Abstract
Avibactam is a novel β-lactamase inhibitor that restores the activity of otherwise hydrolyzed β-lactams against Gram-negative bacteria expressing different classes of serine β-lactamases. In the last decade, β-lactam-avibactam combinations were tested against a variety of clinical isolates expressing multiple commonly encountered β-lactamases. Here, we analyzed isogenic Escherichia coli strains expressing selected single β-lactamase genes that were not previously tested or were not characterized in an isogenic background. The activities of ceftazidime, ceftaroline and aztreonam alone and in combination with 4 mg/L of avibactam, as well as comparator agents, were assessed against an unique collection of isogenic strains of E. coli carrying selected extended-spectrum, inhibitor-resistant, and/or carbapenem-hydrolyzing bla genes. When combined with avibactam, ceftazidime, ceftaroline or aztreonam MICs were reduced for 91.4%, 80.0% and 80.0% of isolates, respectively. The data presented adds to our understanding of the microbiologic spectrum of these β-lactams with avibactam and serve as a reference for further studies.
Keywords: β-lactamase, avibactam, ESBL, carbapenemase, inhibitor-resistance
1. Introduction
The prevalence of extended-spectrum β-lactamases (ESBLs), AmpCs and KPC-carbapenemases among Gram-negative bacteria is increasing worldwide. As a result, the use of β-lactam-β-lactamase inhibitor combinations such as piperacillin-tazobactam and “last-line” β-lactams such as carbapenems is challenged. These agents were formerly active against the majority of Gram-negative pathogens encountered in the clinic and were used empirically without much concern for resistance in the treatment of serious Gram-negative infections (Boucher, et al., 2009; Livermore, Warner, et al., 2011). Regrettably, this situation has changed in recent years, and a wide variety of these pathogens manifested resistance to piperacillin-tazobactam, expanded-spectrum cephalosporins, and carbapenems (Livermore, Warner, et al., 2011). The Gram-negative pathogens most associated with resistance to these antibiotics include Klebsiella pneumoniae, Klebsiella oxytoca, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. (Boucher, et al., 2009). A recent survey showed that 12.2% of Enterobacteriaceae isolated from 72 US hospitals harbored β-lactamases, with CTX-M (53.5%), SHV (25.1%), KPC (16.8%) and CMY (9.1%) accounting for the majority of these enzymes (Castanheira, Farrell, Krause, Jones, & Sader, 2014). To overcome the growing problem of microbial resistance, researchers and pharmaceutical companies are adopting a number of strategies, including the development of both new classes of agents and new β-lactam-β-lactamase inhibitor combinations (Boucher, et al., 2013; Shlaes, 2013).
Avibactam is a novel non-β-lactam β-lactamase inhibitor of serine β-lactamases developed to restore β-lactam efficacy (Drawz, Papp-Wallace, & Bonomo, 2014). Previous microbiological studies using clinical isolates expressing different combinations of β-lactamases showed that avibactam recovered the antibacterial activity of ceftazidime in vitro and in vivo against strains producing class A, class C (i.e., AmpC), and some class D enzymes (reviewed in (Drawz, et al., 2014). However, the interpretation of the minimum inhibitory concentration (MIC) data derived from a clinical isolate is difficult to assess as multiple resistance mechanisms can be present. Isolates may possess multiple different β-lactamases, but also non-β-lactamase-mediated mechanisms such as changes in permeability or upregulation of efflux systems. Expression of individual β-lactamases in an isogenic E. coli background allows for the direct evaluation and comparison of the activities of β-lactams and β-lactam-β-lactamase inhibitor combinations against a single β-lactamase. To this end, a unique testing panel of isogenic E. coli strains expressing selected class A, C, and D β-lactamases that were not previously tested or were not characterized in an isogenic background was designed to assess β-lactam-avibactam potency. The strains selected included 17 that resulted in nonsusceptibility to piperacillin-tazobactam, and 5 with decreased susceptibility to meropenem.
