Abstract
Mice lacking functional neurokinin-1 receptors (NK1R-/-) display abnormal behaviours seen in Attention Deficit Hyperactivity Disorder (hyperactivity, impulsivity and inattentiveness). These abnormalities were evident when comparing the behaviour of separate (inbred: ‘Hom’) wildtype and NK1R-/- mouse strains. Here, we investigated whether the inbreeding protocol could influence their phenotype by comparing the behaviour of these mice with that of wildtype (NK1R+/+) and NK1R-/- progeny of heterozygous parents (‘Het’, derived from the same inbred strains). First, we recorded the spontaneous motor activity of the two colonies/genotypes, over 7 days. This continuous monitoring also enabled us to investigate whether the diurnal rhythm in motor activity differs in the two colonies/genotypes. NK1R-/- mice from both colonies were hyperactive compared with their wildtypes and their diurnal rhythm was also disrupted. Next, we evaluated the performance of the four groups of mice in the 5-Choice Serial Reaction-Time Task (5-CSRTT). During training, NK1R-/- mice from both colonies expressed more impulsive and perseverative behaviour than their wildtypes. During testing, only NK1R-/- mice from the Hom colony were more impulsive than their wildtypes, but NK1R-/- mice from both colonies were more perseverative. There were no colony differences in inattentiveness. Moreover, a genotype difference in this measure depended on time of day. We conclude that the hyperactivity, perseveration and, possibly, inattentiveness of NK1R-/- mice is a direct consequence of a lack of functional NK1R. However, the greater impulsivity of NK1R-/- mice depended on an interaction between a functional deficit of NK1R and other (possibly environmental and/or epigenetic) factors.
Keywords: 5-Choice Serial Reaction-Time Task, ADHD, attention, diurnal motor rhythm, epigenetics, hyperactivity, impulsivity, NK1 receptor, perseveration, TACR1 gene
Mice with functional ablation of the substance P-preferring (NK1) receptor gene (NK1R-/-) (de Felipe et al. 1998) express locomotor hyperactivity, compared with their wildtypes (Fisher et al. 2007; Herpfer et al. 2005; Yan et al. 2010). Typically, they also express a greater incidence of premature responses (a form of impulsivity), %omissions (failure to respond in the task, which can indicate inattentiveness) and perseveration (repetitive nose-pokes), when tested for the first time in the 5-Choice Serial Reaction-Time Task (5-CSRTT; Dudley et al. 2013; Yan et al. 2011).
Hyperactivity, impulsivity and inattentiveness are diagnostic criteria for Attention Deficit Hyperactivity Disorder (ADHD). The possibility that the abnormal behaviours of NK1R-/- mice echo those seen in ADHD patients is supported by an association between polymorphisms in, or near, the TACR1 receptor gene (the human equivalent of the Nk1r gene) and increased vulnerability to ADHD (Sharp et al. 2014; Yan et al. 2010).
All these studies were carried out on homozygous wildtype and NK1R-/- mice, maintained as two inbred strains, which have been housed separately. Whereas this approach reduces overbreeding, there is a risk that genetic drift, or differences in the environment of the two inbred strains, such as maternal physiology or interactions with littermates, influence their behaviour as adults (Crews et al. 2004; Crusio et al. 2009; Sasaki et al. 2014; Tarantino et al. 2011). Here, we aimed to establish whether any of the differences in the behaviour of inbred NK1R-/- mice and their wildtype counterparts could be attributed directly to a lack of functional NK1R, alone, or whether additional (e.g. environmental and/or epigenetic) factors influence their phenotype. To investigate this possibility, we compared the behaviour of the homozygous progeny (wildtype and NK1R-/-) of inbred, homozygous breeding-pairs (‘Hom’ colony) with the behaviour of the homozygous progeny of heterozygous breeding pairs (‘Het’ colony), derived from the same wildtype and NK1R-/- mouse inbred strains.
First, we monitored the spontaneous motor activity of wildtype and NK1R-/- mice from the two colonies. Given the extensive evidence that the normal sleep / arousal pattern is disturbed in patients with ADHD (Kooij & Bijlenga 2013; van Veen et al. 2010), we were also interested in establishing whether the diurnal rhythm of motor activity is similarly disrupted in NK1R-/- mice. To that end, we used activity sensors that enabled us to monitor the movement of the mice over the entire 24 h cycle.
In a second series of experiments, we compared the performance of the two colonies and two genotypes in the 5-CSRTT: this procedure is widely used to evaluate animals' visual attention and response control (Robbins 2002). We aimed to determine whether the impulsivity, inattentiveness and perseveration of NK1R-/- mice reported previously (Dudley et al. 2013; Yan et al. 2010) is also evident in NK1R-/- mice bred from heterozygous parents.
Materials and methods
These experiments were authorized under the UK Animals (Scientific Procedures) Act 1986 and received approval from the local Animal Welfare and Ethical Review Body at University College London.
Animals
We studied two colonies of mice, both held at a facility at UCL and derived from a single colony of mice (129/Sv x C57BL/6J background strain, crossed with an outbred MF1 strain), that was developed many generations ago (described fully in: de Felipe et al. 1998). Given the constraint of the maximum number of mice that we could train and test each day in the 5-CSRTT (24), we opted to study males, only. This was partly because ADHD is more prevalent in males. However, we also needed to be able to compare the results of this experiment with those from all our previous studies in which we have compared the behaviour of the two male genotypes in the 5-CSRTT.
The first (‘Hom’) colony comprised two inbred homozygous lines: mice with functional ablation of the Nk1r gene (NK1R-/-; ‘KO-Hom’) and their wildtype counterparts (NK1R+/+; ‘WT-Hom’), which have been maintained as separate strains since their production (approximately 17 years ago). The second (‘Het’) colony derived from the same inbred (homozygous) strains after cross-breeding wildtype and NK1R-/- mice from the Hom strains to produce heterozygous offspring (F1). Heterozygous breeding-pairs were then used to produce litters containing wildtype (NK1R+/+; ‘WT-Het’), NK1R-/- (‘KO-Het’) and NK1R+/− mice (F2). Only the homozygous progeny were used in these experiments. The genotypes of the WT-Het and KO-Het (NK1R+/+ and NK1R-/-) mice were confirmed using tissue (ear-punch) samples and performing the polymerase chain reaction (PCR) followed by electrophoresis.
The two genotypes from the Hom colony were housed separately such that every home-cage contained up to four WT-Hom or four KO-Hom littermates. By contrast, the two (homozygous) genotypes from the Het colony were housed together as mixed litters, containing up to four WT-Het (NK1R+/+) / KO-Het (NK1R-/-) littermates (NK1R+/− mice were removed and culled at weaning (age 3 weeks)). In the Het colony, cages contained at least one mouse of each genotype. Other aspects of housing and husbandry were the same for the two colonies. Briefly, both colonies were housed in the same holding room at 21 ± 2°C, 45 ± 5% humidity, with a 12:12 h light:dark cycle (lighting increased in steps from 0700 h to 0800 h and reduced in steps from 1900 h to 2000 h). The home-cages incorporated the same environmental enrichment and sawdust bedding (3Rs Bedding Pty, Ltd., London W2, UK) and were cleaned twice weekly. Water and food [2018 global Rodent Diet (Harlan)] were freely available for mice used to monitor activity in the home cage, but mice destined for the 5-CSRTT were subject to restricted diet (see below).
