Abstract
Background
Respiratory syncytial virus (RSV) is a cause of acute chest syndrome (ACS) in sickle cell disease (SCD), but its clinical course and acute complications have not been well characterized. We compared RSV to seasonal influenza infections in children with SCD.
Procedure
We defined cases as laboratory-confirmed RSV or seasonal influenza infection in inpatients and outpatients <18 years of age with SCD from 1 September 1993 to 30 June 2011. We used Fisher’s exact test to compare proportions, Student’s t-test or Wilcoxon rank-sum test to compare continuous variables, and logistic regression to evaluate associations.
Results
We identified 64 children with RSV and 91 with seasonal influenza. Clinical symptoms, including fever, cough, and rhinorrhea were similar for RSV and influenza, as were complications, including ACS and treatments for SCD. In a multivariable logistic regression model, older age (OR 1.2 per year, 95% CI [1.02–1.5], P = 0.04), increased white blood cell count at presentation (OR 1.1 per 1,000/µl increase, 95% CI [1.03–1.3], P = 0.008), and a history of asthma (OR 7, 95% [CI 1.3–37], P = 0.03) were independently associated with increased risk of ACS in children with RSV. The hospitalization rate for children with SCD and RSV (40 per 1,000 <5 years and 63 per 1,000 <2 years) greatly exceeds the general population (3 in 1,000 <5 years).
Conclusions
We conclude that RSV infection is often associated with ACS and similar in severity to influenza infection in febrile children with SCD.
Keywords: acute chest syndrome, influenza, respiratory syncytial virus, sickle cell disease
INTRODUCTION
Sickle cell disease (SCD) is an inherited hemoglobinopathy that affects 1 in 2,500 children in the United States and is associated with anemia, frequent episodes of acute pain, and serious pulmonary complications, such as acute chest syndrome (ACS) and pulmonary hypertension [1]. ACS, defined as a new pulmonary infiltrate in a patient with SCD and fever and respiratory symptoms or signs, is a frequent cause of hospitalization and respiratory failure in those with SCD [2–4]. ACS is often triggered by lower respiratory tract infections in young children [4,5]. In the National Acute Chest Syndrome Study, of 671 episodes of moderate to severe ACS, respiratory syncytial virus (RSV) (26 episodes) was the most common infectious etiology after Mycoplasma pneumoniae and Chlamydia pneumoniae. Influenza was only identified in four participants [4]. Unrecognized viral infections likely contribute to excessive antimicrobial use and health care utilization, since children with SCD and fever are routinely treated with broad-spectrum antibiotics and often hospitalized because of their increased risk of invasive pneumococcal infections [6].
RSV is the most common cause of lower respiratory tract infection in infants in the United States. From November to April, RSV is associated with 20% of hospitalizations, 18% of emergency department visits, and 15% of outpatient visits for acute respiratory infections in children; 3 in 1,000 children age <5 years are hospitalized [7]. Infection with RSV during infancy, although self-limited, may be associated with the development of reactive airway disease or asthma [8–10].
The clinical spectrum and complications of RSV infection in children with SCD have not been reported previously, whereas influenza has been associated with frequent and severe illness in SCD [11,12]. A recent study of 60 children with SCD and acute respiratory infections identified RSV in 8% and influenza in 19% [13]. Based on the largest published study of ACS etiology, which found RSV to be the most common viral etiology of ACS and third most common infectious etiology among children and adults combined [4], we hypothesized that RSV infection would cause more severe disease than seasonal influenza in children with SCD, especially in young children, in whom both RSV infection and infectious etiologies of ACS are more likely. To test this hypothesis we compared influenza and RSV infections in children with SCD.
METHODS
Study Population
We identified patients age <18 years with SCD and influenza or RSV infection by searching discharge and outpatient databases at Johns Hopkins Hospital (JHH) using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes (Table I). We also reviewed the pediatric hematology division’s clinical database. Patients who had a diagnosis of SCD (confirmed by review of the medical record for quantification of abnormal hemoglobin by high performance liquid chromatography) and a positive laboratory test for RSV or influenza from September 1, 1993 to June 30, 2011 were included. We collected data from electronic and paper medical records using a structured form. We used the division of pediatric hematology’s clinical database to estimate the number of patients <2, <5, and <18 years of age with SCD that received care at JHH during the study period.
