Abstract
Objective
To evaluate whether a series of pharmacologic agents with potential neuroprotective effects accelerate and/or improve facial function recovery after facial nerve crush injury.
Study Design
Randomized animal study
Methods
Eighty female Wistar-Hannover rats underwent head fix implantation and daily conditioning. Animals then underwent standardized crush injury to the main trunk of the facial nerve, and were randomized to receive treatment with Atorvastatin (n=10), Sildenafil (n=10), Darbepoetin (n=20), or corresponding control agent (n=40). The return of whisking function was tracked throughout the recovery period.
Results
Darbepoetin treatment showed improved whisking amplitude on postoperative days 15, 18, 21, and at week 5. Additionally, Darbepoetin treated animals had higher whisking velocity and acceleration on postoperative days 18, 21, and week 5. Sildenafil treatment showed improved whisking velocity over controls on postoperative day 12, and improved whisking acceleration on postoperative day 12 and 13 compared with controls, however this marginal benefit was not seen on the subsequent postoperative days. Atorvastatin treatment had no effect on facial function recovery after facial nerve crush injury, compared with controls. By week eight, the Darbepoetin and control treated animals had achieved complete recovery.
Conclusion
Amongst the several potentially neuroprotecitve agents evaluated, only Darbepoetin administration resulted in accelerated facial function recovery after facial nerve crush injury. Further efforts to define the mechanism of action and translate these findings to the use of Darbepoetin in the care of patients with traumatic facial paralysis are needed.
INTRODUCTION
Facial paralysis can be a debilitating condition, with significant functional and aesthetic implications. Management of this condition can be directed toward its underlying cause, toward addressing its functional consequences, or both. In most treatment algorithms, there are few medical options. The discovery of an agent that could facilitate or accelerate facial nerve recovery after crush or transection injury would be invaluable. Our laboratory has developed a rat model of facial nerve injury, whereby facial function is objectively quantified, by the tracking of whisker movements during recovery. This model lends itself to the evaluation of pharmacologic interventions after facial nerve manipulation. Herein, we investigate the effect of three pharmacologic agents on the rate and extent of facial function recovery after facial nerve crush injury.
Many pharmacologic agents, have been shown experimentally to have a beneficial effect on nerve regeneration. Amongst these agents, several are currently in clinical use for other indications, and establishment of their benefit in nerve injury models would pave the way for new indications. Statins, for example, are inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, and have been shown to decrease cardiovascular mortality [1, 2]. They have also been found to have a positive effect on peripheral and central nerves after injury [3] [4]. The exact mechanism by which statins may exert their beneficial effect on injured nerves has yet to be elucidated. Sildenafil (Viagra) is a selective phosphodiesterase type 5 (PDE5) inhibitor. It has been approved for the treatment of erectile dysfunction but there is evidence to suggest that it may also play a role in promoting neurogenesis. PDE5 is an important enzyme involved in the hydrolysis of cGMP. PDE5 inhibition will result in an increase in intracellular cGMP concentration [5]. cGMP has been demonstrated to be involved in the potentiation of neurogenesis.[6–8]. Darbepoetin is an analogue of Erythropoietin (Epo) and is an erythropoiesis-stimulating agent. These drugs have been shown to promote red blood cell survival, stimulate hematopoiesis and angiogenesis, and promote proliferation of smooth muscle fibers and vessel endothelium [9]. They are approved for the treatment of patients with anemia related to chronic renal insufficiency or the administration of chemotherapy. Numerous studies have reported that Epo administration benefits nerve recovery after central and peripheral nerve injury [9–11]. Epo has been shown to prevent axonal degeneration after nerve injury, and to facilitate the return of erectile function after cavernous nerve transaction injury in the rat model [12].
Based on these observations, we hypothesized that Atorvastatin, Sildendifil and Darbepoetin might accelerate facial function recovery after facial nerve crush injury. If effective, these agents could be safely transitioned to use in clinical practice for the management of facial nerve injuries.