Activity monitoring
Five male mice, aged 8–14 weeks at the start of testing, were used from each group (WT-Hom, KO-Hom, WT-Het, KO-Het). Mice from the Hom colony were taken from two separate breeding-pairs for each genotype, while mice from the Het colony were taken from three separate breeding-pairs. Animals were age-matched across the four experimental groups as closely as possible. Between 0900 h and 1000 h on the first day of the experiment, the animals were moved to individual cages, which were placed below an activity sensor attached to the base of the cage above (20 cm above the floor of the lower cage). Their position was configured to ensure that they monitored the whole area of the cage floor that was accessible to the mice. Animals were allowed to habituate to their individual housing conditions throughout day 1. As a precaution, data collected over this 24 h period were excluded from subsequent analyses. In order to correct for any effect of the position of the cage in the rack, mice from all four test groups were monitored simultaneously and were balanced across five activity sensors.
The sensors are activated by turns of the body and rearing, as well as gross ambulatory movement, but not vegetative movements or those associated with respiration and muscle twitches during sleep. A full description of the design and specifications of the apparatus and software is to be published elsewhere (Brown, personal communication). Briefly, these activity-monitoring devices incorporated passive infra-red (PIR) sensors (Panasonic AMN 32111; Premier Farnell UK Limited), which detect the movement of sources of heat. These were used alongside a light-dependent resistor (LDR). The PIR sensors were configured as digital inputs to an Arduino Uno (Rev3) microcontroller board, (http://arduino.cc/en/Main/ArduinoBoardUno) and the state of each PIR was recorded at 100 millisecond intervals. At the end of each 60 second interval, the percentage activity for each PIR sensor was calculated and sent as a serial message alongside a measurement of relative environmental light from the LDR. Serial messages were captured by a laptop, running companion software that saved the data from the sensor, alongside a timestamp, as a tab-delimited text file. Data were then transferred to a Microsoft Excel file for further statistical analysis using InVivoStat (version 2.2.0.0.; Clark et al. 2011).
5-Choice Serial Reaction-Time Task
Owing to the large number of mice needed for this study (48 in total), the experiment was carried out in two replicate steps, each of which involved the training and testing of 6 mice from each experimental group (WT-Hom, KO-Hom, WT-Het, KO-Het). As a precaution, the WT-Het and KO-Het mice in the second half of the experiment were re-derived from the two inbred strains, rather than using a new batch of descendents of the same heterozygous breeding-pairs. Mice from the Hom colony were taken from four breeding-pairs for each genotype (two for each half of the experiment), while mice from the Het colony were taken from six breeding-pairs (three for each half of the experiment).
The mice were aged 6–8 weeks and weighed 27–41 g at the start of training (mean age/start weight for each group: WT-Hom, 6.5 ± 0.1 weeks/34.55 ± 0.71 g; KO-Hom, 6.6 ± 0.1 weeks/31.85 ± 0.53 g; WT-Het, 7.9 ± 0.2 weeks/38.40 ± 1.00 g; KO-Het, 7.8 ± 0.2 weeks/34.80 ± 0.60 g) and were housed in fixed groups of 2 - 4 littermates per cage, throughout. All animals were subject to restricted food supply to stabilize their body weight at 90% free-feeding weight. They were brought into the laboratory at the same time every day (Monday to Friday: 0900 h to 0930 h) and weighed before training/testing in the 5-CSRTT. Half the animals were trained and tested in the morning (1000 h to 1200 h) while the remainder were trained and tested in the afternoon (1300 h to 1500 h). Animals from the two colonies and two genotypes were balanced across the morning and afternoon sessions.
Details of the protocol are reported fully elsewhere (Dudley et al. 2013; Yan et al. 2011). In brief, after habituation to the 5-CSRTT apparatus (Med Associates, St. Albans, VT, USA), the animals were trained to nose-poke in response to a light stimulus in one of five holes in one wall of the chamber: a correct response was rewarded with sweetened milk (0.01 ml of 30% condensed milk solution), delivered from a magazine in the opposite wall of the chamber. Animals were trained in a series of six stages and were required to attain specific performance criteria at each stage. These criteria were based on: the total number of trials completed; the number of correct trials completed; number of premature responses; accuracy; and omissions. Successive stages of training were made progressively more difficult by: decreasing the duration of the light stimulus (stimulus duration, ‘SD’); increasing the amount of time the animal had to wait before the light stimulus appears (intertrial interval, ‘ITI’); and decreasing the length of time during which the animal was allowed to respond to the light stimulus (limited hold, ‘LH’). See Table1 for details of the performance variables, task parameters and progression criteria used at each stage of training.
Table 1.
(a) Task parameters and progression criteria for each stage of the training procedure of the 5-CSRTT. (b) Performance variables measured in the 5-CSRTT
| (a) | ||||
|---|---|---|---|---|
| Stage parameters |
||||
| Stage | SD (seconds) | LH (seconds) | ITI (seconds) | Progression criteria |
| 1 | 30 | 30 | 2 | ≥30 correct trials |
| 2 | 20 | 20 | 2 | ≥30 correct trials |
| 3 | 10 | 10 | 5 | ≥50 correct trials |
| 4 | 5 | 5 | 5 | ≥50 correct trials; ≥ 75% accuracy; |
| ≤25% omissions; total trials – premature = 100 | ||||
| 5 | 2.5 | 5 | 5 | ≥50 correct trials; ≥ 75% accuracy; |
| ≤25% omissions; total trials – premature = 100 | ||||
| 6 | 1.8 | 5 | 5 | ≥50 correct trials; ≥ 75% accuracy; |
| ≤25% omissions; total trials – premature = 100 | ||||
| (b) | |
| Premature responses/100 trials | [premature responses/(correct + incorrect responses + omissions)] × 100 |
| %Omissions | [total omissions/(correct + incorrect responses + omissions)] × 100 |
| Perseveration score | total nose-pokes into the same hole following a correct response |
| Total trials | correct responses + incorrect responses + omissions |
| %Accuracy | [correct responses/(correct + incorrect responses)] × 100 |
| Latency to correct response (seconds) | latency to nose-poke into the correct hole after the onset of the light stimulus |
| Latency to collect reward (seconds) | latency to collect the milk reward following a correct response |
ITI, intertrial interval; LH, limited hold; SD, stimulus duration.