TABLE I.
International Classification of Disease Version 9 (ICD-9) Codes to Identify Patients With Sickle Cell Disease and Influenza or Respiratory Syncytial Virus Infection
| Diagnosis | ICD-9 |
|---|---|
| Sickle cell disease | 282.41, 282.42, 282.6, 282.61, 282.62, 282.63, 282.64, 282.68, 282.69 |
| Respiratory syncytial virus | 079.6, 466.11, 480.1 |
| Influenza | 487, 487.0, 487.1, 487.8, 488, 488.1, 488.11, 488.12, 488.19 |
Definitions
We defined RSV as laboratory-confirmed RSV infection and influenza as laboratory-confirmed infection with influenza A or B. We excluded those with dual infections. We excluded pandemic influenza (H1N1 during the 2008 and 2009 respiratory viral seasons) because of known increased disease severity in children with SCD [11]. We defined ACS as a new pulmonary infiltrate involving ≥1 complete lung segment with fever (≥38.5°C) or at least one respiratory symptom (tachypnea, cough, wheezing, or chest pain) [4]. We defined severe pain as pain requiring ≥2 doses of opiates and asthma exacerbation as wheezing requiring treatment with a bronchodilator or associated with a diagnosis of asthma. Cases occurring from January 1 to June 30 were included in the respiratory virus season of the previous year. We adjusted costs to 2006 dollars using a 5% discount rate.
Diagnosis of RSV and Influenza
Routine testing in children for RSV and influenza included rapid antigen-based (immunochromatographic lateral flow) tests for influenza and RSV followed by direct fluorescence antigen (DFA) in addition to viral culture (shell vial and conventional cell culture) from 1993 to 2005. Rapid antigen-based (immunochromatographic lateral flow) tests for influenza and RSV were used to identify these infections from the September 2005 until October 2009. When rapid antigen-based tests were either negative or not performed, DFA was used to rapidly detect RSV and influenza with viral culture (shell vial and conventional cell culture) only if DFA was negative. Since November 2009, DFA has been used as the initial test and shell vial culture or PCR for respiratory viruses are used (depending on patient characteristics) in those with negative DFA. All testing was performed as part of routine patient care.
