Abstract
Introduction
Gay men with prostate cancer (GMPCa) may have differential health-related quality of life (HRQOL) and sexual health outcomes than heterosexual men with prostate cancer (PCa), but existing information is based on clinical experience and small studies.
Aims
Our goals were to: (i) describe HRQOL and examine changes in sexual functioning and bother; (ii) explore the psychosocial aspects of sexual health after PCa; and (iii) examine whether there were significant differences on HRQOL and sexual behavior between GMPCa and published norms.
Methods
A convenience sample of GMPCa completed validated disease-specific and general measures of HRQOL, ejaculatory function and bother, fear of cancer recurrence, and satisfaction with prostate cancer care. Measures of self-efficacy for PCa management, illness intrusiveness, and disclosure of sexual orientation were also completed. Where possible, scores were compared against published norms.
Main Outcome Measures
Main outcome measures were self-reported sexual functioning and bother on the Expanded Prostate Cancer Index.
Results
Compared with norms, GMPCa reported significantly worse functioning and more severe bother scores on urinary, bowel, hormonal symptom scales (Ps < 0.015–0.0001), worse mental health functioning (P < 0.0001), greater fear of cancer recurrence (P < 0.0001), and were more dissatisfied with their PCa medical care. However, GMPCa reported better sexual functioning scores (P < 0.002) compared with norms. Many of the observed differences met criteria for clinical significance. Physical functioning HRQOL and sexual bother scores were similar to that of published samples. GMPCa tended to be more “out” about their sexual orientation than other samples of gay men.
Conclusions
GMPCa reported substantial changes in sexual functioning after PCa treatment. They also reported significantly worse disease-specific and general HRQOL, fear of recurrence, and were less satisfied with their medical care than other published PCa samples. Sexual health providers must have an awareness of the unique functional and HRQOL differences between gay and heterosexual men with PCa.
Keywords: Prostate Cancer, Gay Men, Quality of Life, Symptom, Functioning
Introduction
Gay men represent at least 3% of the male population in the United States [1], although this may be a large underestimation of the broader category of men who have sex with men, but do not self-identify as gay [2]. Indeed, Blank suggested that at least 5,000 gay men develop prostate cancer (PCa) each year; moreover, at least 50,000 gay men are PCa survivors in the United States. [3]. Some researchers have proposed that gay men’s experience of PCa may differ fundamentally from that of heterosexual men [4].
Until recently, data have been quite limited on health-related quality of life (HRQOL) in gay men with prostate cancer (GMPCa). A pilot study of 15 men treated with radiation or surgery reported lower scores on the urinary and bowel domains of the Expanded Prostate Cancer Index Composite (EPIC) and the ejaculatory function and bother scores [5]. In another small study, gay men undergoing anti-androgen treatment reported worse scores on the International Index of Erectile Function than heterosexual men receiving the same treatment [6]. In a larger study, men in the United States, Australia, Canada, United Kingdom, and other countries reported no significant differences in sexual function scores between heterosexual (N = 460) and nonheterosexual men (N = 96), while nonheterosexual men reported significantly worse sexual bother, ejaculatory function, and ejaculatory bother [7]. In an unpublished study, gay men reported significantly lower urinary and bowel functioning than heterosexual men [8]. Treatment regret was higher in men with poorer urinary and sexual functioning.
While sexual functioning data are mixed, there may be sexual concerns faced only by gay men. Erectile dysfunction (ED) treatments typically focus on creating erections rigid enough for vaginal penetration. However, anal penetration requires a greater rigidity [9]. After anal penetration, the insertive partner also may have difficulty maintaining their erections, if penetration forces blood from the penis [9]. In addition, some gay men report damage to the anus and rectum during PCa treatment that makes receptive anal intercourse painful or reduces the sensation [9]. In a qualitative study, gay men without PCa were asked about their attitudes toward PCa and PCa treatment [10]. Ejaculation was described as crucial to satisfying sex and maintaining relationships with partners. Participants reported feeling disenfranchised by the emphasis on vaginal penetration in ED treatment and research.
This sense of disenfranchisement and being invisible can be seen in other ways. GMPCa may be reluctant to disclose their sexual orientation to a healthcare provider, precluding a partners’ involvement in treatment decision-making [11]. Stigma around homosexuality may be related to negative experiences in the healthcare system, such as providers failing to ask about sexual orientation and assuming heterosexuality [11]. These negative experiences with the healthcare system are likely related to the poorer health outcomes experienced by lesbian, gay, and bisexual persons [12].