Once stable performance had been achieved at Stage 6 of training (‘baseline’ performance: >50 correct trials, >75% accuracy, <25% omissions and total trials = 100), subjects were tested in two ways, so as to challenge different aspects of cognitive performance. One used a fixed, long intertrial interval (LITI), in which the ITI was increased from 5 seconds to 7 seconds. The other used a variable intertrial interval (VITI: 2, 5, 10, 15 seconds), in which the ITIs were delivered in a random sequence. To eliminate any potential carry-over effects of previous experience of the tests, the sequence of testing in the VITI and LITI was counterbalanced across all experimental factors (Colony, Genotype and Time-of-Day). The VITI and LITI tests were carried out on Fridays, only. On the intervening days (Monday–Thursday), the animals carried out Stage 6 of the training procedure, to ensure that baseline performance was restored before the next test day.
By the end of the study, four mice (1 WT-Hom, 2 WT-Het and 1 KO-Het) had not reached the criteria for baseline performance for Stage 6 and so were excluded from all statistical analyses (including training).
Statistical analysis
Statistical analyses were carried out using InVivoStat (Clark et al. 2011). For both experiments, diagnostic plots for normality of the data-set and equality of the variance of the samples were checked and, when necessary, the data were transformed [square-root(score) or Log10(score + 1)] to optimize the homogeneity of variance across the four groups. Because the aim of the experiment was to investigate the effects of breeding strategy on pups' behaviour, we defined ‘Colony’ and ‘Genotype’ as fixed factors (i.e. ‘treatments’) in the analysis. We could not assume that all offspring within a single cage would be affected in the same way by these factors (which would include differences such as parental care, interaction with littermates, and/or maternal physiology) and so individual mice, which received these ‘treatments’, were regarded as the experimental unit.
Although Colony was treated as a between-subjects factor, if there was no interaction between Colony and the variable of interest, data for the two colonies were collapsed for analysis of the effect of Genotype, which was treated as another between-subjects factor. Mead's Resource Equation was used routinely to confirm that sample sizes were large enough to detect statistically significant differences.
Locomotor activity
Only data collected between days 2 and 7 were included in the statistical analysis (see above). For each animal, data captured at 60-second intervals were grouped into 6 h time bins, in order to compare animals' activity during the first and second half of the light and dark phases (Early Light Phase: 0700 h to 1259 h; Late Light Phase: 1300 h to 1859 h; Early Dark Phase: 1900 h to 0059 h; Late Dark Phase: 0100 h to 0659 h). In order to determine whether activity changed across days 2–7, each 6 h time bin was first analysed using mixed model anova with ‘Day’ as the within-subjects factor and ‘Colony’ and ‘Genotype’ as between-subjects factors. If there was no interaction between Day and Colony and/or Genotype, time-matched data for each 6 h time bin were pooled across days 2–7 to produce a mean activity for that time bin (N = 1 for each animal). Mean activity of each colony and genotype was then compared across the different time bins using repeated measures anova with ‘Time Bin’ as the within-subjects factor and ‘Colony’ and ‘Genotype’ as the between-subjects factors. Because changes in locomotor activity during the early dark phase, the late dark phase and early light phase of days 2–7 did not interact with either colony or genotype, the data for these six days were pooled for each time bin. However, during the late light phase, changes in activity over days 2–7 depended on Colony (Colony*Day: F5,80 = 3.17, P = 0.012) and so, for this phase, Day was treated as a separate factor in the analysis.
5-CSRTT
For the training phase, the analysis compared data from the first day of each stage of training, using 4-way mixed model anova, with ‘Colony’, ‘Genotype’ and ‘Time-of-Day’ (morning or afternoon) as the between-subjects factors and ‘Stage (of training)’ as the within-subjects factor. Data from the testing phase of the experiment (VITI and LITI) were first analysed using 3-way single measures anova, with ‘Colony’, ‘Genotype’ and ‘Time-of-Day’ treated as between-subjects factors. Post hoc 2-way, 1-way anova or the LSD test were used for more detailed comparisons of specific test groups. Data points that deviated from the mean by more than 3× standard deviations were treated as outliers and removed from analysis. This was necessary only for the VITI, with one mouse eliminated from the analysis of perseveration (1× WT-Hom), %accuracy and latency to correct response (1× KO-Hom), and latency to collect reward (1× KO-Hom). As a consequence, the statistical analyses compared groups of N = 10-12. Statistical significance was set at P < 0.05.
Results
Both genetic and environmental/epigenetic factors influence locomotor activity, but at different phases of the 24 h cycle
Double-plotted actograms, showing the activity of one mouse from each group (WT-Hom, KO-Hom, WT-Het, KO-Het) across days 1–7, are shown in Fig. 1a.
Figure 1.
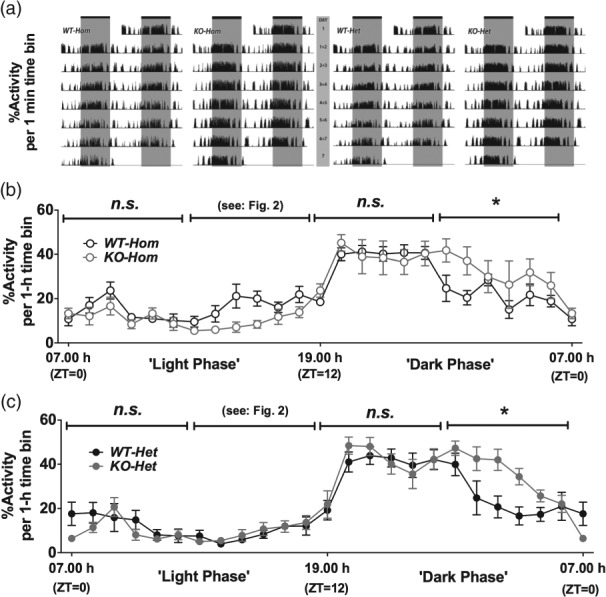
Spontaneous motor activity across the 24 h light:dark cycle. (a) Double-plotted actograms displaying the % activity (per 1 min time bin) of one mouse from each group (WT-Hom, KO-Hom, WT-Het, KO-Het) across days 1–7, (b) mean % activity (per 1 h time bin) of WT-Hom mice and KO-Hom mice across the 24 h cycle, and (c) mean % activity (per 1-h time bin) of WT-Het mice and KO-Het mice across the 24 h cycle. (a) Areas in white represent the light phase of the 24 h light:dark cycle, whereas areas in grey represent the dark phase of the 24 h light:dark cycle. (b) and (c) Circles depict mean ± SEM for each hourly time-point and lines above the graphs represent 6 h time bins used for statistical analysis. For each individual animal, data from time-matched samples were pooled across six consecutive days to produce the mean ± SEM for each time bin (N = 1 for each animal; N = 5 per experimental group). The light intensity in the holding room was increased or reduced in steps between 07.00 h and 08.00 h and 19.00 h and 20.00 h, respectively; *P < 0.05; n.s., non-significant; ZT, zeitgeber time (light cue).
The activity of the two colonies did not differ during the entire dark phase or the early light phase of the 24 h cycle. However, during the late dark phase, the activity of NK1R-/- mice, from both colonies, was greater than that of their respective wildtypes [Genotype*Time Bin: F2,32 = 7.97, P = 0.002, LSD: (Hom): P = 0.046 and (Het): P = 0.031; Fig. 1b,c, respectively].
