Abstract
Background
Studies of sex differences in long-term mortality after acute myocardial infarction (AMI) have reported mixed results. A systematic review is needed to characterize what is known about sex differences in long-term outcomes and to define gaps in knowledge.
Methods and Results
We searched the Medline database from 1966 to December 2012 to identify all studies that provided sex-based comparisons of mortality after AMI. Only studies with at least five years of follow-up were reviewed. Of the 1,877 identified abstracts, 52 studies met inclusion criteria, of which 39 were included in this review. Most studies included less than one-third women. There was significant heterogeneity across studies in patient populations, methodology, and risk adjustment, which produced substantial variability in risk estimates. In general, most studies reported higher unadjusted mortality for women compared with men at both 5 and 10 years after AMI; however, many of the differences in mortality became attenuated after adjustment for age. Multivariable models varied between studies; however, most reported a further reduction in sex differences after adjustment for covariates other than age. Few studies examined sex-by-age interactions; however several studies reported interactions between sex and treatment, whereby women have similar mortality risk as men after revascularization.
Conclusions
Sex differences in long-term mortality after AMI are largely explained by differences in age, comorbidities, and treatment utilization between women and men. Future research should aim to clarify how these differences in risk factors and presentation contribute to the sex gap in mortality.
Keywords: myocardial infarction, sex, women, mortality, follow-up studies, epidemiology
Numerous studies have examined sex differences in the outcomes of patients with acute myocardial infarction (AMI);1–5 however, most of these studies have focused on outcomes in the first year leaving considerable uncertainty about long-term events. In general, studies of short-term outcomes have reported higher crude mortality for women after AMI, which are largely explained by differences in age and comorbidities between men and women. In addition, these studies have identified an age-sex interaction, whereby younger women are at particularly high risk of mortality after AMI even after adjustment for other prognostic factors.4, 5 Significantly less is known about sex differences in mortality over the long term. Although several studies have addressed this topic, they differ considerably with respect to inclusion criteria, methodology, and follow-up, making it difficult to interpret these studies at first glance. Whereas some studies show higher mortality for women after AMI, others have reported no difference or even a survival advantage for women. As such, it remains unclear whether sex differences in mortality persist over the long term and which factors contribute to the gap in mortality, if any. As cardiac care improves and patients are living longer after AMI, it has become increasingly important to evaluate the literature on sex differences in long-term outcomes in order to characterize what is known and to define gaps in knowledge. This information can then be used to generate new hypotheses and to inform future research into this field. Additionally, a review of existing studies would help to determine whether the gap in mortality has changed over time by comparing studies published at different time points and whether the gap varies according to initial treatment (coronary intervention or medical management). Clarifying these issues are critical to our understanding of sex differences in coronary heart disease and for improving cardiac care and outcomes in women. In this article, we systematically review the existing literature on sex differences in long-term mortality after AMI in order to summarize study findings, assess heterogeneity across studies, and identify areas where research is needed.
METHODS
Search Strategy
We searched the Medline database from 1966 to December 2012 to identify studies that provided sex-based comparisons of mortality after AMI. The search strategy included the following terms in either the title or abstract of the article: (“myocardial infarction” or “heart attack” or “acute coronary syndrome” or “AMI”) AND (“gender” or “sex” or (“men and women”) or (“women and men”)) AND (“mortality” or “death” or “survival” or “outcome”).
Study abstracts were reviewed for mention of AMI and either mortality or survival as an outcome measure. Full-length articles for abstracts meeting these criteria were retrieved and reviewed separately by two reviewers to identify studies that met the full inclusion criteria. To be included in this review, studies had to 1) include both men and women, 2) follow patients for a maximum follow-up of at least five years, and 3) report at least one of the following: a) mortality or survival rates by sex; b) sex-specific relative risks or hazards ratio for mortality; c) significance tests for comparing mortality or survival in men and women. We chose to examine only studies with at least five years of follow-up in order to focus on mortality well beyond the acute phase. When two or more studies had overlapping study populations, we selected the study with the most in-depth sex analysis. Only published data were reviewed.