Materials and Methods
HEAD FIXATION AND BEHAVIORAL ADAPTATION
Female Wistar-Hannover rats (Charles River Laboratories, Wilmington, Massachusetts) weighing 200 to 250 grams were used in accordance with Massachusetts Eye and Ear Infirmary guidelines for animal care and use. After animals arrived to our facility, they were handled for several days prior to surgical manipulation. Animals were anesthetized with an intramuscular injection of ketamine (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, Iowa) and medetomidine hydrochloride (0.5 mg/kg) (Orion Corporation, Espoo, Finland). They underwent surgical insertion of a lightweight titanium head implant that provided a set of four external attachment points for rigid head fixation [13, 14]. After implantation, animals were conditioned to a body restraint apparatus by brief daily placement into a fitted sack. By the third week, head restraint was added to the daily conditioning regimen. After the third week, the animals were sufficiently conditioned to undergo head/body restraint without struggling or signs of stress. Thereafter, pre-surgical baseline testing was performed.
FACIAL NERVE CRUSH SURGICAL PROCEDURE
Animals were anesthetized, and the left infra-auricular area was shaved and sterilely prepared. The left facial nerve was approached through an infra-auricular incision in each animal. Left facial nerve exposure involved incision, removal of the parotid gland, and visual identification of the main trunk of the facial nerve as it emerged anterior to the posterior belly of the digastric muscle. Nerve crush was conducted in a manner that has been previously validated [15], proximal to its trifurcation, for 30 seconds using a jeweler’s microforceps, and the crush injury was repeated for an additional 30 seconds in the same location. The wound was then irrigated with saline, closed in a single layer with absorbable suture, and the anesthetic reversed with a subcutaneous injection of 0.05mg/kg of atipamezole hydrochloride. Animals were allowed to recover on a warming pad, and were monitored for food and water intake, post-operative discomfort, and weight gain. Through visual inspection, complete loss of whisking function was verified on postoperative day one.
ADMINSTRATION OF COMPOUNDS
For animals receiving atorvastatin (n = 10), a single tablet of Atorvastatin was dissolved in methanol and saline (80 mg atorvastatin dissolved in 46ml saline with 24 μL methanol). Animals received atorvastatin (10mg/kg) administered by oral gavage twice daily. The dose of atorvastatin was titrated to 10mg/kg given twice daily, beginning four days prior to facial nerve crush surgery. A corresponding control group (n=10) was treated with the same mixture of methanol and saline (no drug), administered by oral gavage twice daily at the same time points.
For the sildenafil-treated animals (n = 10), a single tablet of Sildenafil (100mg) was crushed, weighed and mixed in dough. Animals were given oral doses of sildenafil (4mg/kg) on the day of facial nerve crush surgery and for the first six post operative days (n=10). A comparable control group (n=10) was given one gram of drug-free dough at the same time points (n=10).
For the Darbepoetin-treated animals (n = 20), the drug was administered at a 10 mcg /kg dose subcutaneously, once per week. The initial dose of Darbepoetin was administered approximately one hour prior to facial nerve crush surgery. A corresponding control group (n=20) was treated with an equivalent volume of saline subcutaneously at the same time points.
FUNCTIONAL RECOVERY TESTING
Baseline whisking evaluation was performed pre-operatively, and initial post-surgical evaluation was performed six days after animals recovered from facial nerve manipulation. Our previously validated testing apparatus was employed to monitor whisking recovery. Briefly, on the day of testing, animals were placed in the body restraint device, C-1 whiskers were marked bilaterally using a rectangular strip of polyimide tubes (SWPT-045, SWPT-008, Small Parts Inc.), and then placed into the monitoring apparatus. The horizontal movement of the marked C-1 whiskers was independently tracked using commercial laser micrometers (MetraLight, Santa Mateo CA) and a data acquisition computer. A computer-controlled air valve was used to deliver 10 second sustained flows of scented air toward the snout in order to elicit whisking behavior at two random time points during each 5-minute data recording session per animal [13, 14]. The apparatus generated data regarding the kinematics of whisker movement.
DATA ANALYSIS
Automated Whisking software calculated whisking amplitude, velocity and acceleration for all whisks in the testing session. The three whisks of largest amplitude were detected, and the amplitude, velocity and acceleration of these most vigorous whiskes were averaged for each animal, for each day of testing, on both sides of the face. If the animal had fewer than three whisks during a testing period, a “zero” was entered for the data point. On any given testing day, an animal was excluded from analysis if it exhibited significant distress or discomfort. A group average of each whisking parameter was calculated, and Excel data analysis software was used to perform independent, two sample t-tests for the different time points between the experimental and control groups, under the hypothesis that each agent would improve recovery of facial function. Statistical significance was present if p < 0.05 on one or two tailed t-tests.
