Abstract
Summary
Recent evidence has linked long-term bisphosphonate use with insufficiency fractures of the femur in postmenopausal women. In this case–control study, we have identified a significant association between a unique fracture of the femoral shaft, a transverse fracture in an area of thickened cortices, and long-term bisphosphonate use. Further studies are warranted.
Introduction
Although clinical trials confirm the anti-fracture efficacy of bisphosphonates over 3–5 years, the long-term effects of bisphosphonate use on bone metabolism are unknown. Femoral insufficiency factures in patients on prolonged treatment have been reported.
Methods
We performed a retrospective case–control study of postmenopausal women who presented with low-energy femoral fractures from 2000 to 2007. Forty-one subtrochanteric and femoral shaft fracture cases were identified and matched by age, race, and body mass index to one intertrochanteric and femoral neck fracture each.
Results
Bisphosphonate use was observed in 15 of the 41 subtrochanteric/shaft cases, compared to nine of the 82 intertrochanteric/femoral neck controls (Mantel–Haenszel odds ratio (OR), 4.44 [95% confidence interval (CI) 1.77–11.35]; P=0.002). A common X-ray pattern was identified in ten of the 15 subtrochanteric/shaft cases on a bisphosphonate. This X-ray pattern was highly associated with bisphosphonate use (OR, 15.33 [95% CI 3.06–76.90]; P< 0.001). Duration of bisphosphonate use was longer in subtrochanteric/shaft cases compared to both hip fracture controls groups (P=0.001).
Conclusions
We found a significantly greater proportion of patients with subtrochanteric/shaft fractures to be on long-term bisphosphonates than intertrochanteric/femoral neck fractures. Bisphosphonate use was highly associated with a unique X-ray pattern. Further studies are warranted.
Keywords: Bisphosphonate, Femoral shaft, Hip fracture, Low energy, Osteoporosis, Subtrochanteric
Introduction
Bisphosphonates are the most commonly prescribed drugs for the treatment of osteoporosis and are highly effective in reducing the risk of vertebral and hip fractures in clinical trials of 3–5 years duration [1–3]. However, recent case series have implicated a link between prolonged bisphosphonate therapy and atypical fractures of the femur. Odvina et al. [4] identified five patients who sustained low-energy proximal femur or femoral shaft fractures while on long-term alendronate therapy. All patients demonstrated histomorphometric evidence of severely suppressed bone turnover. Goh et al. [5] and Kwek et al. [6] recently reported on a similar subset of women with low-energy subtrochanteric fractures who were on long-term alendronate treatment. Visekruna et al. [7] identified three patients with femoral fractures who also showed severe suppression of bone turnover on histomorphometry. Each patient had a history of long-term alendronate use plus concomitant anti-remodeling therapy or medications known to alter bone metabolism. Lastly, we have identified a unique radiographic pattern in patients with low-energy subtrochanteric and femoral shaft fractures that is highly associated with long-term alendronate use [8, 9]. Preliminary reports of atypical fractures while on bisphosphonates prompt further investigation [10].
Bisphosphonates are currently thought to act through the induction of osteoclast apoptosis and inhibition of bone resorption [11–13]. Since osteoblastic bone formation follows osteoclastic bone resorption during normal bone remodeling, the inhibition of resorption is accompanied by a decrease in bone formation [14]. Bisphosphonates have also been demonstrated to act via prolongation of secondary mineralization leading to increased bone density on dual-energy X-ray absorptiometry, which may decrease bone toughness due to a higher mineral content [15, 16]. The impact of bisphosphonates on bone resorption, formation, and mineralization has become an important question particularly in the setting of long-term treatment.
Bisphosphonate treatment has been linked to the accumulation of microdamage, which may alter tissue material properties and bone mechanical behavior. While bone strength is increased at cancellous sites following long-term alendronate treatment in animal models, turnover in cancellous and cortical bone is suppressed, increased microdamage is present, and bone toughness is reduced [17–20]. In the laboratory, microdamage accumulation from fatigue loading of cortical bone has been linked to reduced elastic modulus, whole bone strength, and fracture toughness [21–23]. Microdamage has been observed in human autopsy samples but has not been linked to specific treatments [24, 25]. Furthermore, bisphosphonates have the potential to weaken bone through failure of aging collagen. Alteration of both enzymatic and non-enzymatic collagen cross-links through bisphosphonate treatment may contribute to increased fragility of bone [26–28].
A potential link between prolonged bisphosphonate use and low-energy femoral fractures is suggested by evidence of suppression of bone turnover, accumulation of micro-damage, and failure of aging collagen. We performed a case–control study to identify an association between low-energy femoral fractures and prolonged bisphosphonate therapy.