Data Extraction and Methodologic Quality
Two trained reviewers extracted the data using a standardized form to ensure systematic data collection. Variables abstracted included information on study participants, outcomes, covariates, and interactions or stratified analyses. We collected data on 1) unadjusted and adjusted mortality risk ratios or risk differences, and 2) sex-specific mortality rates. When risk ratios (relative risks, odds ratios, or hazard ratios) were not reported, we calculated them from the sex-specific crude rates (i.e. cumulative incidence or incidence rates), whenever possible6–24 Similarly, when age-adjusted risk estimates were not reported but sufficient data were provided,15, 17–20, 24 we calculated Mantel-Haenszel pooled odds ratios across age strata using the frequency procedure in SAS. Breslow-Day tests for heterogeneity were evaluated in all cases to ensure that it was appropriate to calculate a pooled risk estimate. Study quality was evaluated using a modified STROBE checklist, which included information on study design, patient selection, follow-up, statistical analyses, covariate adjustment, and potential sources of biases.25
Analyses
To evaluate whether long-term mortality after AMI differed between men and women, we examined unadjusted, age-adjusted, and multivariate adjusted mortality estimates. Study heterogeneity was evaluated through visual inspection of forest plots and calculation of an I2 statistic. Originally we had planned to perform a meta-analysis of the study results; however, after examining the literature, it became clear that a meta-analysis was not appropriate due to heterogeneity in study populations, outcome assessment, and covariates in multivariable models. Heterogeneity across studies was evaluated both qualitatively and quantitatively using I-squared statistics. Multiple sub-group analyses were evaluated to identify potential sources of heterogeneity and determine whether estimates could be pooled across some studies; however, all comparisons yielded I2-statistics >80%. In general, an I2 statistic >75% reflects high heterogeneity, indicating that it may not be appropriate to calculate a pooled estimate. Therefore, we chose to qualitatively report and compare individual relative risks. Given previous literature indicating an age-sex interaction, we investigated whether a similar interaction was present in studies of long-term mortality. We also reviewed studies for interactions between sex and treatment when available.
Finally, we examined whether specific time trends could be observed in the risk estimates. Adjusted risk ratios for women versus men were plotted by the midpoint of the study recruitment period, and a line was fit to evaluate trends over time. All statistical analyses were performed using a combination of ReviewManager version 5.1.4 (Cochrane Collaboration, Oxford, UK) and SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
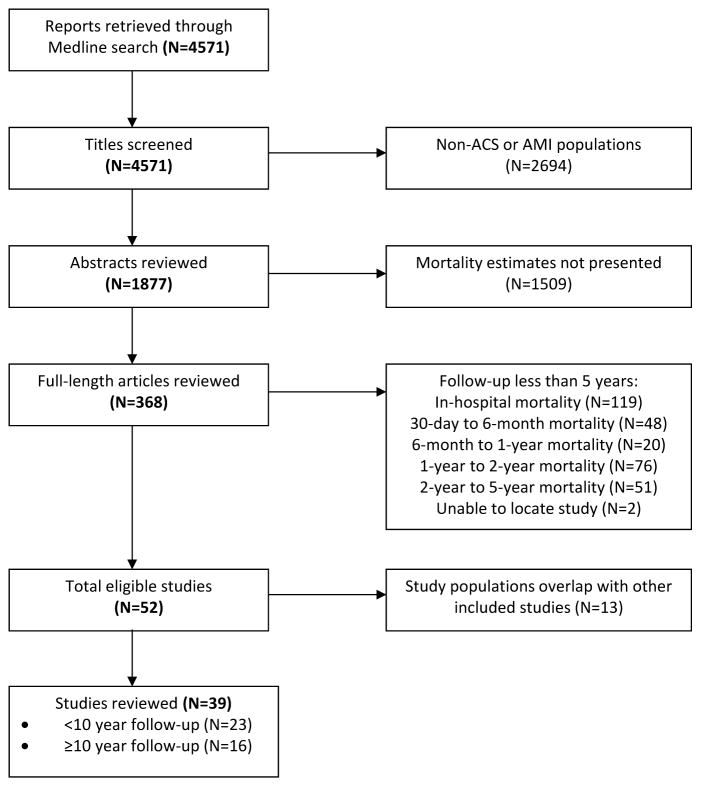
The initial search yielded 4,571 titles, from which 1,877 abstracts were reviewed and 52 articles met the full inclusion criteria. Of these, 13 were subsequently excluded for overlapping study populations, leaving a total of 39 studies included in this review (Figure 1). A description of each study is listed in Table 1. Twenty-three studies followed patients for a maximum of five to ten years after AMI, whereas the remaining 16 studies had maximum follow-up periods longer than ten years. The 12 studies that were excluded for overlapping study populations along with the reason for exclusion are listed in Supplementary Table S1.