Many gay or bisexual men who are diagnosed with PCa may be starting their cancer journey from a much more vulnerable position than their heterosexual counterparts and experience poorer outcomes. However, empirical support for these clinical and anecdotal claims is mixed. Therefore, we carried out an Internet-based study of a convenience sample of GMPCa to determine the extent to which study participants reported significantly different HRQOL and psychosocial characteristics compared with published reports. Based on our clinical experience, we expected that GMPCa would report greater decrements in sexual functioning and report being more bothered by these changes than the published data from primarily heterosexual samples. We also expected that GMPCa would report lower satisfaction with their PCa treatment because of frequent complaints by GMPCa participating in support groups.
Materials and Methods
Study Population
We obtained institutional review board approval from Baylor College of Medicine, Houston, TX, USA and Ryerson University, Toronto, ON, Canada. The eligibility criteria included: (i) self-identification as a gay or bisexual man; and (ii) diagnosis of PCa in the prior 4 years. The time period was selected to ensure participants had been diagnosed and treated recently enough to accurately report current HRQOL and recall changes in sexual behavior since treatment. Given the difficulty in recruiting this hard-to reach population, recruitment methods included: postings to electronic listservs targeting PCa survivors, posting flyers in community centers and at support groups, and advertising the study in the local media.
Procedure
Interested participants called the study’s toll-free number and underwent a brief phone-based screening interview to determine eligibility. Verbal informed consent was obtained after screening, and eligible participants were e-mailed a unique identification code and a link to the web-based survey. Online data were collected via a web survey site that used the same secure-socket layers technology as used in electronic commerce. No names or other identifying information were collected online. All data were collected by self-report. Each participant received $20 for completing the survey.
Instruments
Demographic and Medical Information
Demographic information (age, ethnicity, education, nationality, education level) and medical characteristics (PSA level, Gleason score, PCa treatment, T-stage at diagnosis, and comorbidities) were assessed using the same questions as another large PCa patient-reported outcome study [13].
Disease-Specific Quality of Life
The EPIC [14] was used to assess urinary, bowel, sexual, and hormonal symptom frequency and perceived bother. The scales have established test– retest reliability (r ≥ 0.80) and internal consistency (α ≥ 0.82) for the summary scores for each of the four domains.
General HRQOL
The Physical Health Composite Scale and Mental Health Composite Scale from the Medical Outcomes Study Short Form-36 [15] (SF-36) were used to measure HRQOL. These scales have well-established reliability and validity [16].
Change in Sexual Activity
Three items assessed change in sexual activity since PCa treatment. Participants rated: (i) the extent to which their sexual behavior changed (ranging from 1 = “decreased a lot” to 3 = “increased a lot”); (ii) the frequency of being the insertive partner for sexual activity (ranging from 1 = 0% to 4 = 100%) before PCa treatment; and (iii) similarly, after PCa treatment. Men were also able to indicate if they were not sexually active before or after PCa treatment. As no previously validated measures were available, we developed these questions for this study.
Ejaculatory Function and Bother
Three items from the Male Sexual Health Questionnaire Short-Form (MSHQ) [17], which were validated in several probability samples, some of which included gay men, assessed the degree of dysfunction in ejaculation ability, volume, and strength. Items were summed to form an ejaculatory functioning score. Greater scores reflect better function. The scale has established reliability [17]. Another item assessed bother, ranging from 0 = no problem with ejaculation to 5 = extremely bothered.
Satisfaction with PCa Care
One item on the EPIC [14] assessed “Overall, how satisfied are you with the treatment you received from your PCa,” which ranged from “extremely dissatisfied” to “extremely satisfied.”
Self-Efficacy for PCa Symptom Management
Eleven items assessed the extent to which men felt confident in controlling their PCa-related problems (e.g., urine leakage, understanding their treatment), ranging from 1 = not at all certain to 5 = completely certain. Items are summed to form a total score. The scale has established good internal consistency and has been shown to predict HRQOL [18].
Disease-Specific Anxiety
Fear of cancer recurrence was measured with Kornblith’s five-item scale used in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE™) studies [13]. Raw scale scores were reversed and transformed to a 0 to 100 scale, with higher scores reflecting greater fear of recurrence. The reliability and validity of this scale have been previously established [19].
Illness Intrusiveness Rating Scale (IIRS)
Illness intrusiveness refers to perceived disruption from illness and treatment, and their impact on valued life activities that affect HRQOL [20]. The rating scale contains 13 questions that ask how much one’s cancer interferes with a range of life domains. Ratings range from 1 = “Not very much” to 7 = “Very much.” The measure has been validated in a wide range of diseases and has shown good psychometric properties [21].
Sexual Orientation Disclosure or “Outness Level”
Ten items from the Outness Inventory [22] assessed the degree to which men were open about their sexual orientation in various life domains (e.g., family, everyday life). Each item is rated on a seven-point scale ranging from 1 = person definitely does not know about my sexual orientation to 7 = person definitely knows about my sexual orientation. All items are summed to form an overall “outness” score. The scale has demonstrated internal consistency [22].