During the late light phase, differences in the activity of the two colonies changed over days 2–7 and interacted with genotype (Fig. 2). This is because the activity of both KO-Hom and WT-Het mice decreased over this time (LSD: P < 0.05 for all), whereas the activity of KO-Het mice progressively increased (LSD: P < 0.05 for all). Notwithstanding these changes, the activity of WT-Hom mice, throughout days 2-7, was greater than that of all other groups of mice (LSD: P < 0.05 for all), which did not differ from each other.
Figure 2.
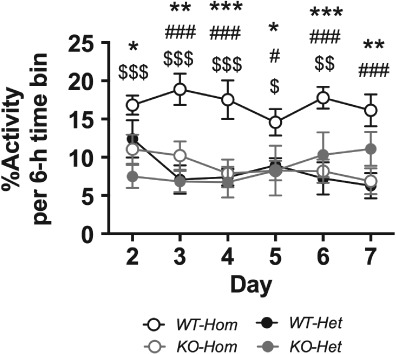
Spontaneous motor activity during the late light phase of the light:dark cycle across days 2−7. Circles depict mean ± SEM for this time bin across days 2–7 (N = 1 for each animal; N = 5 per experimental group). *P < 0.05, **P < 0.01, ***P < 0.05 (cf. WT-Hom vs. KO-Hom); #P < 0.05, ##P < 0.01, ###P < 0.001 (cf. WT-Hom vs. WT-Het); $P < 0.05, $$P < 0.01, $$$P < 0.001 (cf. WT-Hom vs. KO-Het).
NK1R-/- mice from both colonies display a higher incidence of premature responses and perseveration during training in the 5-CSRTT
During the training phase, there was no overall difference in the total number of sessions needed for the two colonies to reach the graduation criteria (Fig. 3a,b). Of all the behaviours measured, only latency to collect the reward, which was slightly longer in Hom mice (F1,36 = 4.34, P = 0.044, Fig. 4p), differed in the two colonies (Fig. 4).
Figure 3.
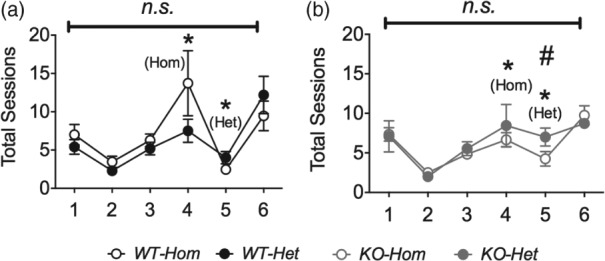
Total sessions required to pass training. Lines above graphs indicate colony comparisons. Circles show mean ± SEM. Numbers below graphs indicate stage of training. *P < 0.05 (cf. WT vs. KO); #P < 0.05 (cf. Hom vs. Het); n.s., non-significant. N = 10–12 per group.
Figure 4.
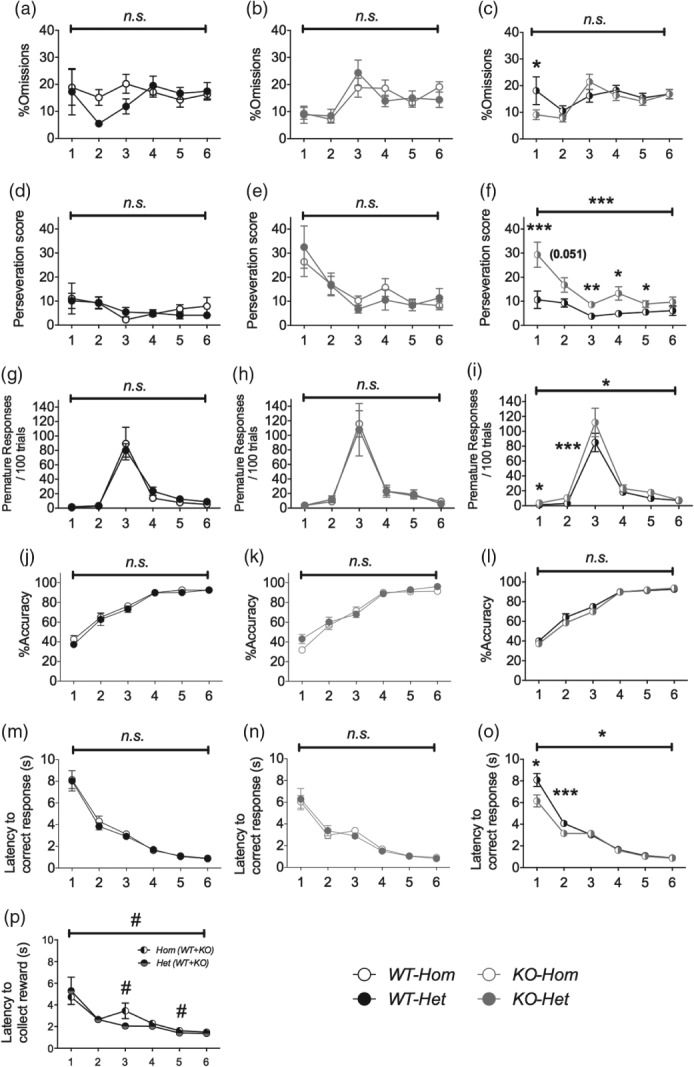
Training in the 5-Choice Serial Reaction-Time Task. There was no main effect of Colony on %omissions (a and b), perseveration (d and e) or premature responses (g and h). The effect of Genotype on %omissions depended on Stage: wildtypes showed greater %omissions than NK1R-/- mice at stage 1 but not at any later stages of training (c). Overall, perseveration (f) and premature responses (i) were greater in NK1R-/- mice compared with wildtype mice. %Accuracy did not differ in the two colonies (j and k) or the two genotypes (l). Latency to correct response also did not differ in the two colonies (m and n), but was greater in wildtype mice compared with NK1R-/- mice overall, although this depended on stage (o). Latency to collect reward differed in the two colonies, with mice from the Hom colony taking longer than mice from the Het colony (p). Lines above graphs indicate comparisons between the two colonies or genotypes. Circles show mean ± SEM. Numbers below graphs indicate stage of training. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., non-significant; N = 10–12 per group.
When comparing genotypes on the first day of each Stage of training, NK1R-/- mice from both colonies showed a higher incidence of perseveration (F1,36 = 15.53, P < 0.001, Fig. 4f) and premature responses (F1,36 = 6.00, P = 0.019, Fig. 4i). There was no overall genotype difference in %omissions, despite a greater incidence of this deficit in wildtypes from both colonies during Stage 1 (F5,180 = 2.33, P = 0.044, Fig. 4c). Latency to correct response was also longer in wildtypes from both colonies (F1,36 = 5.63, P = 0.023), especially during the early stages of training (F5,180 = 3.31, P < 0.007, Fig. 4o).
At the end of Stage 6 of training, there were no colony or genotype differences for any of the behavioural measures.