The SHV variants selected include those with amino acid substitutions at positions 8, 43, 69, 164, 197, 187, 234 and 238, and are representative of the most common ESBL or inhibitor resistant (IR) SHV variants (e.g., SHV-2, -5, -7, -12 and -30) found in the US (Castanheira, et al., 2014). The TEM variants chosen include those with amino acid substitutions at positions 104, 164, 238 and 240, representative of the most common TEM ESBLs (e.g., TEM-10, -12 and -26) variants. Previously untested KPC variants (i.e., KPC-5, -6, -7, and -8) with substitutions at positions 49, 104, 240, and 274 were included. In addition, several AmpC (i.e., ADC-7, CMY-32, CMY-33, PDC-3, P99, and FOX-4) and two OXA variants (i.e., OXA-1 and OXA-24/40) were assayed; four of these clinically important AmpCs (FOX-4, CMY-32 and -33, and ADC-7) were never previously evaluated against ceftazidime-avibactam in an isogenic E. coli background.
Ceftazidime, ceftaroline (the active metabolite of ceftaroline fosamil) and aztreonam were selected for study with avibactam to assess the degree of inhibition of β-lactamases as these different partner β-lactams agents are in various stages of clinical development with avibactam (http://www.clinicaltrials.gov).
2. Materials and methods
2.1. Strains and Plasmids
Thirty-four isogenic E. coli recombinants carrying single selected β-lactamase genes were assembled. Cloning and/or origins of 25 of the bla genes have been previously described (Bethel, et al., 2006; Blazquez, Negri, Morosini, Gomez-Gomez, & Baquero, 1998; Drawz, et al., 2010; Drawz, Taracila, Caselli, Prati, & Bonomo, 2011; Endimiani, Doi, et al., 2010; Endimiani, Hujer, et al., 2010; Giakkoupi, et al., 2001; Giakkoupi, Tzelepi, Tassios, Legakis, & Tzouvelekis, 2000; Helfand, et al., 2003; A. M. Hujer, Hujer, Helfand, Anderson, & Bonomo, 2002; K. M. Hujer, et al., 2005; Mallo, et al., 2010; Nukaga, et al., 1995; Rice, et al., 2000; Sun, et al., 2001; Winkler, et al., 2013). The other 9 recombinants containing blaSHV-7, blaSHV-14, blaSHV-26, blaSHV-30, blaSHV-106, blaSHV-129, blaSHV-141, blaSHV-154, and blaSHV-161 recombinants were produced for this study by conducting site-directed mutagenesis of the pBC SK(−) phagemid carrying blaSHV-1 using the methods described by Hujer et al. (A. M. Hujer, et al., 2002).
2.2. Susceptibility testing
MICs were determined by broth microdilution using custom frozen panels (ThermoFisher Scientific, Cleveland, OH) according to Clinical Laboratory Standards Institute (CLSI) methods (Clinical and Laboratory Standards Institute, 2015a). MIC values were obtained in triplicate and modal values determined. β-Lactam agents tested (doubling dilution concentration range in mg/L) included ceftazidime (0.06–128), ceftaroline (0.03–64), aztreonam (0.06–64), meropenem (0.015–32) and piperacillin-tazobactam (0.06/4–128/4). Ceftazidime, ceftaroline and aztreonam were also tested at the same concentrations in the presence of a fixed concentration of 4 mg/L of avibactam. MICs were interpreted according to current CLSI breakpoints for these compounds in the absence of the inhibitor (Clinical and Laboratory Standards Institute, 2015b). Avibactam and ceftaroline were provided by AstraZeneca as kind gifts.
3. Results
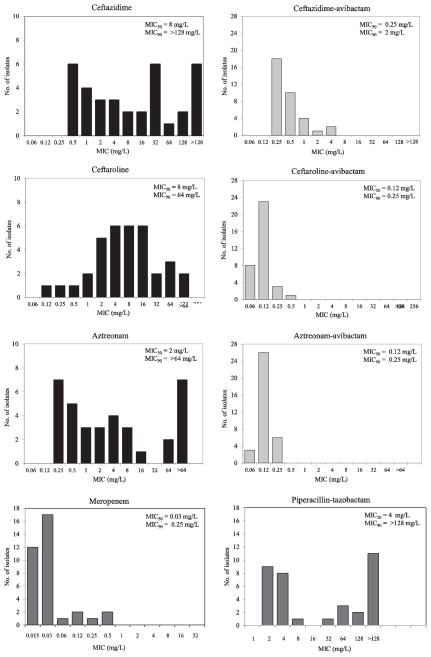
Modal MIC values of the agents tested are presented in Table 1, and MIC distributions of ceftazidime, ceftaroline and aztreonam alone and in the presence of a fixed concentration of 4 mg/L of avibactam are shown in Figure 1.