Understanding of Changes in Sexual Functioning
In addition to the previously described validated measures, we also asked respondents a single open-ended question about their understanding of any sexual changes. We were interested in learning about the difficulties respondents faced in their own words and to understand whether there were aspects of the sexual changes not well characterized by the validated questionnaires. Our intention was to supplement the quantitative survey data with a brief opportunity to contextualize a respondent’s experience. However, this was not a true mixed-methods study.
Data Analyses
Quantitative data were analyzed with SPSS statistical software Version 17 (IBM Corporation, Armonk, NY, USA). Outcome variables were defined as each domain’s mean scores. To compare our data with published data, we carefully selected studies that contained similarly-aged men who had been treated for PCa within a comparable time frame. For example, comparisons on any EPIC construct were made against the EPIC validation cohort, who were approximately 2 years posttreatment and had a mean age 67.2 years, with equal numbers who received brachytherapy, external beam radiation, radical prostatectomy, and hormonal therapy. Comparisons for the SF-36 and fear of recurrence measures were made against the CaPSURE™ cohort, which is a national, observational registry of 14,000 PCa patients recruited from community and academic-based urology practices. All scores were converted to T-values; t-tests compared means and standard errors (SEs) for the current sample against published studies. As in prior studies, we considered HRQOL differences of 10 points or more to be clinically significant [23].
Responses to the open-ended question were tabulated to learn how respondents described their sexual difficulties. We report these qualitative findings descriptively, rather than thematically, because of the exploratory nature of this question and the brief written responses it elicited.
Results
Table 1 lists demographic and medical information for our sample. Mean age of participants (N = 92) was 57.8 years. Most participants were located in the United States, Caucasian, in partnered relationships, and had completed at least college/university. At diagnosis, the average PSA level was 8.75 and mean Gleason score was 6.1. Common PCa treatments included radical prostatectomy (55.4%), external beam radiation (27.2%), and anti-androgen therapy (25%). Table 1 shows the most frequently self-reported comorbidities. The mean time since diagnosis was 1.91 years (SE = 0.15) and no data were collected on treatment end date. No significant differences on any demographic, medical characteristic, or outcome variable were found between U.S. and Canadian participants.
Table 1.
Participant characteristics (N = 92)
| N | Mean (SE) | |
| Age (years) | 89 | 57.8 (0.97) |
| % | ||
| Nationality | ||
| United States | 77 | 83.7 |
| Canada | 15 | 16.3 |
| Relationship status | ||
| Married/living with partner | 42 | 45.7 |
| Primary partner | 16 | 17.4 |
| No primary partner | 21 | 22.8 |
| Dating one/more people | 6 | 6.5 |