Figure 1.
Flow chart for study retrieval and selection.
Table 1.
Characteristics of included studies
| Study, Year | Study Name, Recruitment Period | Location | Inclusion Criteria | Sample Size (% Women) | Maximum Length of Follow-Up |
|---|---|---|---|---|---|
| Alter, 200227 | OMID, 1992–1993 | Canada | -- | 25,697 (34.8) | 5 years |
| Chang, 20039 | Alberta Ministry of Health, 1993–2000 | Canada | First AMI | 22,967 (31.2) | 5 years |
| Fukui, 198710 | n/a, 1975–1984 | Japan | -- | 790 (23.3) | 5 years |
| Howland, 198012 | n/a, 1970–1972 | US | -- | 224 (27.7) | 5 years |
| Kambara, 199515 | KYSMI, 1983–1987 | Japan | Coronary angiography within 3 months | 1000 (18.7) | 5 years |
| Kishpaugh, 198117 | n/a, 1970–1972 | US | -- | 2020 (24.9) | 5 years |
| Koek, 200636 | Dutch National Hospital Discharge Register, 1995 | The Netherlands | First AMI | 21,565 (32.9) | 5 years |
| Lowel, 200019 | MONICA, 1985–1992 | Germany | First AMI Aged <74 years |
2210 (25.6) | 5 years |
| Machon, 201020 | IBERICA, 1997–2000 | Spain | -- | 1677 (26.8) | 5 years |
| Perers, 200741 | Goteborg Swedish Register, 1995–1999 | Sweden | STEMI, aged <80 years | 546 (30.6) | 5 years |
| Hosmane, 200934 | n/a, 2002–2006 | US | Cardiac arrest followed by emergent PCI | 98 (29.6) | 5.5 years |
| Anabitarte, 200928 | Triemli, 1995-? | Switzerland | PCI | 978 (19.0) | 6 years |
| Hellermann, 200533 | Rochester Epidemiology Project, 1979–1981 | US | Heart failure | 791 (52.7) | 6.6 years |
| Setoguchi, 200843 | n/a, 1999–2000 | US | -- | 1625 (80.5) | 6.6 years |
| Bufe, 20107 | n/a, 1999–2001 | Germany | PCI | 500 (24.8) | 7 years |
| Karp, 200716 | Quebec Hospital Discharge Database, 1998–2004 | Canada | -- | 38,543 (38.2) | 7 years |
| Abdulla, 200126 | TRACE, 1990–1992 | Denmark | -- | 6676 (33.0) | 8 years |
| Grundtvig, 201132 | n/a, 1998–2005 | Norway | -- | 2281 (36.8) | 8 years |
| Iribarren, 200513 | n/a, 1995–2002 | US | -- | 30,324 (33.2) | 8 years |
| Van Jaarsveld, 200644 | Groningen Longitudinal Aging Study, 1993–1998 | The Netherlands | -- | 198 (38.4) | 8 years |
| Martin, 198338 | Perth Coronary Register, 1970–1971 | Australia | Aged 30–69 years | 666 (25.1) | 9 years |
| Reynolds, 201242 | OAT, 2000–2006 | US | Total occlusion of infarct- related artery | 2201 (22.0) | 9 years |
| Schreiner, 200123 | FINMONICA, 1983–1990 | Finland | Aged 25–64 years | 4900 (25.4) | 9 years |
| Capewell, 20008 | Scottish Morbidity Record, 1986–1995 | Scotland | First AMI | 117,718 (41.8) | 10 years |
| Galatius-Jensen, 199630 | Danish Verparamil Infarction Trial, 1979–1981 | Denmark | Aged <76 years | 3073 (24.0) | 10 years |
| He, 199411 | n/a, 1974–1986 | China | First AMI | 895 (32.8) | 10 years |
| Langorgen, 200918 | Western Norway CV Registry, 1979–2001 | Norway | First AMI | 11878 (35.7) | 10 years |
| Murabito, 199339 | Framingham Heart Study, 1951–1986 | US | -- | 385 (30.7) | 10 years |