The incidence of premature responding, but not omissions or perseveration, during the VITI test differs in NK1R-/- mice from the Hom and Het colonies
Neither %omissions nor perseveration differed in the two colonies in the VITI test (Fig. 5a,b). However, perseveration was greater in NK1R-/- mice, from both colonies, compared with their wildtypes (F1,40 = 5.47, P = 0.024). There were also genotype differences in %omissions, but these depended on whether animals were tested in the morning or afternoon (Genotype*Time of day: F1,39 = 8.28, P = 0.006). Specifically, the two genotypes did not differ when tested in the morning but, in the afternoon, %omissions were higher in wildtypes than NK1R-/- mice (LSD: P = 0.001).
Figure 5.
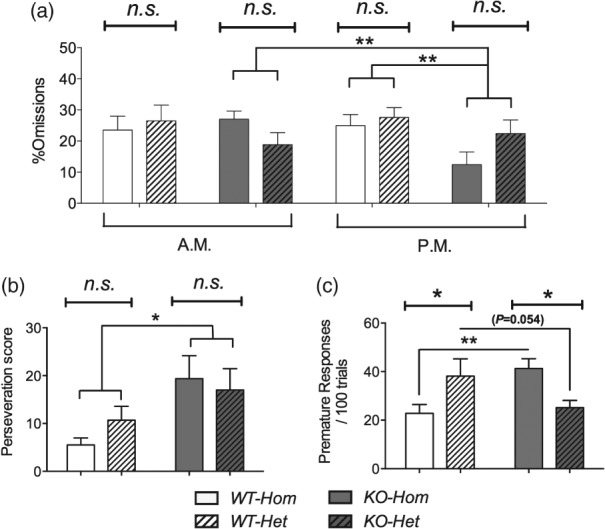
Variable intertrial interval (VITI) in the 5-Choice Serial Reaction-Time Task: attention, perseveration and impulsivity. There was no main effect of colony on either %omissions or perseveration (a and b). However, %omissions were lower in NK1R-/- mice when tested in the afternoon (a) and NK1R-/- mice displayed greater perseveration than wildtype mice, overall (b). The effect of genotype on premature responses depended on colony: in the Homs colony, premature responses were greater in NK1R-/- mice compared with wildtypes, whereas this was not the case for the Het colony (c). Lines indicate comparisons between the main factors or groups. Bars show mean ± SEM. *P < 0.05, **P < 0.01; n.s., non-significant. N = 10–12 per group.
Genotype differences in premature responses depended on Colony (Genotype*Colony: F1,39 = 11.98, P = 0.001, Fig. 5c). As in previous studies, KO-Hom mice expressed more premature responses than WT-Hom mice (LSD: P = 0.006), but KO-Het mice just missed the criterion for carrying out fewer premature responses than WT-Het mice (LSD: P = 0.054).
There were no colony differences in %accuracy, total number of trials completed, latency to correct response or latency to collect reward in the VITI test (Fig. 6a–d) and none of these measures differed in the two genotypes. Overall, %accuracy was slightly higher (c. 3%) in the afternoon than in the morning (F1,34 = 5.05, P = 0.031, Fig. 6a).
Figure 6.
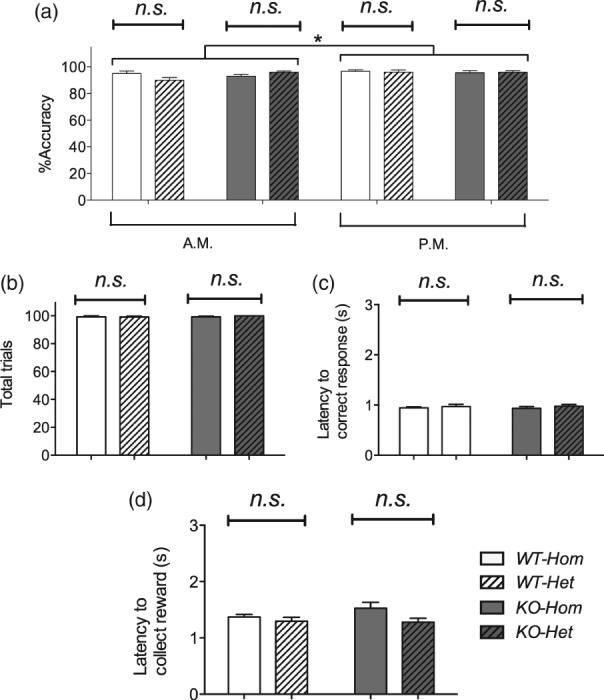
VITI in the 5-Choice Serial Reaction-Time Task: accuracy, total trials and latencies. %Accuracy was slightly higher when animals were tested in the afternoon compared with the morning (a). There was no main effect of any of the main factors on total trials (b), latency to correct response (c) or latency to collect reward (d). Lines indicate comparisons between the main factors or groups. Bars show mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., non-significant. N = 10–12 per group.
The behaviour of NK1R-/- mice from Hom and Het colonies does not differ in the LITI test
When the two colonies were tested with the LITI, there was no difference in %omissions, perseveration or premature responses (Fig. 7a–c). Perseveration and premature responses also did not differ in the two genotypes. However, as in the VITI (see above), a genotype difference in %omissions depended on time of day (Genotype*Time of day: F1,39 = 8.51, P = 0.006). Regardless of colony, %omissions were greater in NK1R-/- mice than their wildtypes when they were tested in the morning (LSD: P = 0.035). Moreover, %omissions by NK1R-/- mice was greater in the morning than the afternoon (LSD: P = 0.003).
Figure 7.
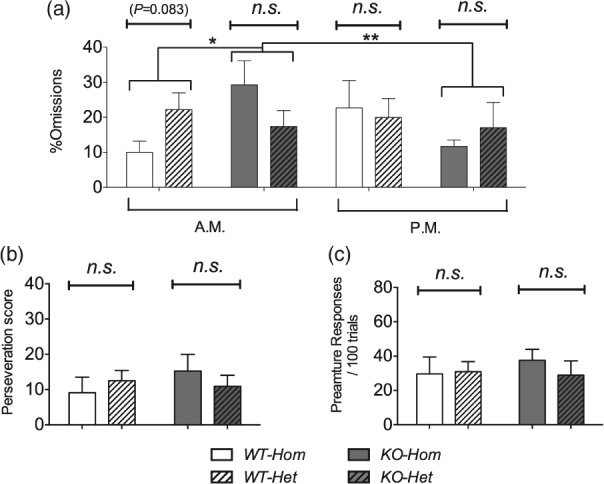
Long intertrial interval in the 5-Choice Serial Reaction-Time Task: attention, perseveration, impulsivity. %Omissions did not differ in the two colonies (a). %Omissions were greater in NK1R-/- mice when tested in the morning but not in the afternoon. There was no main effect of colony, genotype or time-of-day on perseveration (b) or premature responses (c). Bars show mean ± SEM. Lines indicate comparisons between the main factors or groups. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., non-significant. N = 10–12 per group.