Materials and methods
Patient selection
We extracted data from a level I trauma center medical records database for femoral fractures. Cases included in this study were postmenopausal women who presented with low-energy subtrochanteric or femoral shaft fractures between November 2000 and February 2007. Low energy was defined as a fall from standing height or less. All patients in the Neviaser et al. [9] study who met inclusion criteria were included in the study as cases, while the medical record was searched for appropriate controls. Controls were defined as postmenopausal women with low-energy intertrochanteric or femoral neck fractures who presented within this same time period. Intertrochanteric and femoral neck fractures were chosen as controls because they are fractures of the same bone and also occur through similar low-energy mechanisms in a similar patient population. All fractures were confirmed via X-ray and classified using the Müller AO classification [29]. Patients were excluded if they were found to have pre-existing osteomalacia, active malignancy, prior bone metastases, osteogenesis imperfecta, fibrous dysplasia, abnormal renal function defined as serum creatinine >1.5 mg/dl, hepatic failure, hyperthyroidism, hyperparathyroidism, vitamin D deficiency defined as serum 25-hydroxyvitamin D concentration <25 ng/ml (when available), or a history of peptic ulcer or esophageal disease within 1 year. Patients who were taking medications known to affect bone metabolism (hormone replacement therapy, glucocorticoids, anticonvulsants, etc) were also excluded.
Of the 70 radiographically verified subtrochanteric/shaft fracture cases in Neviaser et al. [9], 41 met criteria for inclusion. The medical record was searched, and cases were matched to one intertrochanteric and femoral neck control each, by age (within a decade), body mass index (±3 kg/m2), and race, for a total of 82 intertrochanteric/ femoral neck controls. Appropriate intertrochanteric/femoral neck controls could not be found for three subtrochanteric/ shaft cases, which qualified for the study, and these were excluded from further analysis.
Information gathered from the medical record included demographics, past medical history, medication history including dosages, frequencies, and duration of use, laboratory data, social history, and history of prior femoral fracture. Patients were contacted by phone to ascertain accuracy of the medical record. If patients were unavailable, primary medical doctors were contacted. Bone densitometry scores and complete laboratory work-up including parathyroid hormone (PTH), bone alkaline phosphatase, and N-telopeptide were not available for the majority of patients and thus were not included in our analysis. These patients were from an urban medical center where metabolic bone studies were not routinely carried out, as they are not covered by the current diagnosis-related group for fracture care. This study was approved by the institutional review board of involved institutions.
X-ray analysis
To determine fracture type, all injury radiographs were retrieved and classified using the AO classification. These radiographs were reviewed by three orthopedic surgeons, who identified presence or absence of a pattern we have found in patients on long-term bisphosphonate use [8, 9]. A positive X-ray pattern was defined as a simple subtrochanteric/shaft fracture with cortical thickening and beaking of the cortex. Absence of the pattern was only assigned to a subtrochanteric/shaft case only when all three reviewers were in agreement. To accurately compare cortical hypertrophy, cortical thickness was measured just distal to site of each fracture and normalized to bone diameter at that site to account for differences in magnification. Normalized cortical thickness therefore has no units. During acquisition of this data, measurements were made while blinded to all patient information, including bisphosphonate history.
Statistical analysis
Statistical analysis consisted of descriptive statistics using means, medians, and standard deviations for continuous variables and frequencies and percentages for discrete variables. All demographic and clinical variables were assessed statistically for an association with the occurrence of subtrochanteric/shaft fracture. Categorical data were analyzed using Mantel–Haenszel chi-squares for matched analysis and Pearson’s chi-square or Fisher’s exact test as appropriate for unmatched analysis. Independent samples t tests and one-way analysis of variance were used to compare continuous variables. Mann–Whitney and Kruskal–Wallis were used for non-parametric data where appropriate. Correlations were calculated using the Spearman rank correlation. For those subjects who had a subtrochanteric/ shaft fracture, the time on bisphosphonate to fracture was assessed and alpha was set to 0.05 for all analyses. Relative risk estimates are represented as odds ratios (OR), the probability of an event in the cases versus the controls, with 95% confidence intervals (CI).
Results
Of the 70 radiographically verified subtrochanteric/shaft fracture cases in Neviaser et al. [9], 41 met the inclusion criteria. Malignancy was the most common reason for exclusion, of which multiple myeloma was the most common malignancy present. Other reasons for exclusion included, in order of decreasing frequency, medications known to affect bone metabolism, a peri-menopausal status, vitamin D deficiency, recent history of peptic ulcer disease/ gastroesophageal reflux disease, Paget’s disease, liver disease, and periprosthetic fracture. Some patients had multiple reasons to be excluded. Several patients from the Neviaser et al. [9] were excluded because they were male. The baseline characteristics for both subtrochanteric/shaft cases and intertrochanteric/femoral neck controls are summarized in Table 1. Serum Ca2+ concentration corrected for serum albumin tended to be lower in the subtrochanteric/ shaft cases but was not significantly different than intertrochanteric/femoral neck controls or intertrochanteric controls (P=0.06, P=0.15, respectively). In contrast, corrected calcium was significantly lower in subtrochanteric/shaft cases versus femoral neck controls (P=0.04). Seventeen percent of the subtrochanteric/shaft cases had a history of prior femoral fracture in the contralateral femur. History of prior femoral fracture was not significantly different between the subtrochanteric/shaft cases and the intertrochanteric/femoral neck controls (P=0.25). Metabolic bone disease studies were available for only a small number of patients and thus were not included in the analysis.