Statistical Analysis
We used Intercooled Stata 11.0 (College Station, TX) for all analyses. Continuous variables were compared by Student’s t-test or the Wilcoxon rank-sum test, and dichotomous variables by Fisher’s exact test. We used univariate and multivariate logistic regression, adjusted for clustering by patient, to characterize associations. The Johns Hopkins Medicine Institutional Review Board reviewed this research and classified it as exempt.
RESULTS
Clinical Features
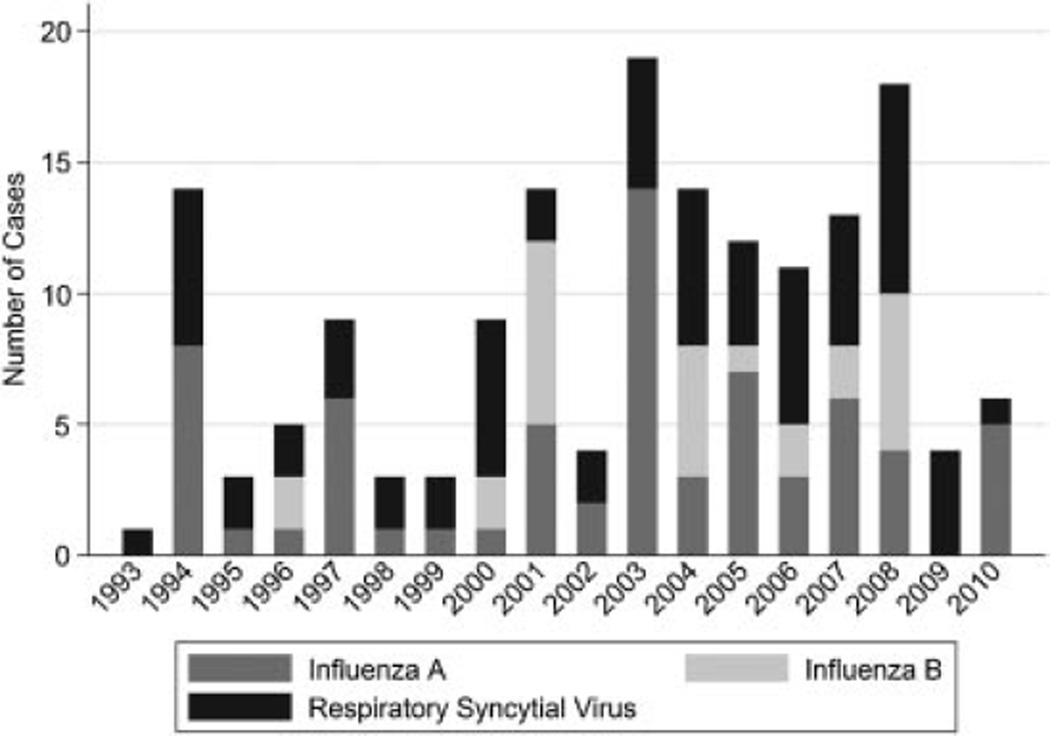
We identified 64 children with RSV and 91 children with influenza within the study period (Fig. 1). RSV infection was rapidly detected in 54 children by an antigen-based test (14) or by DFA (38), whereas influenza was rapidly detected in 54 children by an antigen-based test (15) or DFA (34) as two children were positive by both tests. Culture led to the diagnosis of additional 10 RSV and 37 influenza infections. Those with RSV infection were significantly younger than those with influenza (median age 1.9 years vs. 4.8 years) and more likely to be admitted to the hospital (Table II). Symptoms such as fever (87% vs. 90%), cough (97% vs. 93%), subjective shortness of breath (28% vs. 32%, not available for younger children), diarrhea (15% vs. 14%), and emesis (40% vs. 24%) were similar in those with RSV versus influenza. Respiratory signs more common at presentation with RSV than influenza included wheezing (22% vs. 8%, P = 0.023), rales (32% vs. 13%, P = 0.008), and retractions (19% vs. 6%, P = 0.049) (Table II). The proportion with a previous diagnosis of asthma was similar [RSV (23%) and influenza (28%, P = 0.5)] (Table II), despite the fact that the influenza group was older on average and asthma is often not diagnosed until after age two years. The proportion with evidence of a concomitant non-respiratory infection was also similar [RSV (6.3%) and influenza (8.9%, P = 0.34)]. These included otitis media [9], bacteremia [1], pyelonephritis [1], and group A streptococcal pharyngitis [1].
Fig. 1.
Cases by year and virus at Johns Hopkins Hospital, 1993–2010 seasons.
TABLE II.