| Separated/Divorced | 2 | 2.2 |
| Widowed | 3 | 3.3 |
| Education | ||
| <High school | 1 | 1.1 |
| High school degree | 4 | 4.3 |
| Some college | 20 | 21.7 |
| College/University | 27 | 29.3 |
| Graduate school | 37 | 40.2 |
| Ethnicity | ||
| African American | 5 | 5.4 |
| Asian | 1 | 1.1 |
| Caucasian | 84 | 91.3 |
| Other | 2 | 2.2 |
| Treatment | ||
| Prostatectomy | 51 | 55.4 |
| External radiation | 25 | 27.2 |
| Brachytherapy | 7 | 7.6 |
| Hormonal therapy | 23 | 25.0 |
| No treatment | 8 | 8.7 |
| Other treatment | 6 | 6.5 |
| Mean (SE) | ||
| Years since diagnosis | 1.91 (0.15) | |
| Clinical characteristics at diagnosis | Mean (SE) | |
| PSA level | 75 | 8.75 (1.11) |
| Gleason score | 61 | 6.1 (0.19) |
| T-Stage | N | % |
| Low (T1c–T2a) | 39 | 70.9 |
| Intermediate (T2b–T2c) | 6 | 10.9 |
| High (T3–T4) | 10 | 18.2 |
| Comorbid conditions | ||
| Arthritis | 24 | 26.1 |
| Diabetes | 12 | 12.5 |
| Cardiovascular disease | 11 | 11.6 |
| HIV | 6 | 7 |
| Changes in sexual behavior since PCa treatment | ||
| No change | 7 | 7.6 |
| Changed a little | 13 | 14.1 |
| Changed a lot | 51 | 55.4 |
| No sexual activity | 16 | 17.4 |
| Frequency of sexual activity after PCa treatment | ||
| No change | 9 | 9.8 |
| Changed a little | 22 | 23.9 |
| Changed a lot | 34 | 37.0 |
| No sexual activity | 21 | 22.8 |
| Change in frequency of sexual activity | ||
| Decreased a lot | 37 | 40.2 |
| Decreased a little | 18 | 19.6 |
| Increased a little | 0 | 0.0 |
| Increased a lot | 1 | 1.1 |
PCa = prostate cancer; SE = standard error
Changes in Patterns of Sexual Functioning
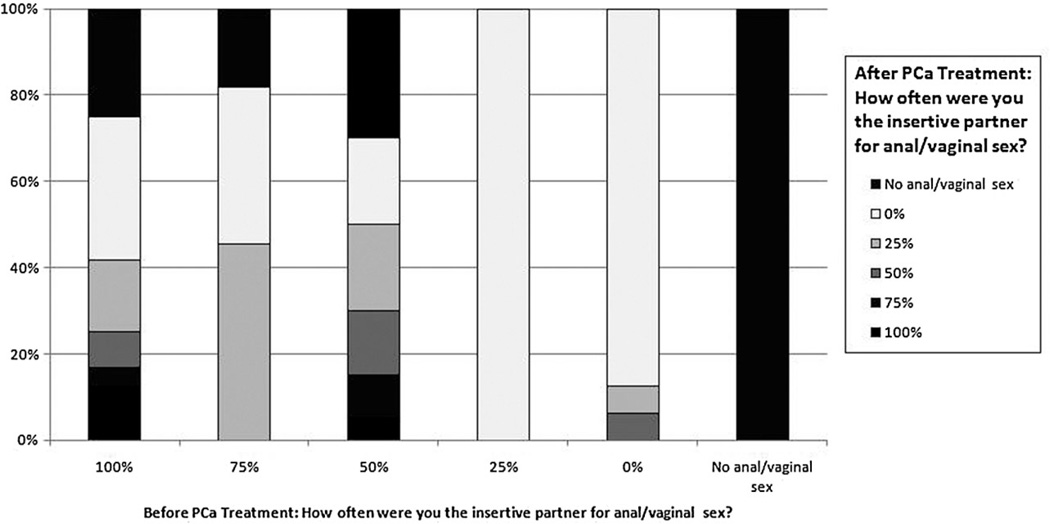
The majority of participants (55.4%; Table 1) reported substantial changes in sexual behavior since PCa treatment, with 40.2% of men reporting the frequency of sexual activity had decreased “a lot.” Figure 1 compares the frequency of being the insertive partner for intercourse before and after PCa treatment. Most men reported substantial differences between their pre- and posttreatment sexual functioning. For example, among the men reporting they were the insertive partner for intercourse 100% of the time pretreatment, only about 40% said they were the insertive partner after treatment and less than 20% reported being the insertive partner 100% of the time.
Figure 1.
Change in sexual behavior before and after PCa treatment.
Disease-Specific Quality of Life
Table 2 displays the mean scores and SEs for GMPCa compared with published means and SEs for each of the symptoms and psychosocial outcome variables. GMPCa had worse functioning and more severe bother scores compared with the urinary, bowel, and hormonal symptom validation data (Ps < 0.015–0.0001) [14]. As we expected, GMPCa also reported worse sexual functioning scores (P < 0.002) than other published samples [14,17]. Contrary to our expectations, they did not report significantly worse sexual bother scores. Compared with CaPSURE™ data, GMPCa reported significantly worse mental health functioning (P < 0.0001) on the SF-36, but not physical health functioning [24]. Moreover, GMPCa reported significantly higher fear of recurrence (P < 0.0001) [19].
Table 2.