Table 1.
Activity of β-lactams alone and in combination with avibactam
| MIC in mg/L of β-lactams alone and in combination with avibactam at a fixed concentration of 4 mg/L |
Ratio of MIC of β-lactam alone to β- lactam/avibactam combinationb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | β-lactamase | Meropenema | Piperacillin- tazobactama |
Ceftazidimea | Ceftazidime- avibactamb |
Ceftarolinea | Ceftaroline- avibactamb |
Aztreonama | Aztreonam- avibactamb |
Ceftazidime | Ceftaroline | Aztreonam |
| Nonec | ≤0.015–0.03 | 1–2 | 0.25 | 0.12 | 0.06–0.12 | ≤0.03 | ≤0.06–0.12 | ≤0.06 | 2 | 2 | 1 | |
| A | SHV-2 | 0.03 | 4 | 4 | 0.25 | 8 | 0.12 | 4 | 0.12 | 16 | 64 | 32 |
| SHV-7 | 0.03 | > 128 | > 128 | 1 | 64 | 0.12 | > 64 | 0.25 | >128 | 512 | >256 | |
| SHV-8 | 0.03 | 2 | 32 | 0.5 | 8 | 0.12 | 1 | 0.12 | 64 | 64 | 8 | |
| SHV-1 S130G |
≤0.015 | > 128 | 0.5 | 0.25 | 0.12 | 0.12 | 0.25 | 0.12 | 2 | 1 | 2 | |
| SHV-14 | ≤0.015 | > 128 | 1 | 0.25 | 2 | 0.06 | 0.25 | 0.12 | 4 | 32 | 2 | |
| SHV-26 | 0.03 | > 128 | 2 | 0.5 | 4 | 0.12 | 0.5 | 0.12 | 4 | 32 | 4 | |
| SHV-30 | ≤0.015 | 2 | 4 | 0.25 | 16 | 0.12 | 2 | ≤0.06 | 16 | 128 | 32 | |
| SHV-49 | 0.03 | > 128 | 0.5 | 0.25 | 0.25 | 0.12 | 0.25 | ≤0.06 | 2 | 2 | 4 | |
| SHV-84 | ≤0.015 | 4 | 0.5 | 0.25 | 2 | 0.12 | 0.25 | 0.25 | 2 | 16 | 1 | |
| SHV-102 | 0.03 | > 128 | 16 | 1 | > 64 | 0.12 | 8 | 0.12 | 16 | >512 | 64 | |
| SHV-106 | ≤0.015 | 8 | 8 | 0.25 | 16 | 0.06 | 4 | 0.12 | 32 | 256 | 32 | |
| SHV-120 | 0.03 | > 128 | 8 | 0.5 | 4 | 0.12 | 1 | 0.12 | 16 | 32 | 8 | |
| SHV-1 G238S, E240K, R275L, & N276D |
≤0.015 | 2 | 64 | 0.25 | 2 | 0.06 | 16 | 0.12 | 256 | 32 | 128 | |
| SHV-141 | ≤0.015 | 2 | 1 | 0.25 | 1 | 0.06 | 0.5 | 0.12 | 4 | 16 | 4 | |
| SHV-154 | 0.03 | 4 | > 128 | 0.5 | 64 | 0.12 | > 64 | 0.25 | >256 | 512 | >256 | |
| SHV-161 | 0.03 | > 128 | 2 | 0.25 | 4 | 0.12 | 0.25 | 0.12 | 8 | 32 | 2 | |
| TEM-10 | 0.03 | 4 | > 128 | 2 | 32 | 0.12 | > 64 | 0.25 | >64 | 256 | >256 | |
| TEM-15 | 0.03 | 2 | 16 | 0.5 | 8 | 0.12 | 8 | 0.12 | 32 | 64 | 64 | |
| TEM-17 | ≤0.015 | > 128 | 2 | 0.25 | 8 | 0.06 | 1 | 0.12 | 8 | 128 | 8 | |
| TEM-19 | 0.03 | 4 | 0.5 | 0.25 | 2 | 0.12 | 0.25 | 0.12 | 2 | 16 | 2 | |
| TEM-26 | 0.06 | 2 | > 128 | 1 | 64 | 0.06 | 64 | 0.12 | >128 | 1024 | 512 | |
| TEM-191 | 0.03 | > 128 | 1 | 0.5 | 1 | 0.12 | 0.5 | 0.25 | 2 | 8 | 2 | |
| KPC-5 | 0.12 | 64 | 32 | 0.5 | 8 | 0.12 | > 64 | 0.12 | 64 | 64 | >512 | |
