10. doi: 10.1152/ajpendo.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The experimental protocol.