Table 1.
Baseline characteristics of subtrochanteric/shaft cases and intertrochanteric/femoral neck controls
| Characteristics | ST/S cases (n=41) | IT/FN controls (n=82)a | IT controls (n=41) | FN controls (n=41) | P value ST/S vs IT/FNb |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 81.2 (11.0) | 81.4 (11.7) | 81.3 (11.8) | 81.5 (11.7) | 0.93 |
| Median | 81 | 82 | 82 | 82 | |
| Range | 55–100 | 50–100 | 52–100 | 50–100 | |
| Race | |||||
| White (%) | 97.6 | 97.6 | 97.6 | 97.6 | |
| Asian (%) | 2.4 | 2.4 | 2.4 | 2.4 | |
| BMI (SD) (kg/m2) | 24.9 (4.8) | 24.0 (4.2) | 23.6 (4.0) | 24.4 (4.5) | 0.33 |
| Corrected calcium (SD) (mg/dL)c | 9.26 (0.37) | 9.44 (0.51) | 9.38 (0.37) | 9.50 (0.63) | 0.06 |
| History of femoral fracture (%) | 17.1 | 9.8 | 7.3 | 12.2 | 0.25 |
| Bisphosphonate use (%) | 36.6 | 11 | 14.6 | 7.3 | 0.002 |
ST/S subtrochanteric/shaft, IT intertrochanteric, FN femoral neck, y years, SD standard deviation, BMI body mass index
Equal numbers of IT and FN fractures
P values were calculated using the t test and Fisher’s exact test
Serum calcium was corrected for serum albumin concentration
Fifteen of the 41 subtrochanteric/shaft cases were found to be on a bisphosphonate, significantly greater than the nine of 82 patients in the intertrochanteric/femoral neck controls on a bisphosphonate (OR 4.44 ([95% CI 1.77–11.35]); P=0.002, Table 1). The rate of bisphosphonate use in the subtrochanteric/shaft cases was significantly higher than the rate in both intertrochanteric and femoral neck groups alone (P=0.01, P=0.001 respectively). Bisphosphonate use did not differ between intertrochanteric and femoral neck control groups (P=0.28). The rate of use of antiresorptive agents expected in this population of post-menopausal women is 10–14%, according to the National Health and Nutrition Examination Survey, 1999–2002 [30]. History of femoral fracture and corrected calcium were not significantly associated with subtrochanteric/shaft cases. Due to the nature of the investigation, we were unable to determine if patients complained of prodromal pain.
Alendronate was the bisphosphonate reported in each of the subtrochanteric/shaft cases being treated, which differed significantly from that expected based on the reported rates of bisphosphonates prescribed for osteoporosis during the evaluation period (P=0.042) [31]. Furthermore, for all patients on a bisphosphonate in the study, one patient in the femoral neck controls was taking risedronate, while another in the intertrochanteric controls took etidronate and then alendronate; all others were on alendronate. This was also significantly different from expected distributions (P=0.048).
A unique X-ray fracture pattern was observed in ten of the 41 subtrochanteric/shaft cases. This pattern was a simple transverse or oblique fracture with beaking of the cortex on one side and cortical thickening around the site of fracture, as seen in Fig. 1a,b. We name this pattern “simple with thick cortices.” This simple with thick cortices pattern was highly associated with bisphosphonate use, as ten of the 15 subtrochanteric/shaft cases who were on a bisphosphonate were identified to have the pattern. Only three of the 26 subtrochanteric/shaft cases who were not on bisphosphonate were found to have the simple with thick
Fig. 1.
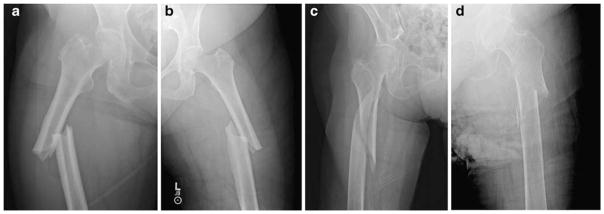
Representative radiographs of subtrochanteric/shaft cases on a bisphosphonate with evidence of the simple with thick cortices pattern with comparison radiographs of subtrochanteric/shaft fracture cases not on a bisphosphonate. a, b Representative radiographs of ten of the 41 subtrochanteric/shaft cases that were associated with bisphosphonate use. Prominent cortical thickening near the fracture site and beaking of the cortex on one side can be seen. a An 83-year-old women with a 9-year history of alendronate use. b A 61-year-old woman with a 9-year history of alendronate use. c, d Representative radiographs of subtrochanteric/shaft fractures in women not on bisphosphonate. Fractures are more complex in nature, cortical thickening is minimal, and there is no identifiable beaking at the fracture site. These fractures are not consistent with our definition of the characteristic X-ray pattern associated with prolonged bisphosphonate use. c An 83-year-old woman with no history of bisphosphonate use. d A 60-year-old woman with no history of bisphosphonate use cortices pattern. The calculated OR for simple with thick cortices pattern and its association with bisphosphonate use was 15.33 ([95% CI 3.06–76.90]; P<0.001). Representative radiographs of the subtrochanteric/shaft cases with and without the pattern associated with bisphosphonate use can be seen in Fig. 1.