Comparison of quality of life scores between current sample and published samples of men with prostate cancer
| Current sample | Comparator sample | |||||
|---|---|---|---|---|---|---|
| N | Mean (SE) | N | Mean (SE) | t-Score | P | |
| EPIC urinary | ||||||
| Function | 90 | 67.2 (2.8) | 252 | 86.5 (1.0) [14] | 7.94 | 0.0001 |
| Bother | 90 | 68.9 (3.1) | 252 | 75.8 (1.3) [14] | 2.43 | 0.015 |
| EPIC sexual | ||||||
| Function | 89 | 38.7 (2.6) | 252 | 29.5 (1.5) [14] | 3.07 | 0.002 |
| Bother | 88 | 40.1 (3.6) | 252 | 41.1 (1.9) [14] | 0.26 | 0.79 |
| EPIC bowel | ||||||
| Function | 90 | 77.6 (2.2) | 252 | 87.9 (0.9) [14] | 5.30 | 0.0001 |
| Bother | 90 | 77.5 (3.0) | 252 | 85.3 (1.2) [14] | 2.92** | 0.004 |
| SF-36 | ||||||
| MCS | 86 | 43.9 (1.4) | 730 | 51.9 (1.4) [24] | 7.02 | 0.0001 |
| PCS | 86 | 48.3 (1.1) | 730 | 48.9 (0.4) [24] | 0.52 | 0.60 |
| EPIC hormonal | ||||||
| Function | 85 | 73.5 (2.4) | 252 | 84.0 (1.0) [15] | 4.80 | 0.0001 |
| Bother | 90 | 52.4 (2.1) | 252 | 88.7 (0.9) [15] | 18.95 | 0.0001 |
| Fear of recurrence | 91 | 49.2 (2.3) | 333 | 20.0 (1.1) [25] | 12.86 | 0.0001 |
| Outness level | ||||||
| To family | 90 | 5.7 (0.2) | 414 | 5.21 (0.1) [22] | 2.87 | 0.004 |
| To world | 79 | 4.7 (0.2) | 414 | 5.07 (0.1) [22] | 2.01 | 0.04 |
| Total | 79 | 4.9 (0.2) | 223 | 4.90 (0.1) [22] | 0.00 | 1.00 |
EPIC = Expanded Prostate Cancer Index Composite; MCS = Mental Health Component Score; PCS = Physical Health Component Score; SE = standard error; SF-36 = Short-Form-36 Health Survey
We were unable to locate other published studies of PCa patients for the MSHQ ejaculatory functioning or bother scale scores. Mean ejaculatory bother scores on the MSHQ for GMPCa was 2.8 (SE = 0.16), which reflects a “moderate” amount of bother. The mean MSHQ ejaculatory functioning scale score was 4.5 (SE = 0.38), which reflects very low levels of ejaculatory functioning in GMPCa. In prior research, men with lower urinary tract symptoms and benign prostatic hyperplasia reported average ejaculatory functioning scores of 12.4 [17]. While not directly comparable given that the latter sample did not have PCa, the means vary by 8 points, suggesting a clinically significant difference [23].
Psychosocial Variables
Respondents reported being significantly more “out” about their sexual orientation to their family and to the world at large (Table 2; both P < 0.05) than gay men in other published samples [22]. PCa symptom management self-efficacy, illness intrusiveness, and satisfaction with care were also examined. GMPCa rated their overall self-efficacy for PCa symptom management as a moderate amount of confidence (Table 3, no comparator data were available for this measure). We examined their self-efficacy for producing an erection and for having a satisfying sexual relationship. Both were significantly lower than their overall self-confidence in managing symptoms (Ps < 0.001). Respondents indicated that PCa had a moderate impact on their lives on the overall IIRS score and on the questions about the respondents’ sex life. The reported interference on the relationship with a spouse or partner was small to moderate. A substantial minority of men reported being dissatisfied (11.8%) or being “uncertain” if they were satisfied (34.8%) with PCa treatment. Notably, the percentage of men who reported that they were “satisfied” with their PCa care (64.7%) was, as we expected, comparatively lower than that described in another study of surgical patients (84%) [25].
Table 3.
Mean score on other psychosocial variables
| Variable | Mean (SE) |
|---|---|
| Symptom management self-efficacy | |
| Overall | 3.5 (0.09) |
| Self-efficacy for producing an erection | 2.7 (0.15) |
| Self-efficacy for having a satisfying sexual relationship | 3.0 (0.13) |
| Illness Intrusiveness Rating Scale | |
| Overall | 30.93 (2.09) |
| Intrusion on sexual life | 4.74 (0.25) |
| Intrusion on relationship with spouse or partner | 2.49 (0.27) |
SE = standard error
Open-Ended Responses Describing Sexual Difficulties Posttreatment
Forty-five men responded to the optional question about changes in sexual functioning after treatment. In their written responses to the open-ended question, men briefly reported a variety of problems beyond erectile difficulties (Table 4). The most common response to the question was simply change in their erectile ability (49%). Responses grouped in the “other” category included age concerns and men indicating they did not know what caused the change. Men reported painful erections, climacturia, and low libido after treatment (Table 5, quote 1). Others reported changes in self-image (Table 5, quotes 2–3). Some men reported changing their sexual repertoire in response to their changed erectile ability, with mixed results (Table 5, quote 4). Some men reported that their partner was struggling with the change in their relationship (Table 5, quote 5). Several men reported that the lack of ejaculation (Table 5, quote 6). While many of these responses are similar to concerns expressed by heterosexual men, it is important to document these issues among G/B men and identify areas for future research (e.g., partner concerns, changes from insertive to receptive partner, etc.).
Table 4.
Frequency of categories of responses (total sum to more than 100% because of multiple responses by the same individual)
| Category | N | Percentage |
|---|---|---|
| ED | 22 | 49 |
| Other | 9 | 20 |
| MH | 9 | 20 |
| Partner | 8 | 18 |
| Desire | 8 | 18 |
| Climacturia | 4 | 9 |
| Pain | 4 | 9 |
| Ejaculation | 1 | 2 |
N = 45
ED = erectile dysfunction; MH= mental health
Table 5.