As in the VITI test, there were no colony differences in %accuracy, total number of trials completed, latency to correct response, or latency to collect reward and none of these measures differed in the two genotypes (Fig. 8a–d).
Figure 8.
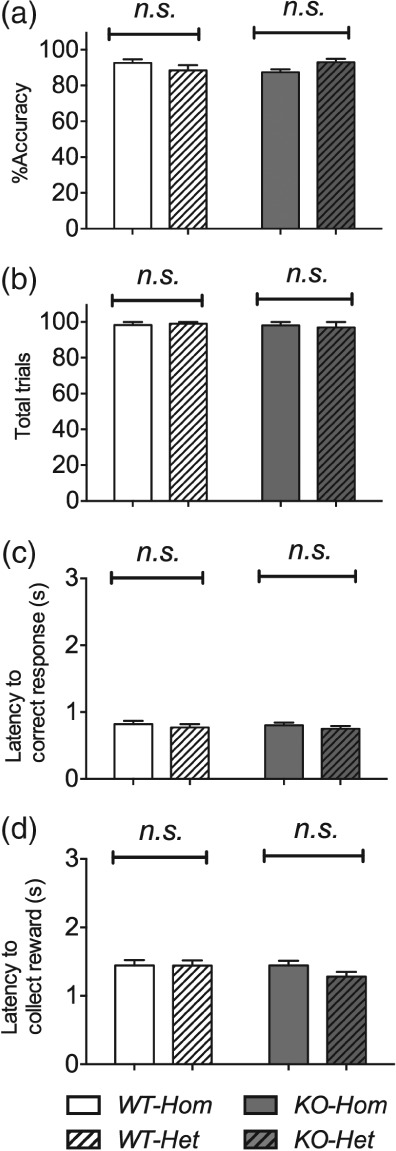
Long intertrial interval in the 5-Choice Serial Reaction-Time Task: accuracy, total trials and latencies. None of these behavioural measures differed in the two colonies. There was also no main effect of any of the other experimental factors (a–d). Lines at top of graphs indicate colony comparisons. Bars show mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., non-significant. N = 10–12 per group.
Discussion
Genotype and breeding environment influence different aspects of the 24 h cycle of motor activity
Both colonies of NK1R-/- mice were more active than their respective wildtypes during the late dark (active) phase of the 24 h cycle. This finding is in line with our report that acute treatment with an NK1R antagonist induced hyperactivity in inbred wildtypes (Yan et al. 2010) and suggests that a lack of functional NK1R, alone, is sufficient to induce hyperactivity.
A second finding was that the diurnal regulation of motor activity was disrupted in NK1R-/- mice. There are many reasons to expect abnormal light-entrained activity rhythms when NK1R function is impaired. Light activates the retinohypothalamic tract (RHT), which projects to the suprachiasmatic nucleus (SCN), directly, and indirectly via the thalamic intergeniculate leaflet (‘IGL’; Morin et al. 2003). Substance P is found within the core and/or in peripheral zones of the SCN (Hartwich et al. 1994; Piggins et al. 2001) and both substance P and NK1R are expressed in the IGL (Morin et al. 1992; Piggins et al. 2001). NK1R antagonism suppresses excitatory postsynaptic currents in the SCN, evoked by optic nerve stimulation (Kim et al. 1999, 2001), whereas treatment with an NK1R antagonist selectively blocks the ability of light to induce phase advances of locomotor activity (Challet et al. 2001). It is interesting that phase advances occur in response to light exposure during the late active phase, which is when we observed differences in motor activity in NK1R-/- mice.
NK1R are also expressed by some serotonergic neurones in the raphé nuclei (Lacoste et al. 2006), which is thought to gate motor activity (Jacobs & Azmitia 1992). Central serotonergic transmission contributes to motor entrainment of the circadian rhythm (Edgar et al. 1997; Meyer-Bernstein et al. 1997), most likely through activation of serotonergic receptors in the SCN (e.g. Horikawa et al. 2000). It follows that the abnormal serotonergic transmission in NK1R-/- mice (Froger et al. 2001) could also help to explain this disruption of their 24 h motor rhythm.
A third finding was that the activity of WT-Hom mice during the late light phase was greater than that of all other groups of mice. This difference depends on functional NK1R because these WT-Hom mice were also more active than the KO-Homs at this time. Yet, there was no difference in the activity of WT-Het and KO-Het mice and so we infer that environmental and/or epigenetic factors either blunted the activity of the WT-Hets or increased that of WT-Homs. There is extensive evidence that early-life experience affects adult behaviour (see Meaney 2010). Epidemiological studies also suggest that a range of environmental factors interact with a genetic vulnerability for ADHD (see Elia et al. 2014).
The abnormal diurnal motor rhythm of NK1R-/- mice has a clear equivalent in ADHD. Many patients experience delayed sleep onset at the end of the active phase (Kooij & Bijlenga 2013; van Veen et al. 2010) while some ADHD patients also experience delayed waking at the end of the resting phase (Cortese et al. 2013). Here, an obvious difference is that mice from the Hom colony were bred, weaned and housed separately, but mice from the Het colony were bred and weaned by NK1R+/− dams and housed in mixed litters. The extent to which interactions between pups and their mother and/or littermates account for the differences in behavioural phenotypes of the two colonies merits further investigation. Such studies could shed light on whether an interaction between early-life experiences and polymorphism(s) of TACR1 disrupt motor rhythms in ADHD patients.
Impulsivity of NK1R-/- mice in the 5-CSRTT (VITI) is influenced by environmental/epigenetic factors
NK1R-/- mice, from either colony, carried out more premature responses overall than their wildtypes, especially during the early stages of training. This finding points to a lack of functional NK1R as a factor that can exacerbate impulsivity. Because there were no differences in any behavioural measure at the end of training, it seems that neither the two colonies nor two genotypes differed in their ability, or their motivation, to carry out the task at this stage of the protocol. Nonetheless, we cannot be certain that the food restriction increased motivation to the same extent in the two colonies/genotypes.
When tested in the VITI, KO-Hom mice carried out more premature responses compared with their wildtypes. Because this increase did not occur in the LITI, the greater incidence of premature responses in KO-Hom mice seems to depend on the stimulus being unpredictable, rather than an increase in motivation to respond. Whatever the explanation, it is striking that there was no increase in premature responses in KO-Het mice. These findings suggest that the incidence of this behaviour is determined by an interaction between NK1R and other (e.g. environmental and/or epigenetic) factors.
There is extensive evidence for interactions between mother and pups that influence cognitive performance (e.g. Hao et al. 2011). Early-life experiences also affect susceptibility to psychiatric disorders in later life (Anda et al. 2006; Lima et al. 2010). As yet, there is no evidence that epigenetic changes affect NK1R (TACR1) function. However, repeated cocaine administration does decrease DNA methylation of the TACR3 gene (Nk3r gene in mice: Barros et al. 2013). Detailed comparison of the influence of breeding strategy on the behaviour of NK1R-/- mice in the two colonies could help to identify the cause(s) of excessive impulsivity.