The normalized cortical thickness for subtrochanteric/ shaft cases not on a bisphosphonate averaged 0.19±0.048 (mean±SD), significantly different when compared to 0.30± 0.091 for those cases on a bisphosphonate (P<0.001). In comparison, mean normalized cortical thickness for inter-trochanteric/femoral neck controls on a bisphosphonate was 0.19±0.064, significantly different from those subtrochanteric/shaft cases on a bisphosphonate (P=0.004) while not different from subtrochanteric/shaft cases not on a bisphosphonate. Duration of bisphosphonate use was significantly correlated to normalized cortical thickness for subtrochanteric/shaft cases on a bisphosphonate (Spearman’s rank correlation, ρ, 0.7, P<0.001, Fig. 2). When analysis of normalized cortical thickness was restricted to those subtrochanteric/shaft cases that were on a bisphosphonate, the thickness in those with presence of the simple with thick cortices pattern was 0.36±0.048, significantly greater than 0.20±0.034 for those subtrochanteric/shaft cases without the pattern (P<0.001).
Fig. 2.
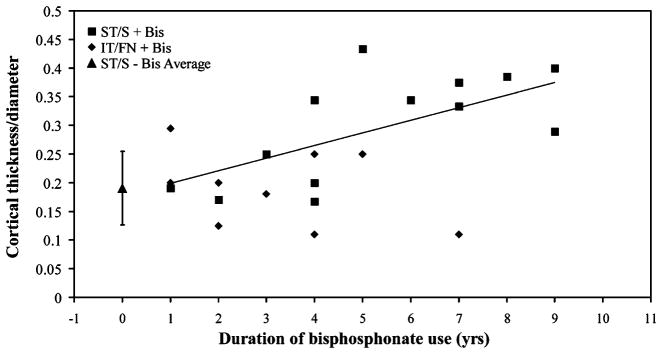
Correlation of duration of bisphosphonate use with normalized cortical thickness. Cortical thickness normalized to diameter for all subtrochanteric/shaft cases, and those intertrochanteric/femoral neck on bisphosphonate treatment was correlated to duration of bisphosphonate use. While blinded to all patient information, including bisphosphonate history, normalized cortical thickness was measured distal to the fracture site in each case. Duration represents length of time on bisphosphonate up to the date of fracture. The mean normalized cortical thickness of all subtrochanteric/shaft cases not on a bisphosphonate was 0.19±0.048, represented as a triangle data point with error bars depicting ±SD. Spearman’s rank coefficient, ρ, for correlation of subtrochanteric/shaft cases cortical thickness/ diameter with duration of bisphosphonate use was 0.7, yielding P<.001. ST/S+Bis subtrochanteric/shaft cases on a bisphosphonate, IT/FN+Bis intertrochanteric/femoral neck controls on a bisphosphonate, ST/S–Bis Average mean value for subtrochanteric/shaft not on a bisphosphonate
Differences in X-ray patterns observed between subtrochanteric/shaft cases on a bisphosphonate and those not on a bisphosphonate are reflected in the Müller AO classifications (Table 2). Subtrochanteric/shaft cases on a bisphosphonate tended to be simple transverse or oblique, while those in subtrochanteric/shaft cases not on a bisphosphonate were of more varied patterns.
Table 2.
AO classifications of the subtrochanteric/shaft cases
| Classification | ST/S +Bis | ST/S–Bis | |
|---|---|---|---|
| 31 | A3.1 | 7 | |
| A3.2 | |||
| A3.3 | |||
| 32 | A1.1 | 1 | 1 |
| A1.2 | 2 | ||
| A1.3 | |||
| A2.1 | 2 | 7 | |
| A2.2 | 4 | 1 | |
| A2.3 | |||
| A3.1 | 3 | 1 | |
| A3.2 | 4 | ||
| A3.3 | 1 | ||
| 33 | B1.1 | 3 | |
| B1.2 | |||
| B1.3 | |||
| B2.1 | 1 | 3 | |
| B2.2 | |||
| B2.3 |
ST/S subtrochanteric/shaft, +Bis patients on a bisphosphonate, –Bis patients not on a bisphosphonate
To elucidate the impact of the duration of bisphosphonate use on the formation of the simple with thick cortices pattern, subtrochanteric/shaft cases on a bisphosphonate were divided into two groups based on the presence or absence of the pattern (Table 3). These two groups were similar in race distribution, body mass index, history of osteoporosis and femoral fracture, and serum-corrected calcium levels. Cases with a history of bisphosphonate use with presence of the simple with thick cortices pattern were significantly younger (70.4±10.6) than those on a bisphosphonate without the X-ray pattern (82.5±9.3, P= 0.05). Length of time on a bisphosphonate was significantly different between these two groups. Subtrochanteric/shaft cases with presence of the simple with thick cortices pattern were associated with a prolonged duration of bisphosphonate use, with an average of 7.3±1.8 years (n=10), while those without the pattern had an average duration of 2.8± 1.3 years of use (n=5, P<0.001). Patients in the intertrochanteric/femoral neck control group who were on bisphosphonate had an average duration of 3.3 years, which was also significantly shorter than subtrochanteric/shaft cases on a bisphosphonate with the simple with thick cortices pattern (P<0.001). There was no significant difference in history of femoral fractures between sub-trochanteric/shaft cases with or without the simple with thick cortices pattern.