| KPC-6 | 0.12 | 64 | 32 | 0.5 | 16 | 0.25 | > 64 | 0.12 | 64 | 64 | >512 | |
| KPC-7 | 0.5 | 128 | 32 | 0.25 | 16 | 0.12 | 64 | 0.12 | 128 | 128 | 512 | |
| KPC-8 | 0.5 | 128 | > 128 | 4 | 32 | 0.25 | > 64 | 0.12 | >32 | 128 | >512 | |
| C | ADC-7 | ≤0.015 | 32 | 32 | 0.5 | 16 | 0.12 | 4 | 0.12 | 64 | 128 | 32 |
| CMY-32 | ≤0.015 | 2 | 32 | 0.25 | 4 | 0.06 | 8 | 0.12 | 128 | 64 | 64 | |
| CMY-33 | ≤0.015 | 4 | 128 | 1 | 4 | 0.12 | 4 | 0.12 | 128 | 32 | 32 | |
| PDC-3 | 0.03 | 4 | 1 | 0.25 | 0.5 | 0.12 | 0.5 | 0.12 | 4 | 4 | 4 | |
| P99 | 0.03 | 2 | 4 | 0.25 | 4 | 0.12 | 2 | 0.12 | 16 | 32 | 16 | |
| FOX-4 | 0.03 | 4 | 128 | 0.5 | 16 | 0.06 | 2 | ≤0.06 | 256 | 256 | 32 | |
| D | OXA-24/40 | 0.25 | 64 | 0.5 | 0.25 | 2 | 0.5 | 0.25 | 0.12 | 2 | 4 | 2 |
| OXA-1 | 0.03 | > 128 | 0.5 | 0.25 | 8 | 0.25 | 0.5 | 0.12 | 2 | 32 | 4 | |
Grey shaded values indicate modal MIC values ≥ 4-fold higher in E. coli β-lactamase containing recombinants than modal MIC values of the parent E. coli.
Bolded values indicate ≥ 4-fold decrease in modal MIC values of β-lactam-avibactam combinations compared to β-lactams alone or ≥ 4-fold ratio of MIC of β-lactam alone to MIC of β-lactam/avibactam combination
Parent E. coli
Figure 1.
MICs of meropenem, piperacillin-tazobactam, and ceftazidime, ceftaroline and aztreonam alone and combined with avibactam against the E. coli recombinants studied.
Of the strains included in this study, the four blaKPC and the blaOXA-24/40 recombinants showed decreased susceptibility to meropenem (MICs 0.12–0.5 mg/L) (Table 1). Seventeen of the thirty-four strains (i.e., E. coli carrying blaSHV-7, blaSHV-1 S130G, blaSHV-14, blaSHV-26, blaSHV-49, blaSHV-102, blaSHV-120, blaSHV-161, blaTEM-17, blaTEM-191, blaKPC-7, blaKPC-8, and blaOXA-1) were resistant (MICs ≥ 128 mg/L; n=13) or intermediate (MICs 32–64 mg/L; n=4) to piperacillin-tazobactam.
In the panel studied, we observed that of the strains expressing blaESBLs chosen most demonstrated increased MICs against ceftazidime, ceftaroline, and aztreonam when compared to control strains. Similar results were seen with blaAmpC bearing strains. As expected all the blaKPC carrying strains were resistant to ceftazidime, ceftaroline and aztreonam. blaOXA-24/40 and blaOXA-1 producing strains were susceptible to ceftazidime and aztreonam, but MICs were elevated when the strains were tested against ceftaroline.