RESULTS
All animals exhibited normal cage behavior throughout the study. They achieved normal weight gain and did not exhibit aggressive cage behavior. There were no post-operative wound infections after either the head mount implantation, or the facial nerve crush procedure in any of the study groups. Corneal ulcerations were noted in four animals in the control groups, and in one animal in the Atorvastatin group. These were aggressively treated with ophthalmic antibiotic drops, with rapid healing. All animals tolerated administration of drug or control without any noted side effects. Animals that had not conditioned appropriately to the testing apparatus were excluded: For the control animals, there were a total of five exclusions: one dough-receiving animal, three subcutaneous injection animal, and one gavage animal. In the drug-receiving groups, there were two exclusions, both in the atorvastatin group. A single control animal had an ineffective crush injury, so was excluded. All other animals underwent testing throughout the duration of the study.
Animals demonstrated signs of recovery of whisking function starting on postoperative day 12. There was a rapid recovery phase from postoperative day 12 to day 18. After postoperative day 18, whisking recovery tended to plateau.
Administration of Darbepoetin was found to have a modest but statistically significant beneficial effect on whisking recovery after facial nerve crush injury. There was a trend toward higher whisking amplitude early in recovery, but without statistical significance, until POD 15 when there was statistically higher whisking amplitude in the drug-treated group (p < 0.05). This significant improvement in amplitude was consistently seen thereafter on postoperative day 18 (p < 0.05), and day 21 (p < 0.05) (Figure 2). The improved whisking amplitude in the Darbepoetin group was also observed during the plateau phase during the 5th week (p < 0.05) (Figure 1). The difference in whisking velocity was less pronounced, with the Darbepoetin treated animals having a significantly larger whisking velocity than control animals ononly on PODs 18 and 21 (p < 0.05) and postoperative week 5 (p < 0.05) (Figure 1,2). Results were the same when comparing whisking acceleration with dur-treated and control animals (Figures 1). By postoperative week eight the whisking amplitude, velocity and acceleration were similar between the two groups, as full recovery had occurred (Table 1).
Figure 2.
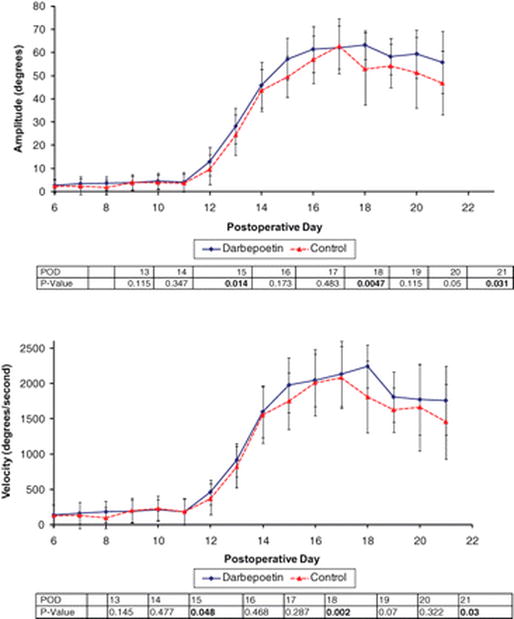
Line graphs showing the effect of darbepoetin treatment on whisking amplitude (a) and velocity (b), following facial nerve crush injury, along with a table of associated P values, (a) Whisking amplitude during recovery was statistically higher in animals receiving darbepoetin (n = 20) compared to control animals (n = 16) on postoperative days 15, 18, and 21 when tested across days 13 to 21. (b) Whisking velocity during recovery was statistically higher in animals receiving darbepoetin compared to control animals on postoperative days 15, 18. and 21.
Figure 1.