| Welin, 200045 | Goteborg Swedish Register, 1985–1987 | Sweden | Aged <65 years | 275 (16.4) | 10 years |
| Johansson, 198335 | Goteborg Swedish Register, 1968–1977 | Sweden | Age <65 years | 1521 (17.2) | 12 years |
| Marrugat, 199321 | REGICOR, 1978–1988 | Spain | Age <74 years | 1216 (15.9) | 12 years |
| Nicolau, 200422 | n/a, 1985–1997 | Brazil | STEMI treated with streptokinase | 686 (19.1) | 12 years |
| Benderly, 19976 | SPRINT, 1981–1983 | Israel | -- | 4808 (23.3) | 13 years |
| Lawesson, 201237 | RIKS-HIA, 1995–2006 | Sweden | STEMI | 54,146 (34.9) | 13 years |
| Chan, 198929 | n/a, 1971–1981 | Scotland | Resuscitation from ventricular fibrillation | 75 (29.3) | 14 years |
| Goldberg, 199331 | Worcester Heart Attack Study, 1975–1981 | US | -- | 3148 (39.1) | 14 years |
| Singer, 199524 | Rochester Epidemiology Project, 1960–1979 | US | -- | 1608 (38.0) | 23 years |
| Nauta, 201240 | n/a, 1985–2008 | The Netherlands | -- | 14,434 (27.9) | 20 years |
| Isaksson, 201114 | Northern Swedish MONICA, 1985–2006 | Sweden | Age 25–64 years | 8630 (21.6) | 23 years |
Abbreviations: AMI, acute myocardial infarction; n/a, not applicable; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Study Quality
Of the 39 studies included in this review,6–24, 26–45 27 (69%) were designed to specifically examine sex differences in long-term prognosis after AMI.6, 7, 9, 11, 13–18, 20–24, 27, 30, 31, 35–37, 39–44 The other 12 studies either included sex as a covariate or evaluated it in secondary analyses. We found significant heterogeneity across studies in study design, patient inclusion criteria, follow-up, and covariates, which are summarized in Supplementary Table S2.
Nine studies (23%) had age-specific inclusion criteria, including four studies that required patients to be less than 65 years of age.14, 23, 35, 45 Although most studies did not specify treatment criteria, three included only patients who had been treated with PCI,7, 28, 34 and one limited its sample to patients treated with streptokinase.22
Follow-up length and measurement varied widely between studies. Of the 39 studies included in this review, 21 measured long-term mortality from admission, nine from discharge, and 15 from timepoints after discharge (Supplementary Table S2). Eight studies reported long-term mortality measured from multiple starting points. Length of follow-up varied from five to 23 years.
Overview of Study Findings
Baseline and Clinical Characteristics
In nearly all studies, women represented less than half of the patients, with 28 studies (72%) containing less than one-third women. Only two studies had samples with a female majority.33, 43 Although neither of these studies specifically sampled women, both used inclusion criteria that were more common in women (post-AMI heart failure and enrollment in a Medicare program for the elderly).