Selected verbatim responses to an open-ended question about changes in sexual functioning after prostate cancer treatment
|
N = 45
Discussion
The interest in GMPCa treatment outcomes has grown since our data collection ended and other studies are beginning to be published [5,7,8,26]. From our data and these other studies, several patterns are emerging. First, gay men may be diagnosed at younger ages in our study and others, leaving a longer potential time to live with treatment-related side effects [7]. Gay men reported significantly worse urinary and bowel function across studies. Sexual functioning and bother and hormonal functioning varied across studies. While EPIC sexual functioning and bother scores were mixed across studies, clear patterns emerged around poorer ejaculatory functioning and greater bother in gay men. Gay men also reported some changes in sexual role (Figure 1); however, some men indicated in their responses to our open-ended question that such changes were problematic, agreeing with responses from previous qualitative work that suggests that sexual repositioning may not be an option for some men [10]. Importantly, these similarities in results emerge in spite of differences in study design. Because of concerns about recall bias, our study only included men who were within 4 years of diagnosis while other studies enrolled men with substantially longer times since diagnosis [7,8]. One study only enrolled men 50 and older. Given the younger age at which gay men may be diagnosed, we chose not to have a minimum age for enrollment [27]. In addition, other studies to date have primarily focused on HRQOL. Our study includes both HRQOL and other important psychosocial variables not found in other studies. These factors increase the strength of our study, relative to others.
Given their generally worse HRQOL, it may not be surprising that men in our sample reported lower satisfaction with PCa medical care compared with other PCa survivors. However, comorbidity rates in this sample were comparable with other samples [28]. In addition, HIV rates were relatively low at 7%; slightly lower than the U.S. incidence rate of 11% for men aged over 50 years [29]. Therefore, this cohort does not appear to possess worse overall health compared with other research samples. This suggests that an examination of social-contextual factors, such as trust in one’s healthcare provider and social support, will be essential to understanding treatment dissatisfaction as well as the lower levels of HRQOL among GMPCa.
Other unique factors may contribute to the observed decrements in functioning and HRQOL. It is well established that the sexual practices of gay men differ from that of heterosexual counterparts, and that sexual “role” can become a significant component of one’s identity [30]. For example, 80% of men who reported being solely in the insertive sexual role prior to PCa treatment were no longer in that role posttreatment. In addition, prior research has documented the cultural significance among gay men around the eroticization of ejaculate and semen [31]. The lack of ejaculate posttreatment may also factor into lower HRQOL levels.
The results of our study and the other recent studies of HRQOL for gay men with PCa have several implications for urologists and other sexual health providers. Sexual rehabilitation may be especially challenging physiologically and difficult emotionally for gay men. The need for a firmer erection for anal penetration may mean that providers should educate survivors about the limitations of oral therapies for ED and consider more invasive treatments sooner with gay men. Frank conversations about possible difficulties with receptive anal sex are also needed. Importantly, appropriate referrals for evaluation and treatment should be made, particularly for survivors treated with radiotherapy. Because of the eroticization of ejaculation for many gay men as a sign of sexual completion [30,31], treatment for PCa is inherently distressing because their ejaculation likely will be impossible after treatment. Sex therapists can help gay men experiencing distress from changes in sexual functioning, including loss of ejaculation, and other physical or psychosocial difficulties. Sex therapists may also be important resources to help men grieve for their loss of key aspects of their sexual identity and diminished spontaneity because of their PCa treatment [32]. Changing from being the insertive partner during sex to being receptive may be acceptable for some survivors, but one study suggests that this is problematic for others [10]. Moreover, sex therapists can assist survivors in rethinking their options for a fulfilling sexual relationship and deciding what adjustments they may be willing to make.
Limitations of the current study include a highly self-selected, small sample, cross-sectional design, and lack of information about physician–patient relationships. The clinical data were gathered via self-report, which may not reflect the actual clinical records and resulted in more missing data than studies that have the ability to extract information from patient records. Although we compared our findings against other North American published samples that were comparable regarding age and time since diagnosis, the samples were not perfectly matched. Ideally, future studies should recruit an age and treatment-matched heterosexual sample of men as a comparator. Our sample was comprised primarily of self-identified, highly educated Caucasian men comfortable being “out” as gay, and willing to complete an online survey. Given that “outness” and higher education have both been shown to predict better physical and mental health outcomes, our data may underestimate the problems faced by older men who were raised in an era where being gay or bisexual was less socially acceptable. As social norms continue to change for gay men, future cohorts of GMPCa may experience less psychosocial burden than was reported in our sample. Conversely, GMPCa with lower HRQOL or who were particularly unhappy with their treatment may be more likely to frequent support groups or participate in listservs, and therefore more likely to participate in this study. Finally, we did not collect data on time since treatment (patients were, on average, 1.9 years postdiagnosis) and cannot comment on how this variable might have affected patient-reported outcomes.