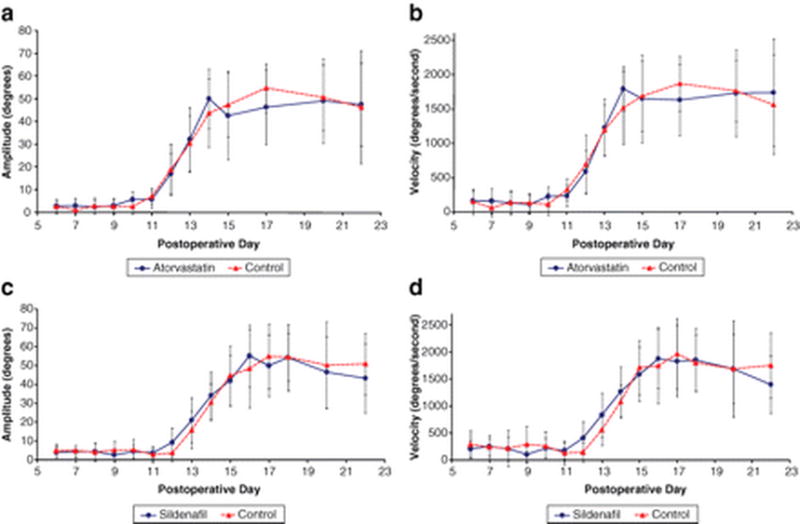
Line graphs showing the effect of treatment with atorvastatin (a, b) and sildenafil (c, d) on whisking amplitude and velocity following facial nerve crush injury, (a, b) There is no difference in whisking amplitude or velocity in the atorvastatin-treated animals (n = 10; plots a–b) compared to matched controls (n = 9) or in the sildenafil-treated animals (n = 10; plots c–d) compared to the matched control animals (n = 10). POD, postoperative day.
Table 1.
Whisking Amplitude and Velocity at Weeks 5 and 8 After Facial Nerve Crush Injury
| Darbepoetin | Control | P Value | |
|---|---|---|---|
| Week 5 | |||
| Amplitude, degree ± SD | 73.5 ± 11.0 | 53.2 ± 15.2 | .025 |
| Velocity, degree/s ± SD | 1825.6 ± 598.8 | 1516.0 ± 408.6 | .048 |
| Week 8 | |||
| Amplitude, degree ± SD | 73.5 ± 11.0 | 70.2 ± 20.5 | .36 |
| Velocity, degree/s ± SD | 2062.3 ± 606.1 | 1977.7 ± 741.1 | .403 |
There was no difference in the rate or extent of recovery of whisking function between animals treated with Atorvastatin and control animals. (Figure 1). Similarly, animals treated with Sildenafildid not demonstrate any consistent, significant effect on facial function recovery. No significant difference in whisking amplitude was demonstrated between Sildenafil and control groups during recovery from nerve crush injury (Figure 1d). However, whisking velocity was higher in the Sildenafil treated animals than control animalson POD12 (p <0.05). This difference in whisking velocity was not seen on any other postoperative days. Similarly, whisking acceleration was significantly higher only early in recovery on POD12and13 (p < 0.05), however this difference was not demonstrated on other postoperative days during recovery or plateau phase.
DISCUSSION
A number of reports have identified the beneficial effects of neurotrophic factors such as neurotrophin-3 (NT-3), brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) on the injured nerve [16] [17, 18]. Despite promising findings with various agents, no pharmacologic agent (with the exception of corticosteroids) has been deemed safe or efficacious enough to be used in clinical practice. This study evaluates three safe, FDA-approved drugs in current clinical use for other conditions: Atorvastatin, Sildenafil, and Darbepoetin. All three agents have demonstrated potential for use in the setting of peripheral nerve injury. To our knowledge, this is the first report evaluating these three agents in the rat facial nerve model. We found a modest but potentially useful benefit to the administration of Darbepoetin following facial nerve crush injury.
Crush injury to a peripheral nerve results in disruption of nerve fibers with subsequent wallerian degeneration and axonal regeneration. The crush model is a reliable model of axonotmesis [15];. Numerous predictable cellular and molecular changes occur in injured neurons and in their microenvironment, [21, 22] and these events provide targets for potential pharmacologic intervention. After injury, within weeks most axons have regenerated and remyelinated, yielding functional recovery within several months [23]. The predictable regeneration that occurs after crush injury allows evaluation of the effect of different pharmacologic agents on the speed and extent of functional recovery. The rat facial nerve model serves as an excellent paradigm for the study of peripheral nerve injury; the results of which can have direct clinical implications.