MICs of all recombinant strains with raised ceftazidime, ceftaroline or aztreonam MICs compared with the parent E. coli were lowered by the addition of 4 mg/L of avibactam (Table 1 and Figure 1). Sixteen of the thirty-four strains (i.e., E. coli producing blaSHV-7, blaSHV-8, blaSHV-1-G238S, -E240K, -R275L, and -N276D, blaSHV-102, blaSHV-154, blaTEM-10, blaTEM-15, blaTEM-26, blaKPC-5, blaKPC-6, blaKPC-7, blaKPC-8, blaADC-7, blaCMY-32, blaCMY-33, and blaFOX-4) were resistant to ceftazidime (MIC range 16 to >256 mg/L). However, when combined with avibactam, ceftazidime MICs were lowered (range 0.25 to 4 mg/L). blaKPC-8 expressed in E. coli DH10B was singular in that the MIC of ceftazidime-avibactam vs. this tranformant was the highest (4 mg/L).
Twenty-nine of the thirty-four isolates (i.e., E. coli carrying blaSHV-2, blaSHV-7, blaSHV-8, blaSHV-14, blaSHV-26, blaSHV-30, blaSHV-84, blaSHV-102, blaSHV-106, blaSHV-120, blaSHV-1 -G238S, -E240K, -R275L, and -N276D, blaSHV-154, blaSHV-161, blaTEM-10, blaTEM-15, blaTEM-17, blaTEM-19, blaTEM-26, blaKPC-5, blaKPC-6, blaKPC-7, blaKPC-8, blaADC-7, blaCMY-32, blaCMY-33, blaP99, blaFOX-4, blaOXA-24/40, and blaOXA-1) were resistant to ceftaroline (MIC range 2 to >64 mg/L). When avibactam was added, the MICs decreased considerably (MIC range 0.06 to 0.5 mg/L).
Only nine isolates (i.e., E. coli carrying blaSHV-7, blaSHV-1 -G238S, -E240K, -R275L, and -N276D, blaSHV-154, blaTEM-10, blaTEM-26, blaKPC-5, blaKPC-6, blaKPC-7, and blaKPC-8) were resistant to aztreonam (MIC range 16 to >64 mg/L), but when combined with avibactam all MICs were lowered considerably (MIC range 0.12 to 0.25 mg/L).
4. Discussion
Avibactam in combination with the selected β-lactams yielded lower MICs against all E. coli strains expressing selected single class A, C, or D β-lactamases that showed raised MICs of ceftazidime, ceftaroline, or aztreonam. This restored the antibacterial potency of these agents against strains expressing multiple variants of SHV and TEM ESBLs, previously untested class C β-lactamases, and uncommon KPC variants.
As absolute MIC values are affected not only by the hydrolytic activity of the β-lactamase, but also the level of expression, codon usage, protein stability, and extent of localization to the periplasm, use of isogenic recombinant strains minimized these variations. Thus the results reported here reflect both the hydrolytic activity of the β-lactamases (MICs ∝ Vmax/Km) against the β-lactams studied, and inhibition of the β-lactamases by avibactam in a uniform background (Cantu & Palzkill, 1998).
Notable findings of this study include demonstration of activity of the β-lactam-avibactam combinations studied against piperacillin-tazobactam and meropenem nonsusceptible strains. However, although the activities of the β-lactamases and their inhibition by avibactam were demonstrated by testing in the isogenic background, these studies necessarily obscured any effect that might result from differences in cell physiology between strains, species and genera of bacteria. In this regard, we highlight that the combination with avibactam substantially shifts MICs of ceftazidime to lower values in studies of unselected clinical isolates (Flamm, Stone, Sader, Jones, & Nichols, 2014; Sader, Castanheira, Flamm, Farrell, & Jones, 2014; Wang, et al., 2014), implying that these other factors do not currently compromise that activity of avibactam in inhibiting β-lactamases in wild-type strains of multiple species. Interestingly, the E. coli strain producing KPC-8 (with amino acid substitutions of V240G and H274Y) presents an unexpected finding. Further studies are in progress to define the properties of this novel variant; we anticipate that the substitutions that are present enhance the β-lactamase’s kinetic properties towards ceftazidime.
In summary, the β-lactam-avibactam combinations will potentially be a significant addition to the antibiotic armamentarium against pathogens expressing members of the classes of β-lactamases studied. Our study is unique in that we assay the activity of these agents in an uniform isogenic background. We also provide a reference collection for future studies.