Demographic Characteristics and Presenting Symptoms of Children With Respiratory Syncytial Virus (RSV) or Seasonal Influenza and Sickle Cell Disease (SCD), Johns Hopkins Hospital 1993–2011
| Variable | RSV n = 64 | Influenza n = 91 |
P-Value |
|---|---|---|---|
| Age, years | 1.9 (1–3.5) | 4.8 (2, 11) | <0.0001 |
| Male | 50% | 62% | 0.16 |
| Genotype | 0.14 | ||
| HbSS | 75% | 86% | |
| HbSβ-thalassemia | 1.5% | 2% | |
| HbSC disease | 22% | 12% | |
| HbS-other | 1.5% | 0 | |
| Asthma | 23% | 28% | 0.51 |
| Chronic transfusion protocol | 2% | 18% | 0.002 |
| Wheezing | 22% (13/60) | 8% (7/84) | 0.03 |
| Retractions | 19% (10/54) | 6% (3/51) | 0.08 |
| Nasal Flaring | 8% (4/50) | 2% (1/50) | 0.36 |
| Grunting | 0 (0/43) | 2% (1/46) | 0.52 |
Treatment and Outcomes
All children with RSV infection and most children with influenza (100%vs. 89%, P = 0.006), were admitted to the hospital (Table III). Ventilator support was required in some cases of RSV but in no cases of influenza (5% vs. 0% P = 0.07) (Table III) and the only death occurred in a 15-year-old with RSV infection. Outcomes, including length of stay and the proportion who developed ACS or required intensive care unit admission, were similar in the two groups (Table III). In a multivariable logistic regression model, older age (OR 1.2 per year, 95% CI [1.02–1.5], P = .04), increased white blood cell count at presentation (OR 1.1 per each 1,000/µl increase, 95% CI [1.03–1.3], P = 0.008), and a previous diagnosis of asthma (OR 7, 95% CI [1.03–37], P = 0.03) were independently associated with increased risk of ACS in children with RSV. As expected, antiviral agents, effective against influenza but not RSV, were more likely to be administered to patients with influenza compared with those with RSV (40% vs. 5%, P < 0.0001) (Table III). Nearly all children in both groups received antibacterial agents (Table III). Bronchodilators were used in about half of the children and did not differ between the two groups (Table III).
TABLE III.
Treatments and Outcomes for Children With Respiratory Syncytial Virus (RSV) or Seasonal Influenza and Sickle Cell Disease (SCD), Johns Hopkins Hospital 1993–2011
| Variable | RSV n = 64 | Influenza n = 91 |
P-Value |
|---|---|---|---|
| Acute chest syndrome | 26% (15/58) | 15% (13/84) | 0.13 |
| Hospital admission | 100% | 89% | 0.006 |
| Intensive care | 5% | 3% | 0.69 |
| Ventilatory support | 5% | 0% | 0.07 |
| Acute transfusion PRBC* | 13% | 15% | 0.65 |
| Therapy with antiviral agents | 5% | 40% | <0.0001 |
| Therapy with antibacterial agents | 100% | 97% | 0.27 |
| Therapy with bronchodilators | 50% | 40% | 0.25 |
| Length of stay (days)** | 2 (1–3) | 2 (1–3) | 0.15 |
| Hospital charges ($)** | 4,599 (3,352, 6,424) | 4,603 (3,117, 6,695) | 0.54 |
Indicates packed red blood cells.
Indicates median (interquartile range).
Epidemiology
Based on the clinic population at this institution, the estimated rate of hospitalization in patients age <5 years with SCD was 40 per 1,000 person-years (95% CI [30–52]) for those with RSV and 32 (95% CI [24–44]) for those with influenza. The rate for those <2 years was 63 per 1,000 person-years (95% CI [44–87]) for RSV and 39 (95% CI [25–60]) per 1,000 person-years for influenza.
Length of Stay and Costs
Both the median length of stay and cost of hospitalization were similar between the RSV (2 days, $4599) and the influenza (2 days, $4603) groups (Table III).
DISCUSSION
RSV is a common infection in children and is often more severe in young children with chronic cardiopulmonary conditions and prematurity. We found that the rate of hospitalization for RSV in sickle cell disease greatly exceeded (>10-fold) the rate in the general US population (3 in 1,000 for children younger than 5 years) [7]. The morbidity of and rate of hospitalization for influenza was similar to RSV. The rate of hospitalization for RSV for those <2 years (63 per 1,000 person-years) was similar to that of other conditions known to be at high risk for RSV, such cyanotic congenital heart disease (79 per 1,000) [16].
Some clinical features of RSV infection in children with SCD were similar to the general, non-SCD population, including rhinorrhea and lower respiratory signs on exam (19–32% for respiratory findings, compared with a rate of lower respiratory tract involvement for 20–30% of the general population) (Table II). The proportion of children with RSV and SCD who were febrile at admission (55%) was similar to that found in studies of the general population (69%) [7].Within RSV cases, increased white blood cell count and older age (with increasing odds with each year of age) were independently associated with increased risk of ACS. This is unlikely to reflect referral bias, since a similar pattern was not observed in influenza.