Reports in the literaturesuggest that statin administration will result in improved facial function after facial nerve injury. Numerous studies have demonstrated the beneficial effects of statin administration on both central and peripheral nerves after injury. Atorvastatin was found to be neuroprotective, promote neuronal migration and plasticity, and improve functional neurologic recovery in mice subjected to stroke [3] [24, 25]. Simvastatin was shown, in rat CNS explants, to counteract myelin-associated neurite outgrowth inhibition and promote neurite outgrowth [26]. Gholami et al in 2007 demonstrated that administration of Simvastatin prior to ischemic-reperfusion injury of sciatic nerve resulted in improved observer-rated limb function recovery [27], and went on to identify histological features of neuroprotection after administration of Simvastatin, in the context of ischemic-reperfusion injury of the sciatic nerve [4]. Our results do not corroborate this benefit. Sildenafil, an FDA approved, orally available drug with a low side effect profile, was originally designed to combat erectile dysfunction. Investigators have explored its possible neuroregenerative effects based on the known positive effects of NO on neural tissue [8, 28]. Studies have shown that increasing NO levels, or increasing cGMP levels with Sildenafil, induces neurogenesis and promotes functional recovery of the central nervous system after stroke [8, 28]. In the current study, very early in recovery, there was a small improvement in whisking velocity and acceleration in the Sildenafil-treated animals compared with controls, however this difference was very short lived. Despite the supporting evidence, the administration of Sildenafil, after facial nerve crush injury, showed no long lasting, significant benefit in facial function recovery.
In contrast to the above two agents, Darbepoetin administration showed modest benefit in whisking function recovery after facial nerve crush injury. Darbepoetin has three times the half-life of recombinant human Epo (rHuEpo) [31]. This longer half life allows for less frequent dosing while achieving an equivalent beneficial effect on anemia related to chemotherapy administration [32].
Erythropoietin has been found in in vitro and in in vivo models of central and peripheral nerve injury to have a protective and regenerative effect. Epo and its receptor have been localized to the central and peripheral nervous system as well as to the retina. After peripheral nerve injury, Epo production is increased in Schwann cells. While the mechanism by which Epo exerts its effect on nerves is under investigation, it is believed that by binding to its receptor, it activates numerous downstream pathways that may be responsible for its neuroregenerative potential [9]. Epo was found to reduce glutamate toxicity, inhibit neuronal apoptosis and limit axonal degeneration after nerve injury [9, 33]. In rats after transection of the cavernous nerve, administration of rHuEpo and Darbepoetin resulted in improved erectile function [12]. Administration of Epo after crush injury to the sciatic and facial nerves has been reported to improve functional recovery [11, 34]. OUr findings in the current study corroborate previous work on Epo, as well as Darbepoetin. We found that Darbepoetin-treated animals had a modest but statistically significant improvement in all kinematics of whisking at most postoperative time points, compared to their control counterparts. The current work is limited by inclusion of only functional, behavioral data, which by its nature is fluctuating and potentially inconsistent. Despite the fact that all animals were tested in the same apparatus under similar conditions, there are variables beyond investigator control, such has day to day variability in the mood of the animal. Randomization of animals was done in order to distribute any known and unknown confounding variables.In behavioral studies such as this, the standard error is expected to be large, however statistically analysis did reveal real differences in whisking parameters between experimental and control groups. The methodology utilized to collect data in the current study is more rigorous than what has been published previously, where whisker function is rated through visual assessment of video clips.
Conclusions
This study demonstrates that Darbepoetin, but not Atorvastatin or Sildenafil, facilitate facial function recovery after facial nerve crush injury. These findings extend to the facial nerve previous work that has shown Epo and Darbepoetin to be beneficial when administered after central and peripheral nerve injury. These results establish the foundation for future work that may result in the incorporation of Darbepoetin into the management of patients presenting with facial paralysis after traumatic facial nerve injury.