Table 3.
Comparison of subtrochanteric/shaft cases on bisphosphonate with or without the characteristic x-ray pattern
| ST/S on bisphosphonate with X-ray pattern (n=10) | ST/S on bisphosphonate without X-ray pattern (n=5) | P valuea | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 70.4 (10.6) | 82.5 (9.3) | 0.05 |
| Median | 75.7 | 84 | |
| Range | 55–83 | 71–96 | |
| Race | |||
| White (%) | 90 | 100 | |
| Asian (%) | 10 | 0 | |
| BMI (SD) (kg/m2) | 25.0 (4.1) | 23.4 (3.8) | 0.48 |
| History of femoral fracture (%) | 20 | 33.3 | 0.99 |
| Corrected calcium (SD) (mg/dL)b | 9.17 (0.53) | 9.28 (0.19) | 0.62 |
| Duration of bisphosphonate use (SD) (years)c | 7.3 (1.8) | 2.8 (1.3) | <0.001 |
| Normalized cortical thickness (SD)d | 0.36 (0.048) | 0.20 (0.034) | <0.001 |
ST/S, subtrochanteric/shaft, y, years, SD, standard deviation, BMI, body mass index
P values were calculated using the t test and Fisher’s exact test
Serum calcium was corrected for serum albumin concentration
Represents total time on a bisphosphonate up to the date of fracture
Normalized cortical thickness was measured distal to the site of fracture while blinded to bisphosphonate use
To illustrate how the duration of bisphosphonate use differed depending on the fracture type, we determined the duration of bisphosphonate use in patients with subtrochanteric/shaft, intertrochanteric and femoral neck fractures individually (Fig. 3). The number of subtrochanteric/shaft fractures increased with increasing duration of bisphosphonate use, while the numbers of intertrochanteric and femoral neck fractures declined. Through analysis of all patients from subtrochanteric/shaft cases and intertrochanteric and femoral neck fractures, Kruskal–Wallis one-way variance analysis yielded P=0.001. Time on bisphosphonate differed significantly for subtrochanteric/shaft fractures as compared to both intertrochanteric (P=0.01) and femoral neck fractures (P=0.001). There was no significant difference for time on bisphosphonate between intertrochanteric and femoral neck fractures (P=0.3).
Fig. 3.
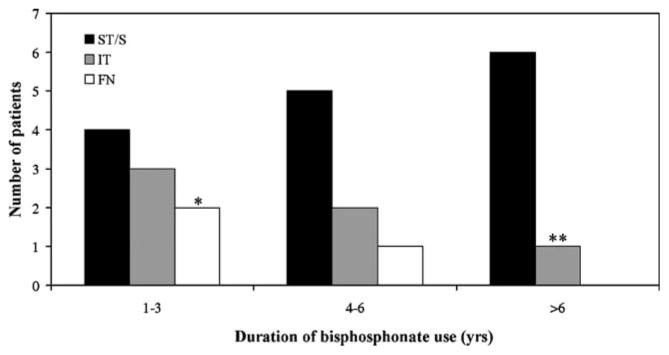
Distribution of all fractures associated with bisphosphonate use. All fractures associated with bisphosphonate use were grouped by the type of femoral fracture and displayed according to duration of bisphosphonate use. Black bars represent subtrochanteric/shaft (ST/S) fractures, gray bars represent intertrochanteric (IT) fractures, and white bars represent femoral neck (FN) fractures. Kruskal–Wallis one-way variance analysis of the duration of bisphosphonate use in patients in all three groups yielded P=0.001. Time on a bisphosphonate differed for subtrochanteric/shaft fractures as compared to both intertrochanteric (P=0.01) and femoral neck fractures (P=0.001). There was no significant difference between time on a bisphosphonate between intertrochanteric and femoral neck fractures (P=0.3). Single asterisk One patient in this group was taking risedronate. Double asterisks This patient was taking etidronate for 5 years and then took alendronate for 2 years
Discussion
The findings of this matched case–control study suggest that prolonged bisphosphonate use is associated with low-energy subtrochanteric/shaft fractures in postmenopausal women who have no obvious secondary causes of bone loss. Furthermore, bisphosphonate use of greater than 5 years was associated with a characteristic fracture of the femur, defined as a simple transverse or oblique fracture with cortical thickening and beaking of the cortex in the subtrochanteric/shaft region. This fracture is an atypical fracture for osteoporotic women [32, 33]. Our results are consistent with studies demonstrating that bisphosphonates protect against intertrochanteric and femoral neck fractures in postmenopausal women. Of the patients with intertrochanteric and femoral neck fractures in our study, 11% were on a bisphosphonate, but the number of fractures decreased with duration of use. Conversely, the number of patients with subtrochanteric/shaft fractures on a bisphosphonate tended to increase with prolonged treatment.