Conclusions
In summary, this study provides additional data about changes in sexual functioning and bother and novel data on the psychosocial aspects of sexual health after PCa for gay men. Our data suggest that further research using both validated psychosocial questionnaires to explore topics such as anxiety, depression, and grief after treatment, and open-ended questions to understand how the experience of GMPCa may be different in yet-unidentified ways.
GMPCa report significantly worse HRQOL and less satisfaction with medical care than other published samples of men with PCa. Given these differences, cultural competence at both the individual and institutional levels is crucial for increasing healthcare system responsiveness to GMPCa [33]. Urologists and other sexual health providers need to consider the approaches they use, sensitively inquire as to how the adverse effects of treatment may differentially affect their gay or bisexual male patients, and provide clinical interventions to manage symptoms to assist GMPCa [4]. When indicated, referral to sex therapy will help gay men with PCa address the potentially significant experience of sexual losses and grief due to PCa treatment and achieve a measure of adaptation to a new sexual paradigm [4].
Acknowledgments
The authors gratefully acknowledge the efforts of Darryl Mitteldorf, Executive Director at MaleCare Cancer Support, and Laura Katz and Joseph Donia in the recruitment of the sample.
Funding Support
This work was partly supported by the Houston VA HSR&D Center of Excellence (HFP90-020). The views expressed reflect those of the authors and not necessarily the views of the Department of Veterans Affairs/Baylor College of Medicine.
Additional support was provided by Baylor College of Medicine Dan L Duncan Cancer Center and the Canadian Institute of Health Research. DML also received support from Mentored Research Scholar Grant 06-083-01-CPPB from the American Cancer Society. TLH received support from a New Investigator Award in Gender and Health from the Canadian Institute of Health Research.
Footnotes
Conflict of Interest:Wittmann owns Pfizer stock ($4,000). All other authors report no conflicts of interest.
Statement of Authorship
-
Conception and DesignDavid M. Latini; Tae L. Hart; David W. Coon
-
Acquisition of DataDavid M. Latini; Tae L. Hart; Marc A. Kowalkowski; Karen Zhang; Justin I. Hersom
-
Analysis and Interpretation of DataDavid M. Latini; Tae L. Hart; Marc A. Kowalkowski; Heather H. Goltz; Daniela A. Wittmann
-
Drafting the ArticleDavid M. Latini; Tae L. Hart; Marc A. Kowalkowski; Heather H. Goltz
-
Revising It for Intellectual ContentDavid M. Latini; Tae L. Hart; Heather H. Goltz; Daniela A. Wittmann; David W. Coon; Justin I. Hersom; Karen Zhang
-
Final Approval of the Completed ArticleTae L. Hart; David W. Coon; Marc A. Kowalkowski; Karen Zhang; Justin I. Hersom; Heather H. Goltz; Daniela A. Wittmann; David M. Latini
References
- 1.Black D, Gates G, Sanders S, Taylor L. Demographics of the gay and lesbian population in the United States: Evidence from available systematic data sources. Demography. 2000;37:139–154. [PubMed] [Google Scholar]
- 2.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: A population-based survey of New York City men. Ann Intern Med. 2006;145:416–425. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 3.Blank TO. The challenge of prostate cancer: Half a man or a man and a half? Generations. 2008;32:68–72. [Google Scholar]
- 4.Blank TO. Gay men and prostate cancer: Invisible diversity. J Clin Oncol. 2005;23:2593–2596. doi: 10.1200/JCO.2005.00.968. [DOI] [PubMed] [Google Scholar]
- 5.Lee TK, Breau RH, Eapen L. Pilot study on quality of life and sexual function in men-who-have-sex-with-men treated for prostate cancer. J Sex Med. 2013;10:2094–2100. doi: 10.1111/jsm.12208. [DOI] [PubMed] [Google Scholar]
- 6.Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S. Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: A possible implication of androgens in male homosexual arousal. BJU Int. 2011;108:110–115. doi: 10.1111/j.1464-410X.2010.09764.x. [DOI] [PubMed] [Google Scholar]
- 7.Wassersug RJ, Lyons A, Duncan D, Dowsett GW, Pitts M. Diagnostic and outcome differences between heterosexual and nonheterosexual men treated for prostate cancer. Urology. 2013;82:565–571. doi: 10.1016/j.urology.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Allensworth-Davies D. School of Public Health. Boston, MA: Boston University; 2012. Assessing localized prostate cancer post-treatment quality of life outcomes among gay men. [Google Scholar]
- 9.Goldstone SE. The ups and downs of gay sex after prostate cancer treatment. In: Perlman G, Drescher J, editors. A gay man’s guide to prostate cancer. Binghamton, NY: The Haworth Medical Press; 2005. pp. 43–55. [Google Scholar]