References
- 1.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 2.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 3.Chen J, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gholami MR, et al. The effects of simvastatin on ischemia-reperfusion injury of sciatic nerve in adult rats. Eur J Pharmacol. 2008;590(1–3):111–4. doi: 10.1016/j.ejphar.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 5.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274(20):13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 6.Hindley S, et al. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J Neurosci Res. 1997;47(4):427–39. [PubMed] [Google Scholar]
- 7.Gonzalez-Hernandez T, Rustioni A. Nitric oxide synthase and growth-associated protein are coexpressed in primary sensory neurons after peripheral injury. J Comp Neurol. 1999;404(1):64–74. [PubMed] [Google Scholar]
- 8.Zhang R, et al. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50(5):602–11. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 9.Lykissas MG, et al. The role of erythropoietin in central and peripheral nerve injury. Clin Neurol Neurosurg. 2007;109(8):639–44. doi: 10.1016/j.clineuro.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. Faseb J. 2001;15(10):1804–6. doi: 10.1096/fj.00-0857fje. [DOI] [PubMed] [Google Scholar]
- 11.Grasso G, et al. Neuroprotective potential of erythropoietin and darbepoetin alfa in an experimental model of sciatic nerve injury. Laboratory investigation. J Neurosurg Spine. 2007;7(6):645–51. doi: 10.3171/SPI-07/12/645. [DOI] [PubMed] [Google Scholar]
- 12.Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol. 2005;174(5):2060–4. doi: 10.1097/01.ju.0000176808.94610.dd. [DOI] [PubMed] [Google Scholar]
- 13.Heaton JT, et al. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171(2):197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadlock T, et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008;118(10):1744–9. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 15.Bridge PM, et al. Nerve crush injuries–a model for axonotmesis. Exp Neurol. 1994;127(2):284–90. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 16.Lee AC, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184(1):295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 17.Sun W, et al. The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30(27):4649–56. doi: 10.1016/j.biomaterials.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JY, et al. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000;12(12):4171–80. [PubMed] [Google Scholar]
- 19.Kurtoglu Z, et al. Effects of trapidil after crush injury in peripheral nerve. Acta Med Okayama. 2005;59(2):37–44. doi: 10.18926/AMO/31967. [DOI] [PubMed] [Google Scholar]
- 20.Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood blow. An in vivo study on rabbit tibial nerve. J Hand Surg [Am] 1981;6(1):3–12. doi: 10.1016/s0363-5023(81)80003-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Peripheral nerve injury induces down-regulation of Foxo3a and p27kip1 in rat dorsal root ganglia. Neurochem Res. 2009;34(5):891–8. doi: 10.1007/s11064-008-9849-8. [DOI] [PubMed] [Google Scholar]
- 22.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- 23.Hoogeveen JF, et al. Hyperthermic injury versus crush injury in the rat sciatic nerve: a comparative functional, histopathological and morphometrical study. J Neurol Sci. 1992;108(1):55–64. doi: 10.1016/0022-510x(92)90188-q. [DOI] [PubMed] [Google Scholar]
- 24.Gertz K, et al. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34(2):551–7. doi: 10.1161/01.str.0000054055.28435.bf. [DOI] [PubMed] [Google Scholar]
- 25.Laufs U, et al. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31(10):2442–9. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 26.Holmberg E, et al. Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma. 2006;23(9):1366–78. doi: 10.1089/neu.2006.23.1366. [DOI] [PubMed] [Google Scholar]
- 27.Gholami M, et al. The Effects of Simvastatin on Functional Recovery of Rat Reperfused Sciatic Nerve. Pakistan Journal of Biological Sciences. 2007;10(23):4256–4260. doi: 10.3923/pjbs.2007.4256.4260. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33(11):2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 29.Firestein BL, Bredt DS. Regulation of sensory neuron precursor proliferation by cyclic GMP-dependent protein kinase. J Neurochem. 1998;71(5):1846–53. doi: 10.1046/j.1471-4159.1998.71051846.x. [DOI] [PubMed] [Google Scholar]
- 30.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Nephrol Dial Transplant. 2001;16(Suppl 3):3–13. [PubMed] [Google Scholar]
- 31.Egrie JC, et al. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol. 2003;31(4):290–9. doi: 10.1016/s0301-472x(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 32.Patton J, Reeves T, Wallace J. Effectiveness of darbepoetin alfa versus epoetin alfa in patients with chemotherapy-induced anemia treated in clinical practice. Oncologist. 2004;9(4):451–8. doi: 10.1634/theoncologist.9-4-451. [DOI] [PubMed] [Google Scholar]
- 33.Keswani SC, et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56(6):815–26. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, et al. High dose erythropoietin promotes functional recovery of rats following facial nerve crush. J Clin Neurosci. 2009;16(4):554–6. doi: 10.1016/j.jocn.2008.06.013. [DOI] [PubMed] [Google Scholar]