Patients with subtrochanteric/shaft fractures and the simple with thick cortices pattern demonstrated increased thickness of bone with absence of cortical thinning typically seen in patients with osteoporosis. We cannot conclude that bisphosphonates increased the thickness of cortical bone in these patients, as current evidence suggests that bisphosphonates increase bone mineral density, leading only to minimal changes in dimensions. It is possible that this subset of patients had thickened cortices before treatment and, when treated with bisphosphonates, were predisposed to this unique fracture. These patients may have been initially given a bisphosphonate for treatment of osteoporosis at the spine while cortical bone in the femur was thickened; a disconnection between the disease at the hip and spine. Conversely, we cannot exclude the possibility that bisphosphonates did contribute to thickening of the cortical bone in these patients, since we did not have pre-treatment radiographs.
It has been proposed that these fractures may propagate through stress fractures [6, 8, 9]. A stress fracture in an area of frozen bone may lead to inhibition of healing and deposition of bone of poor quality. While stress fractures can lead to simple transverse or oblique fractures with beaking, thickened cortices alone could also predispose to this type of fracture. Furthermore, we do not have data on prodromal pain in this patient population. Prospective studies would be required to properly define inciting events of fracture.
Potential mechanisms by which bisphosphonates may predispose to low-energy femoral fractures include alteration of tissue mineral properties, suppression of bone turnover, and increased microdamage accumulation, each of which have the potential to compromise bone strength. Bisphosphonates prolong secondary mineralization of bone and exert strong and lasting suppression of bone turnover beyond discontinuation [3, 4, 7, 11, 14–16, 18, 20, 34–39]. Furthermore, the suppression of bone turnover with bisphosphonate treatment in animals has been linked to the accumulation of microdamage in vivo [17–19, 40, 41]. The presence of microdamage due to alendronate treatment appears to differentially affect the mechanical behavior of cancellous and cortical sites [17, 18, 21, 42]. This selectivity may partially explain the occurrence of low-energy fractures in the cortical bone of the proximal femur and the absence of observed insufficiency fractures at other sites. Although suppression of turnover and microdamage accumulation seem a plausible mechanism to explain these low-energy fractures with bisphosphonate use, increased microdamage in humans on bisphosphonate treatment has yet to be demonstrated at cancellous or cortical sites.
Bisphosphonates have been thought to act principally via suppression of bone turnover and increased bone mineral density. However, recent data suggests that the drugs change tissue composition, altering collagen cross-linking and thus collagen age and maturity. In animal models, human dose equivalents of alendronate and risedronate significantly increased the ratio of enzymatic cross-links pyridinoline to deoxypyridinoline, an indicator of collagen maturity and bone stiffness, with little effect on bone toughness [26]. Pentosidine, a marker of non-enzymatic cross-links, was also significantly increased. Non-enzymatic cross-links have been associated with a decrease in fracture toughness, strain to failure, and an increase in bone brittleness [27]. Furthermore, using nanoscale composite modeling of bone, toughness of collagen microfibrils was reduced when the numbers of non-enzymatic cross-links were increased [28]. Studies using human samples are required to validate this model of aging collagen and increased fragility with bisphosphonate use.
Our data is consistent with several recent case series reporting atypical femoral fractures associated with bisphosphonate use. One report identified nine patients on alendronate who presented with atypical nonvertebral fractures [4]. Histomorphometric analysis yielded decreased bone volume, bone formation rate, and intra- and endocortical surfaces devoid of cellular elements. Six patients exhibited delayed or absent fracture healing while on alendronate, while three of six patients with the diagnosis of postmenopausal osteoporosis had femoral shaft fractures. These patients had an average length of alendronate use of 7 years, which is similar to our timeline. More recently, nine patients on alendronate were identified with subtrochanteric insufficiency fractures [5]. These patients had an average length of alendronate use of 4.2 years and had fracture patterns classified as simple transverse or oblique with cortical thickening, similar to our simple with thick cortices pattern. Similarly, Visekruna et al. reported three patients on alendronate for a duration of 5–10 years with metadiaphyseal femoral fractures. Evidence of delayed fracture healing and severely suppressed bone turnover via histomorphometry and evaluation of biochemical markers was again found [7]. All patients had an 8- to 16-year history of prednisone use, while two patients had additionally used endogenous estrogens, hormone replacement therapy, or raloxifene. These studies, taken together with our findings, may highlight a small subset of the population more susceptible to the effects of prolonged bisphosphonate treatment.