The high rate of hospitalization and associated medical costs may reflect the standard practice of admitting most children <3 years old with SCD and fever, and all those with SCD and high fever (T > 40°C) or pulmonary infiltrates. Given the high proportion of children with SCD with functional asplenia and their vulnerability to life-threatening infections with encapsulated organisms, children are routinely given parenteral antibiotics pending the results of blood cultures at our center and others. RSV infection does not exclude a serious bacterial infection and thus would not prevent hospitalizations in most cases [17]. Whether a known viral source of illness affects total hospitalization cost, length of antibiotic administration, or length of stay may be worth further study.
Our study was limited to a single center with a relatively small sample size and therefore less able to detect small differences between infection with RSV and influenza. We retrospectively extracted data, and therefore could not assess clinical features that were not recorded. Our estimate of the proportion with ACS is imprecise, because some children (6 with RSV and 7 with influenza) did not have a chest radiograph and ACS cannot be reliably excluded in young children without imaging [18]. Since ACS was defined clinically in this review, all cases may not have met the prospective, research-quality definition: ACS may have been overestimated in young children with viral etiologies of chest infiltrates (such as mucus plugging causing atelectasis, for example). Therefore, the true proportion with ACS secondary to RSV infection may have been higher or lower than we report.
Further study is needed to examine the long-term outcomes of RSV infection in patients with SCD and the hypothesis that infection with RSV in young children with SCD may contribute to later asthma diagnosis. Similar studies have been done in other populations, and early-life RSV infection has been associated with subsequent development of asthma, pulmonary function abnormalities and allergic sensitization [14,19]. Many children with SCD have abnormal pulmonary function (i.e., lower airway obstruction and hyper-responsiveness to methacholine) in the absence of other risk factors for asthma, including family history [20,21–24]. Further studies are needed to examine the possible link between RSV infection and pulmonary function abnormalities in children with SCD.
Finally, we present data that RSV is a common cause of hospitalization for young children with SCD and a contributor to increased healthcare utilization, since patients with fever and/or pulmonary infiltrates are routinely treated for bacterial infection. Whether infections could be prevented or lessened in severity is unknown. Daycare attendance has been associated with increased respiratory morbidity and more frequent emergency department visits for children with chronic lung disease of prematurity, a group at increased risk for complications from RSV and other respiratory viruses [25]. Whether daycare avoidance could help decrease the frequency of hospitalization for RSV in children with SCD is not known, however this strategy, as well as the possible benefit of palivizumab in this population may be worth examining until an RSV vaccine becomes available.
ACKNOWLEDGMENTS
We thank the medical microbiology lab for clinical testing and Devin Muntz and Sue Dixon for maintaining the clinical database of children with SCD.
Grant sponsor: SCS; Grant numbers: T32; HL72748; Grant sponsor: JJS; Grant number: 1K23HL078819-01A2; Grant sponsor: National Heart, Lung and Blood Institute (NHLBI) JFC; Grant numbers: U54HL090515
Dr. Casella has received an honorarium and travel expenses in the past and presently receives salary support through Johns Hopkins for providing consultative advice to Adventrx Pharmaceuticals regarding a proposed clinical trial of an agent for treating vasoocclusive crisis in sickle cell disease.
Abbreviations
- ACS
acute chest syndrome
- RSV
respiratory syncytial virus
- SCD
sickle cell disease
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this study.
Contributors’ Statement: S. Christy Sadreameli: Dr. Sadreameli collected data for the study, assisted with the initial data analysis, drafted the initial manuscript and approved the final manuscript as submitted. Megan E. Reller: Dr. Reller performed data interpretation, assisted with manuscript preparation and revision, and approved the final manuscript as submitted. David G. Bundy: Dr. Bundy performed data interpretation, assisted with manuscript preparation and revision, and approved the final manuscript as submitted. James F. Casella: Dr. Casella performed data interpretation, assisted with manuscript preparation and revision, and approved the final manuscript as submitted. John J. Strouse: Dr. Strouse conceptualized and designed the study, collected data, performed the initial data analysis, and helped with data interpretation, manuscript preparation and revision. He approved the final manuscript as submitted.