Ceftazidime-avibactam restores ceftazidime potency against contemporary ceftazidime-resistant Enterobacteriaceae and P. aeruginosa (Flamm, et al., 2014), while aztreonam-avibactam is active against metallo-β-lactamase producers (Livermore, Mushtaq, et al., 2011). The ability of ceftaroline-avibactam to inhibit the growth of cephalosporin-resistant Gram-negative bacteria as well as methicillin-resistant Staphylococcus aureus makes ceftaroline-avibactam a potentially useful broad-spectrum combination for empirical treatment of infections and directed treatment of resistant infections (Kanafani & Corey, 2009).
Acknowledgments
This work was supported by a research grant from AstraZeneca Pharmaceuticals and Actavis (formerly Forest Laboratories) to K.P.W. and R.A.B. M.L.W. has been supported by Medical Scientist Training Program Training Grant, Case Western Reserve University-T32 GM07250. K.P.W. is supported by funds and/or facilities provided by the Cleveland Department of Veterans Affairs and a Veterans Affairs Career Development Award. R.A.B. is supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Department of Veterans Affairs Merit Review Program 1I01BX001974, the Veterans Integrated Service Network 10 Geriatric Research, Education, and Clinical Center (VISN 10 GRECC), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01 AI100560 and R01 AI063517. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. RT and WWN are employees of AstraZeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bethel CR, Hujer AM, Hujer KM, Thomson JM, Ruszczycky MW, Anderson VE, Pusztai-Carey M, Taracila M, Helfand MS, Bonomo RA. Role of Asp104 in the SHV β-lactamase. Antimicrob Agents Chemother. 2006;50:4124–4131. doi: 10.1128/AAC.00848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez J, Negri MC, Morosini MI, Gomez-Gomez JM, Baquero F. A237T as a modulating mutation in naturally occurring extended-spectrum TEM-type β-lactamases. Antimicrob Agents Chemother. 1998;42:1042–1044. doi: 10.1128/aac.42.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Benjamin DK, Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D Infectious Diseases Society of A. 10 × ‘20 Progress--development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Cantu C, 3rd, Palzkill T. The role of residue 238 of TEM-1 β-lactamase in the hydrolysis of extended-spectrum antibiotics. J Biol Chem. 1998;273:26603–26609. doi: 10.1074/jbc.273.41.26603. [DOI] [PubMed] [Google Scholar]
- Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob Agents Chemother. 2014;58:833–838. doi: 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 10. Vol. 2015. Wayne, PA: 2015a. Approved standard M7-A10. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fourth Informational Supplement M100-S25. Wayne, PA: 2015b. [Google Scholar]
- Drawz SM, Bethel CR, Doppalapudi VR, Sheri A, Pagadala SR, Hujer AM, Skalweit MJ, Anderson VE, Chen SG, Buynak JD, Bonomo RA. Penicillin sulfone inhibitors of class D β-lactamases. Antimicrob Agents Chemother. 2010;54:1414–1424. doi: 10.1128/AAC.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58:1835–1846. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawz SM, Taracila M, Caselli E, Prati F, Bonomo RA. Exploring sequence requirements for C(3)/C(4) carboxylate recognition in the Pseudomonas aeruginosa cephalosporinase: Insights into plasticity of the AmpC β-lactamase. Protein Sci. 2011;20:941–958. doi: 10.1002/pro.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endimiani A, Doi Y, Bethel CR, Taracila M, Adams-Haduch JM, O’Keefe A, Hujer AM, Paterson DL, Skalweit MJ, Page MG, Drawz SM, Bonomo RA. Enhancing resistance to cephalosporins in class C β-lactamases: impact of Gly214Glu in CMY-2. Biochemistry. 2010;49:1014–1023. doi: 10.1021/bi9015549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endimiani A, Hujer KM, Hujer AM, Sampath R, Ecker DJ, Bonomo RA. Rapid identification of blaKPC-possessing Enterobacteriaceae by PCR/electrospray ionization-mass spectrometry. J Antimicrob Chemother. 2010;65:1833–1834. doi: 10.1093/jac/dkq207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RK, Stone GG, Sader HS, Jones RN, Nichols WW. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J Chemother. 2014;26:333–338. doi: 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- Giakkoupi P, Hujer AM, Miriagou V, Tzelepi E, Bonomo RA, Tzouvelekis LS. Substitution of Thr for Ala-237 in TEM-17, TEM-12 and TEM-26: alterations in β-lactam resistance conferred on Escherichia coli. FEMS Microbiol Lett. 2001;201:37–40. doi: 10.1111/j.1574-6968.2001.tb10729.x. [DOI] [PubMed] [Google Scholar]