Similar to both of these case series, nearly all patients in both the subtrochanteric/shaft cases and intertrochanteric/ femoral neck controls were prescribed alendronate in our study. Two possibilities could explain this observation. Alendronate may be more strongly associated with these fractures than other bisphosphonates as a result of the differing properties of individual drugs. Zoledronic acid infusion 90 days after surgical repair of hip fractures reduced new fractures and decreased mortality, suggesting that this agent did not have a negative effect on repair [43]. Alternately, it may be due to a class effect, with the predominance of alendronate simply due to regional differences in prescribing patterns and duration of time on the market. The proportion of bisphosphonate use in all patients presenting in the New York City area is unknown; thus, our rates of types of bisphosphonates used are no more than an approximation.
Our study has several limitations. This study was a matched case–control study, which carries several major disadvantages, including selection and information bias. Using the medical record as a source of data carries inherent inaccuracy. Therefore, patients or primary medical doctors were contacted to confirm the accuracy of information. Key indicators of the degree of osteoporosis before fracture, such as bone densitometry scores and a metabolic bone disease evaluation, including PTH and vitamin D, were largely unavailable. These values may be particularly important considering the lower serum calcium concentration in cases versus controls, which was significantly lower when compared to the femoral neck group alone, although this could simply be due to the higher percentage of patients on bisphosphonates. Other risk factors, such as family history of fractures, were also unavailable. Patients with subtrochanteric/shaft fractures could have been prescribed bisphosphonates at a higher rate simply because they looked more frail. In view of the unusual nature of these fractures in older, osteoporotic patients, further studies are needed to characterize risk factors for these fractures and identify potential confounders. Lastly, due to the retrospective nature of this study, nothing is known about the compliance of the patients prescribed bisphosphonates.
This study has important implications for the future treatment of osteoporosis in postmenopausal women. Evaluation of cortical bone may be indicated at the onset of treatment and mid-treatment, as patients with thickened cortices may be at increased risk for low-energy femoral fractures. Furthermore, some patients who present with osteoporosis of the spine may have been given treatment without knowledge of thickened cortical bone. Since these fractures may propagate from stress fractures, patients on a bisphosphonate who present with thigh pain may need further evaluation. Several key questions remain unanswered. Should physicians use caution when treating patients with thick cortical bone at the hip at the initiation of bisphosphonate treatment? When a discrepancy between osteoporosis of the hip and spine exists, should the patients be treated differently? When a patient does sustain a low-energy subtrochanteric or femoral shaft fracture, should the bisphosphonate be continued, substituted with a maintenance dose, discontinued, or replaced with an anabolic agent? A prospective study may serve to answer these questions.
In summary, we found that a significantly greater percentage of patients with low-energy subtrochanteric/ shaft fractures were receiving bisphosphonate therapy as compared to those with intertrochanteric and femoral neck fractures. To our knowledge, this study is the first to date to provide such evidence. Consideration of the long-term effect on bone metabolism is warranted when treating patients undergoing prolonged bisphosphonate treatment. Additional studies are needed to confirm whether prolonged bisphosphonate use increases the risk of subtrochanteric/shaft fractures in postmenopausal women. Clinical data confirm that prolonged bisphosphonate treatment prevents hip fractures, but a small subgroup of patients may be susceptible to long-term effects of bisphosphonates by developing subtrochanteric/shaft fractures. Further studies are warranted to characterize this subgroup.
Acknowledgments
Rose Mary Fisher and the entire Metabolic Bone Disease Service at the Hospital for Special Surgery are acknowledged. Thanks are also due to Dr. Margaret Peterson for statistical expertise and Drs. Felicia Cosman and Richard S. Bockman for their critical review of the manuscript and expert advice.
Funding/support This study was supported by The Charles Cohn Foundation, Inc. and NIH Grant R01-ARO41325 “FT-IR Microscopy of Mineral Structure in Osteoporosis.”
Footnotes
Conflicts of interest Joseph M. Lane has been on speaker’s bureau for Eli Lilly, Proctor and Gamble, GlaxoSmithKline, and Roche Pharmaceuticals.