- 10.Asencio M, Blank T, Descartes L, Crawford A. The prospect of prostate cancer: A challenge for gay men’s sexualities as they age. Sex Res Soc Policy. 2009;6:28–51. [Google Scholar]
- 11.Mitteldorf D. Psychotherapy with gay prostate cancer patients. In: Perlman G, Drescher J, editors. A gay man’s guide to prostate cancer. Binghamton, NY: The Haworth Medical Press; 2005. pp. 57–67. [Google Scholar]
- 12.Institute of Medicine. The health of lesbian gay bisexual, and transgender people: Building a foundation for better understanding. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 13.Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, Carroll PR. The CaPSURE database: A methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the prostate strategic urologic research endeavor. Urology. 1996;48:773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 14.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Kosinski M, Keller S. SF-36 physical and mental health summary scales: A user’s manual. Boston: New England Medical Center; 1994. [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rosen RC, Catania JA, Althof SE, Pollack LM, O’Leary M, Seftel AD, Coon DW. Development and validation of four-item version of male sexual health questionnaire to assess ejaculatory dysfunction. Urology. 2007;69:805–809. doi: 10.1016/j.urology.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 18.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: An examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001;92:1451–1459. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart SL, Latini DM, Cowan JE, Carroll PR. Fear of recurrence, treatment satisfaction, and quality of life after radical prostatectomy for prostate cancer. Support Care Cancer. 2008;16:161–169. doi: 10.1007/s00520-007-0296-x. [DOI] [PubMed] [Google Scholar]
- 20.Devins GM. Illness intrusiveness and the psychosocial impact of lifestyle disruptions in chronic life-threatening disease. Adv Ren Replace Ther. 1994;1:251–263. doi: 10.1016/s1073-4449(12)80007-0. [DOI] [PubMed] [Google Scholar]
- 21.Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Mohr J, Fassinger R. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66–90. [Google Scholar]
- 23.Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007;177:2151–2156. doi: 10.1016/j.juro.2007.01.134. discussion 56. [DOI] [PubMed] [Google Scholar]
- 24.Bellizzi KM, Latini DM, Cowan JE, DuChane J, Carroll PR. Fear of recurrence, symptom burden, and health-related quality of life in men with prostate cancer. Urology. 2008;72:1269–1273. doi: 10.1016/j.urology.2007.12.084. [DOI] [PubMed] [Google Scholar]
- 25.Schroeck FR, Krupski TL, Sun L, Albala DM, Price MM, Polascik TJ, Robertson CN, Tewari AK, Moul JW. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 26.Thomas C, Wootten A, Robinson P. The experiences of gay and bisexual men diagnosed with prostate cancer: Results from an online focus group. Eur J Cancer Care (Engl) 2013;22:522–529. doi: 10.1111/ecc.12058. [DOI] [PubMed] [Google Scholar]
- 27.Heslin KC, Gore JL, King WD, Fox SA. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008;46:1240–1248. doi: 10.1097/MLR.0b013e31817d697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karakiewicz PI, Bhojani N, Neugut A, Shariat SF, Jeldres C, Graefen M, Perrotte P, Peloquin F, Kattan MW. The effect of comorbidity and socioeconomic status on sexual and urinary function and on general health-related quality of life in men treated with radical prostatectomy for localized prostate cancer. J Sex Med. 2008;5:919–927. doi: 10.1111/j.1743-6109.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 29.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, Lin LS, An Q, Mermin J, Lansky A, Hall HI Group HIVIS. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart TA, Wolitski RJ, Purcell DW, Gomez C, Halkitis P. Seropositive Urban Men’s Study T. Sexual behavior among HIV-positive men who have sex with men: What’s in a label? J Sex Res. 2003;40:179–188. doi: 10.1080/00224490309552179. [DOI] [PubMed] [Google Scholar]
- 31.Schilder AJ, Orchard TR, Buchner CS, Miller ML, Fernandes KA, Hogg RS, Strathdee SA. “It’s like the treasure”: Beliefs associated with semen among young HIV-positive and HIV-negative gay men. Cult Health Sex. 2008;10:667–679. doi: 10.1080/13691050802183899. [DOI] [PubMed] [Google Scholar]
- 32.Wittmann D, Foley S, Balon R. A biopsychosocial approach to sexual recovery after prostate cancer surgery: The role of grief and mourning. J Sex Marital Ther. 2011;37:130–144. doi: 10.1080/0092623X.2011.560538. [DOI] [PubMed] [Google Scholar]
- 33.Lambda Legal. When health care isn’t caring: Lambda Legal’s survey of discrimination against LGBT people and people with HIV. New York: Lambda Legal; 2010. [Google Scholar]