- Giakkoupi P, Tzelepi E, Tassios PT, Legakis NJ, Tzouvelekis LS. Detrimental effect of the combination of R164S with G238S in TEM-1 β-lactamase on the extended-spectrum activity conferred by each single mutation. J Antimicrob Chemother. 2000;45:101–104. doi: 10.1093/jac/45.1.101. [DOI] [PubMed] [Google Scholar]
- Helfand MS, Bethel CR, Hujer AM, Hujer KM, Anderson VE, Bonomo RA. Understanding resistance to β-lactams and β-lactamase inhibitors in the SHV β-lactamase: lessons from the mutagenesis of SER-130. J Biol Chem. 2003;278:52724–52729. doi: 10.1074/jbc.M306059200. [DOI] [PubMed] [Google Scholar]
- Hujer AM, Hujer KM, Helfand MS, Anderson VE, Bonomo RA. Amino acid substitutions at Ambler position Gly238 in the SHV-1 β-lactamase: exploring sequence requirements for resistance to penicillins and cephalosporins. Antimicrob Agents Chemother. 2002;46:3971–3977. doi: 10.1128/AAC.46.12.3971-3977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hujer KM, Hamza NS, Hujer AM, Perez F, Helfand MS, Bethel CR, Thomson JM, Anderson VE, Barlow M, Rice LB, Tenover FC, Bonomo RA. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 β-lactamase: defining a unique family of class C enzymes. Antimicrob Agents Chemother. 2005;49:2941–2948. doi: 10.1128/AAC.49.7.2941-2948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanafani ZA, Corey GR. Ceftaroline: a cephalosporin with expanded Gram-positive activity. Future Microbiol. 2009;4:25–33. doi: 10.2217/17460913.4.1.25. [DOI] [PubMed] [Google Scholar]
- Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37:415–419. doi: 10.1016/j.ijantimicag.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Mallo S, Perez-Llarena FJ, Kerff F, Soares NC, Galleni M, Bou G. A tripeptide deletion in the R2 loop of the class C β-lactamase enzyme FOX-4 impairs cefoxitin hydrolysis and slightly increases susceptibility to β-lactamase inhibitors. J Antimicrob Chemother. 2010;65:1187–1194. doi: 10.1093/jac/dkq115. [DOI] [PubMed] [Google Scholar]
- Nukaga M, Haruta S, Tanimoto K, Kogure K, Taniguchi K, Tamaki M, Sawai T. Molecular evolution of a class C β-lactamase extending its substrate specificity. J Biol Chem. 1995;270:5729–5735. doi: 10.1074/jbc.270.11.5729. [DOI] [PubMed] [Google Scholar]
- Rice LB, Carias LL, Hujer AM, Bonafede M, Hutton R, Hoyen C, Bonomo RA. High-level expression of chromosomally encoded SHV-1 β-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2000;44:362–367. doi: 10.1128/aac.44.2.362-367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014;58:1684–1692. doi: 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes DM. New β-lactam-β-lactamase inhibitor combinations in clinical development. Ann N Y Acad Sci. 2013;1277:105–114. doi: 10.1111/nyas.12010. [DOI] [PubMed] [Google Scholar]
- Sun T, Nukaga M, Mayama K, Crichlow GV, Kuzin AP, Knox JR. Crystallization and preliminary X-ray study of OXA-1, a class D β-lactamase. Acta Crystallogr D Biol Crystallogr. 2001;57:1912–1914. doi: 10.1107/s0907444901016274. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, Wang H. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother. 2014;58:1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler ML, Rodkey EA, Taracila MA, Drawz SM, Bethel CR, Papp-Wallace KM, Smith KM, Xu Y, Dwulit-Smith JR, Romagnoli C, Caselli E, Prati F, van den Akker F, Bonomo RA. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J Med Chem. 2013;56:1084–1097. doi: 10.1021/jm301490d. [DOI] [PMC free article] [PubMed] [Google Scholar]