Perseveration and impaired attentional performance of NK1R-/- mice are a direct consequence of a lack of NK1R
Perseveration did not differ in the two colonies but was higher in NK1R-/- mice during training and the VITI test, as in our previous studies (Dudley et al. 2013; Yan et al. 2011). These findings suggest that this behaviour is attributed directly to a lack of functional NK1R. Importantly, they rule out environmental/epigenetic influences on perseveration, in the VITI at least. It is striking that repetitive behaviour (including compulsive checking) is evident in some ADHD patients (Gürkan et al. 2010). The lack of any genotype difference in the LITI test fits with our experience that the incidence of perseveration in the LITI is more variable than in the VITI (cf. Dudley et al. 2013; Yan et al. 2011), suggesting that the LITI and VITI tests must recruit different neuronal processes.
There was no difference in %omissions in the two colonies during training or testing and so we infer that epigenetic factors have no bearing on this behaviour. Interestingly, genotype-dependent differences in this behaviour depended on time of day, as in our previous study (Yan et al. 2011). There is evidence for a circadian rhythm in cognitive performance (e.g. Winocur & Hasher 2004). Performance deficits in ADHD patients similarly depend on time of day (Usami et al. 2013). However, we cannot distinguish whether the changes we report are explained by a disruption of a circadian influence or an extraneous procedural factor. They are unlikely to arise from any genotype-dependent change in motivation to respond because neither the latency to correct response nor latency to collect the reward differed in the morning and afternoon. Whatever the explanation, we infer that this behaviour is not influenced by the breeding environment.
We did not explore the mechanisms that could underlie these behavioural abnormalities. However, a lack of NK1R would blunt both glutamatergic excitation and GABAergic inhibition of noradrenergic projections from the locus coeruleus to the prefrontal cortex, which govern visual attention (see Yan et al. 2009). NK1R are also densely expressed on (cholinergic) interneurons in the striatum (Gerfen 1991), where they mediate the release of acetylcholine and dopamine (Galarraga et al. 1999), both of which have been implicated in impulsive behaviour (see Jupp & Dalley 2014; Moreno et al. 2013). The functional integrity of these interneurons is thought to determine appropriate motor responses to salient environmental stimuli (e.g. Ding et al. 2010) and to constrain impulsivity and perseveration (e.g. Burguière et al. 2013; Christakou et al. 2004; Chudasama et al. 2003).
Conclusions
We infer that the hyperactivity and perseveration of NK1R-/- mice are a direct consequence of a lack of functional NK1R, which also disrupts the diurnal regulation of motor activity. Greater %omissions were evident in NK1R-/- mice only in the morning of the LITI test, indicating that evaluation of this behavioural deficit is confounded by procedural or temporal factors, as yet unidentified. By contrast, the greater impulsivity of NK1R-/- mice was influenced by an interaction between genetic and environmental and/or epigenetic factors in combination with a lack of functional NK1R, the effects of which depend on previous test experience and cognitive context.
This study also reveals that differences in the phenotype of genetically-altered animals can be assigned to genetic, environmental/epigenetic factors, or both, only after head-to-head comparisons of the progeny of homozygous and heterozygous breeding-pairs. It follows that biomarkers for ADHD could differ for each behavioural aspect of the disorder (‘endophenotype’). This is particularly important for research into the aetiology of ADHD because there is strong evidence that both genetic (Faraone et al. 2005) and environmental factors (including maternal behaviour; Elia et al. 2014) increase vulnerability to this disorder.
Acknowledgments
This work was funded by an MRC PhD studentship (A.J.P.) and an MRC CASE studentship (K.P. in partnership with RenaSci Ltd). The authors wish to thank Dr Simon Bate for his invaluable advice on the statistical analysis of the data during the preparation and revision of this manuscript. Stephen Hunt and Clare Stanford are named inventors on an EU patent for the NK1R-/- mouse model of ADHD, held by UCL Business (CS declined the option to receive royalties).
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR. Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros M, Dempster EL, Illott N, Chabrawi S, Maior RS, Tomaz C, De Souza Silva MA, Huston JP, Mill J. Muller CP. Decreased methylation of the NK3 receptor coding gene (TACR3) after cocaine-induced place preference in marmoset monkeys. Addict Biol. 2013;18:452–454. doi: 10.1111/j.1369-1600.2011.00409.x. [DOI] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G. Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E, Dugovic C, Turek FW. Van Reeth O. The selective neurokinin 1 receptor antagonist R116301 modulates photic responses of the hamster circadian system. Neuropharmacology. 2001;40:408–415. doi: 10.1016/s0028-3908(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW. Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A. Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clark RA, Shoaib M, Hewitt KN, Stanford SC. Bate ST. A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J Psychopharmacol. 2011;26:1136–1142. doi: 10.1177/0269881111420313. [DOI] [PubMed] [Google Scholar]
- Cortese S, Brown TE, Corkum P, Gruber R, O'Brien LM, Stein M, Weiss M. Owens J. Assessment and management of sleep problems in youths with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:784–796. doi: 10.1016/j.jaac.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Crews D, Fuller T, Mirasol EG, Pfaff DW. Ogawa S. Postnatal environment affects behaviour of adult transgenic mice. Exp Biol Med. 2004;229:935–939. doi: 10.1177/153537020422900910. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Goldowitz D, Holmes A. Wolfer D. Standards for the publication of mouse mutant studies. Genes Brain Behav. 2009;8:1–4. doi: 10.1111/j.1601-183X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA. Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley JA, Weir RK, Yan YC, Grabowska EM, Grimmé AJ, Amini S, Stephens DN, Hunt SP. Stanford SC. Antagonism of L-type Ca(v) channels with nifedipine differentially affects performance of wildtype and NK1R-/- mice in the 5-Choice Serial Reaction-Time Task. Neuropharmacology. 2013;64:329–336. doi: 10.1016/j.neuropharm.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Reid MS. Dement WC. Serotonergic afferents mediate activity-dependent entrainment of the mouse circadian clock. Am J Physiol. 1997;273:R265–R269. doi: 10.1152/ajpregu.1997.273.1.R265. [DOI] [PubMed] [Google Scholar]
- Elia J, Laracy S, Allen J, Nissley-Tsiopinis J. Borgmann-Winter K. Epigenetics: genetics versus life experience. In: Tannock R, editor; Stanford SC, editor. Behavioural Neuroscience of Attention Deficit Hyperactivity Disorder. Springer; 2014. pp. 317–340. & (eds), &. In. [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA. Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- de Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F. Hunt SP. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- Fisher AS, Stewart RJ, Yan T, Hunt SP. Stanford SC. Disruption of noradrenergic transmission and the behavioural response to a novel environment in NK1R-/- mice. Eur J Neurosci. 2007;25:1195–1204. doi: 10.1111/j.1460-9568.2007.05369.x. [DOI] [PubMed] [Google Scholar]