Conflict of Interest: Dr. Casella has received an honorarium and travel expenses in the past and presently receives salary support through Johns Hopkins for providing consultative advice to Adventrx Pharmaceuticals regarding a proposed clinical trial of an agent for treating vasoocclusive crisis in sickle cell disease. The other authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: National and state estimates. Am J Hematol. 2010;85:77–78. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 2.Charache S, Scott JC, Charache P. “Acute chest syndrome” in adults with sickle cell anemia. Microbiology, treatment, and prevention. Arch Intern Med. 1979;139:67–69. [PubMed] [Google Scholar]
- 3.Vichinsky EP, Styles LA, Colangelo LH, et al. Acute chest syndrome in sickle cell disease: Clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- 4.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 5.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 6.McCavit TL, Quinn CT, Techasaensiri C, et al. Increase in invasive Streptococcus pneumoniae infections in children with sickle cell disease since pneumococcal conjugate vaccine licensure. J Pediatr. 2011;158:505–507. doi: 10.1016/j.jpeds.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bont L, Aalderen WM, Kimpen JL. Long-term consequences of respiratory syncytial virus (RSV) bronchiolitis. Paediatr Respir Rev. 2000;1:221–227. doi: 10.1053/prrv.2000.0052. [DOI] [PubMed] [Google Scholar]
- 9.Simões E, Carbonell-Estrany X, Rieger C, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dakhama A, Lee YM, Gelfand EW. Virus-induced airway dysfunction: Pathogenesis and biomechanisms. Pediatr Infect Dis J. 2005;24:S159–S169. doi: 10.1097/01.inf.0000188155.46381.15. [DOI] [PubMed] [Google Scholar]
- 11.Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116:3431–3434. doi: 10.1182/blood-2010-05-282194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inusa B, Zuckerman M, Gadong N, et al. Pandemic influenza A (H1N1) virus infections in children with sickle cell disease. Blood. 2010;115:2329–2330. doi: 10.1182/blood-2009-12-260836. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan A, Wang WC, Gaur A, et al. Prospective evaluation for respiratory pathogens in children with sickle cell disease and acute respiratory illness. Pediatr Blood Cancer. 2013 Oct 4; doi: 10.1002/pbc.24798. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taussig LM, Wright AL, Holberg CJ, et al. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–675. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 15.Leong M, Dampier C, Varlotta L, et al. Airway hyperreactivity in children with sickle cell disease. J Pediatr. 1997;131:278–283. doi: 10.1016/s0022-3476(97)70166-5. [DOI] [PubMed] [Google Scholar]
- 16.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 17.Levine DA, Platt SL, Dayan PS, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728–1734. doi: 10.1542/peds.113.6.1728. [DOI] [PubMed] [Google Scholar]
- 18.Morris C, Vichinsky E, Styles L. Clinician assessment for acute chest syndrome in febrile patients with sickle cell disease: Is it accurate enough? Ann Emerg Med. 1999;34:64–69. doi: 10.1016/s0196-0644(99)70273-8. [DOI] [PubMed] [Google Scholar]
- 19.Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 20.Field JJ, Stocks J, Kirkham FJ, et al. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139:563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozbek OY, Malbora B, Sen N, et al. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42:1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 22.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, et al. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138:188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 23.Sylvester KP, Patey RA, Rafferty GF, et al. Airway hyperresponsiveness and acute chest syndrome in children with sickle cell anemia. Pediatr Pulmonol. 2007;42:272–276. doi: 10.1002/ppul.20571. [DOI] [PubMed] [Google Scholar]
- 24.Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Curr Opin Pediatr. 2008;20:279–287. doi: 10.1097/MOP.0b013e3282ff62c4. [DOI] [PubMed] [Google Scholar]
- 25.McGrath-Morrow SA, Lee G, Stewart BH, et al. Day care increases the risk of respiratory morbidity in chronic lung disease of prematurity. Pediatrics. 2010;126:632–637. doi: 10.1542/peds.2010-0844. [DOI] [PubMed] [Google Scholar]