Contributor Information
B. A. Lenart, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA
A. S. Neviaser, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Medical Orthopedic Trauma Service, New York Presbyterian Hospital–Weill Cornell Medical Center, New York, NY, USA
S. Lyman, Methodology & Statistics Core, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA
C. C. Chang, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA
F. Edobor-Osula, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA
B. Steele, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA
M. C. H. van der Meulen, Mechanical & Aerospace Engineering, Cornell University, 219A Upson Hall, Ithaca, NY 14853, USA
D. G. Lorich, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA. Medical Orthopedic Trauma Service, New York Presbyterian Hospital–Weill Cornell Medical Center, New York, NY, USA
J. M. Lane, Email: lanej@hss.edu, Hospital for Special Surgery, 535 E. 70th St., New York, NY 10021, USA. Weill Medical College, Cornell University, New York, NY, USA. Medical Orthopedic Trauma Service, New York Presbyterian Hospital–Weill Cornell Medical Center, New York, NY, USA
References
- 1.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 2.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Alendronate Phase III Osteoporosis Treatment Study Group (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 4.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 5.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 6.Kwek EB, Goh SK, Koh JS, Png MA, Howe TS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–231. doi: 10.1016/j.injury.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008;93:2948–2952. doi: 10.1210/jc.2007-2803. [DOI] [PubMed] [Google Scholar]
- 8.Neviaser AS, Lenart BA, Lane JM, Lorich DG. Nontraumatic femoral shaft fractures associated with alendronate use. Orthopedic Trauma Association Annual Meeting; Boston, MA.. 2007. [DOI] [PubMed] [Google Scholar]
- 9.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 10.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–1306. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 11.Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 12.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 13.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RG, Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006;103:7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 15.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 16.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984;304:509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 17.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 18.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Mashiba T, Burr DB. Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int. 2001;69:281–286. doi: 10.1007/s002230010036. [DOI] [PubMed] [Google Scholar]
- 20.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 21.Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R. Does microdamage accumulation affect the mechanical properties of bone? J Biomech. 1998;31:337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 22.Danova NA, Colopy SA, Radtke CL, Kalscheur VL, Markel MD, Vanderby R, McCabe RP, Escarcega AJ, Muir P. Degradation of bone structural properties by accumulation and coalescence of microcracks. Bone. 2003;33:197–205. doi: 10.1016/s8756-3282(03)00155-8. [DOI] [PubMed] [Google Scholar]
- 23.Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37:96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel TE, Schaffler MB, Fyhrie DP. In vivo trabecular microcracks in human vertebral bone. Bone. 1996;19:89–95. doi: 10.1016/8756-3282(96)88871-5. [DOI] [PubMed] [Google Scholar]
- 26.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 27.Ruppel ME, Miller LM, Burr DB. The effect of the microscopic and nanoscale structure on bone fragility. Osteoporos Int. 2008;19:1251–1265. doi: 10.1007/s00198-008-0579-1. [DOI] [PubMed] [Google Scholar]
- 28.Siegmund T, Allen MR, Burr DB. Failure of mineralized collagen fibrils: modeling the role of collagen cross-linking. J Biomech. 2008;41:1427–1435. doi: 10.1016/j.jbiomech.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Schatzker J, Tile M, editors. The rationale of operative fracture care. Springer; London: 1995. [Google Scholar]
- 30.Gehlbach SH, Avrunin JS, Puleo E, Spaeth R. Fracture risk and antiresorptive medication use in older women in the USA. Osteoporos Int. 2007;18:805–810. doi: 10.1007/s00198-006-0310-z. [DOI] [PubMed] [Google Scholar]
- 31.Stafford RS, Drieling RL, Hersh AL. National trends in osteoporosis visits and osteoporosis treatment, 1988–2003. Arch Intern Med. 2004;164:1525–1530. doi: 10.1001/archinte.164.14.1525. [DOI] [PubMed] [Google Scholar]
- 32.Salminen S, Pihlajamaki H, Avikainen V, Kyro A, Bostman O. Specific features associated with femoral shaft fractures caused by low-energy trauma. J Trauma. 1997;43:117–122. doi: 10.1097/00005373-199707000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Salminen ST, Pihlajamaki HK, Avikainen VJ, Bostman OM. Population based epidemiologic and morphologic study of femoral shaft fractures. Clin Orthop Relat Res. 2000;372:241–249. doi: 10.1097/00003086-200003000-00026. [DOI] [PubMed] [Google Scholar]
- 34.Boivin G, Meunier PJ. Changes in bone remodeling rate influence the degree of mineralization of bone. Connect Tissue Res. 2002;43:535–537. doi: 10.1080/03008200290000934. [DOI] [PubMed] [Google Scholar]
- 35.Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- 36.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 37.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 38.Rossini M, Gatti D, Zamberlan N, Braga V, Dorizzi R, Adami S. Long-term effects of a treatment course with oral alendronate of postmenopausal osteoporosis. J Bone Miner Res. 1994;9:1833–7. doi: 10.1002/jbmr.5650091121. [DOI] [PubMed] [Google Scholar]
- 39.Stock JL, Bell NH, Chesnut CH, 3rd, Ensrud KE, Genant HK, Harris ST, McClung MR, Singer FR, Yood RA, Pryor-Tillotson S, Wei L, Santora AC., 2nd Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med. 1997;103:291–297. doi: 10.1016/s0002-9343(97)00130-7. [DOI] [PubMed] [Google Scholar]
- 40.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- 41.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–74. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]