- Froger N, Gardier AM, Moratalla R, Alberti I, Boni C, De Felipe C, Rupniak NM, Hunt SP, Jacquot C, Hamon M. Lanfumey L. 5-hydroxytryptamine (5HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci. 2001;21:8188–8197. doi: 10.1523/JNEUROSCI.21-20-08188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Hernández-López S, Tapia D, Reyes A. Bargas J. Action of substance P (neurokinin-1) receptor activation on rat neostriatal projection neurons. Synapse. 1999;33:26–35. doi: 10.1002/(SICI)1098-2396(199907)33:1<26::AID-SYN3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Substance P (neurokinin-1) receptor mRNA is selectively expressed in cholinergic neurons in the striatum and basal forebrain. Brain Res. 1991;556:165–170. doi: 10.1016/0006-8993(91)90563-b. [DOI] [PubMed] [Google Scholar]
- Gürkan K, Bilgiç A, Türkoglu S, Kiliç BG, Aysev A. Uslu R. Depression, anxiety and obsessive-compulsive symptoms and quality of life in children with attention-deficit hyperactivity disorder (ADHD) during three-month methylphenidate treatment. J Psychopharmacol. 2010;24:1810–1818. doi: 10.1177/0269881109348172. [DOI] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA. Kosten TA. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Front Psychiatry. 2011;3:21. doi: 10.3389/fpsyt.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwich M, Kalsbeek A, Pevet P. Nurnberger F. Effects of illumination and enucleation on substance-P-immunoreactive structures in subcortical visual centers of golden hamster and Wistar rat. Cell Tissue Res. 1994;277:351–361. doi: 10.1007/BF00327783. [DOI] [PubMed] [Google Scholar]
- Herpfer I, Hunt SP. Stanford SC. A comparison of neurokinin 1 receptor knockout (NK1-/-) and wildtype mice: exploratory behaviour and extracellular noradrenaline concentration in the cerebral cortex of anaesthetized subjects. Neuropharmacology. 2005;48:706–719. doi: 10.1016/j.neuropharm.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H. Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nuclei. J Neurosci. 2000;20:5867–5873. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL. Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jupp B. Dalley JW. Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. Br J Pharmacol. 2014;171:4729–4766. doi: 10.1111/bph.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Kim SH, Kim DY, Lee HW, Shin HC, Chung JM, Han HC, Na HS. Hong SK. Electrophysiological evidence for a role of substance P in retinohypothalamic transmission in the rat. Neurosci Lett. 1999;274:99–102. doi: 10.1016/s0304-3940(99)00681-3. [DOI] [PubMed] [Google Scholar]
- Kim DY, Kang HC, Shin HC, Lee KJ, Yoon YW, Han HC, Na HS, Hong SK. Kim YI. Substance P plays a critical role in photic resetting of the circadian pacemaker in the rat hypothalamus. J Neurosci. 2001;21:4026–4031. doi: 10.1523/JNEUROSCI.21-11-04026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JJ. Bijlenga D. The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev Neurother. 2013;13:1107–1116. doi: 10.1586/14737175.2013.836301. [DOI] [PubMed] [Google Scholar]
- Lacoste B, Riad M. Descarries L. Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur J Neurosci. 2006;23:2947–2958. doi: 10.1111/j.1460-9568.2006.04833.x. [DOI] [PubMed] [Google Scholar]
- Lima AR, Mello MF. Mari JdeJ. The role of early parental bonding in the development of psychiatric symptoms in adulthood. Curr Opin Psychiatry. 2010;23:383–387. doi: 10.1097/yco.0b013e32833a51ce. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Blanchard JH. Morin LP. The serotonergic projection from the median raphé nucleus to the suprachiasmatic nucleus modulates activity phase onset, but not other circadian rhythm parameters. Brain Res. 1997;755:112–120. doi: 10.1016/s0006-8993(97)00111-x. [DOI] [PubMed] [Google Scholar]
- Moreno M, Economidou D, Mar AC, Lopez-Granero C, Capriolo D, Theobald DE, Fernando A, Newman AH, Robbins TW. Dalley JW. Divergent effects of D2/3 receptor activation in the nucleus accumbens core and shell on impulsivity and locomotor activity in high and low impulsive rats. Psychopharmacology. 2013;228:19–30. doi: 10.1007/s00213-013-3010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Blanchard J. Moore RY. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the golden hamster. Vis Neurosci. 1992;8:219–230. doi: 10.1017/s095252380000287x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH. Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Samuels RE, Coogan AN. Cutler DJ. Distribution of substance P and neurokinin-1 receptor immunoreactivity in the suprachiasmatic nuclei and intergeniculate leaflet of hamster, mouse, and rat. J Comp Neurol. 2001;438:50–65. doi: 10.1002/cne.1301. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Sasaki A, De Vega W, Sivanathan S, St-Cyr S. McGowan PO. Maternal high-fat diet alters anxiety behaviour and glucocorticoid signalling in adolescent offspring. Neuroscience. 2014;272:92–101. doi: 10.1016/j.neuroscience.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Sharp SI, McQuillin A, Marks M, Hunt SP, Stanford SC, Lydall GJ, Morgan MY, Asherson P, Curtis D. Gurling HM. Genetic association of the tachykinin receptor 1 TACR1 gene in bipolar disorder, attention deficit hyperactivity disorder, and alcohol dependence syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014;165:373–380. doi: 10.1002/ajmg.b.32241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino LM, Sullivan PF. Meltzer-Brody S. Using animal models to disentangle the role of genetic, epigenetic, and environmental influences on behavioural outcomes associated with maternal anxiety and depression. Front Psychiatry. 2011;2:44. doi: 10.3389/fpsyt.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami M, Okada T, Sasayama D, Iwadare Y, Watanabe K, Ushijima H, Kodaira M, Sugiyama N, Sawa T. Saito K. What time periods of the day are concerning for parents of children with Attention Deficit Hyperactivity Disorder? PLoS One. 2013;8:e79806. doi: 10.1371/journal.pone.0079806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC. Van Someren EJ. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67:1091–1096. doi: 10.1016/j.biopsych.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Winocur G. Hasher L. Age and time-of-day effects on learning and memory in a non-matching-to-sample test. Neurobiol Aging. 2004;25:1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Yan TC, Hunt SP. Stanford SC. Behavioural and neurochemical abnormalities in mice lacking functional tachykinin-1 (NK1) receptors: a model of attention deficit hyperactivity disorder. Neuropharmacology. 2009;57:627–635. doi: 10.1016/j.neuropharm.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Yan TC, McQuillin A, Thapar A, Asherson P, Hunt SP, Stanford SC. Gurling H. NK1 (TACR1) receptor gene ‘knockout’ mouse phenotype predicts genetic association with ADHD. J Psychopharmacol. 2010;24:27–38. doi: 10.1177/0269881108100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL, Hunt SP, Stephens DN. Stanford SC. Performance deficits of NK1 receptor knockout mice in the 5-Choice Serial Reaction-Time Task: effects of d-amphetamine, stress and time-of-day. PLoS One. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]