To determine whether men and women with AMI differed with respect to baseline characteristics and treatment, we qualitatively compared studies that reported the prevalence of these risk factors in men and women (Supplementary Table S3). More studies reported a higher percentage of diabetes, congestive heart failure (CHF), hypertension, depression, and renal dysfunction in female patients, whereas smoking and history of AMI tended to be more prevalent in male patients. Additionally, studies tended to report higher Killip classes and lower rates of PCI, CABG, and fibrinolytic therapy in women.
Unadjusted Mortality
Of the 26 studies reporting mortality five to nine years after AMI, 13 (50%) reported significantly higher mortality for women compared with men, two (8%) reported higher mortality for men, and seven (27%) found nonsignificant differences in mortality between men and women (Table 2). Most of the relative risk estimates for studies finding a higher unadjusted mortality in women were of comparable magnitude ranging from 1.23 to 1.88.
Table 2.
Effect of gender on long-term mortality (five or more years) after myocardial infarction
| Unadjusted
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate (%) | Age-Adjusted | Adjusted for Age and Other Covariates | ||||||||
|
|
||||||||||
| Study, Year | Sample Size | Follow-Up* | Women | Men | RR | P or 95% CI | RR | P or 95% CI | RR | P or 95% CI |
| Alter, 2002* | 25,697 | 5 years | -- | -- | Age-specific | -- | -- | -- | -- | -- |
| Capewell, 2000* | 117,718 | 5 years | 58.6 | 45.6 | 1.29 | 1.27–1.30 | -- | -- | -- | -- |
| 10 years | 70.8 | 59.0 | 1.20 | 1.19–1.21 | -- | -- | -- | -- | ||
| Chang, 2003† | 22,967 | 5 years | 38.8 | 26.8 | 1.45 | 1.39–1.51 | -- | -- | 0.99 | 0.93–1.05 |
| Fukui, 1987§ | 651 | 5 years | 27.7 | 23.2 | 1.21 | 0.89–1.64 | -- | -- | -- | -- |
| Howland, 1980* | 224 | 5 years | 54.0 | 40.0 | 1.35 | 1.00–1.82 | 1.03 | 0.67–1.60 | -- | -- |
| Kambara, 1995* | 1,000 | 5 years | 12.4 | 6.6 | 1.88 | 1.18–2.98 | 1.60 | 0.89–2.86 | -- | NS |
| Kishpaugh, 1981 | 2,020* | 5 years | 56.5 | 43.8 | 1.29 | 1.17–1.42 | -- | -- | -- | -- |
| 1,495§ | 5 years | 45.3 | 36.0 | 1.26 | 1.09–1.44 | -- | -- | -- | -- | |
| 1,371# | 5 years | 50.5 | 39.0 | 1.29 | 1.13–1.48 | 1.07 | 0.81–1.41 | -- | -- | |
| Koek, 2006* | 21,565 | 5 years | 46.2 | 33.7 | 1.52 | 1.46–1.59 | 0.94 | 0.90–0.99 | 0.93 | 0.89–0.97 |
| Lowel, 2000 | 2,210* | 5 years | 34.2 | 24.6 | 1.39 | 1.20–1.60 | 1.15 | 0.93–1.43 | -- | -- |
| 2,024§ | 5 years | 25.9 | 18.5 | 1.40 | 1.17–1.68 | 1.12 | 0.87–1.43 | -- | -- | |
| Machon, 2010 | 1,677* | 5 years | 35.0 | 23.5 | 1.49 | 1.27–1.75 | 1.00 | 0.77–1.30 | -- | -- |
| 1,448§ | 5 years | 19.8 | 13.3 | 1.49 | 1.15–1.92 | 1.05 | 0.75–1.45 | -- | -- | |
| Perers, 2007§ | 546 | 5 years | -- | -- | -- | -- | -- | NS | -- | -- |
| Singer, 1995–96 | 1,608* | 5 years | 61.4 | 50.1 | 1.23 | 1.12–1.34 | 0.99 | 0.79–1.25 | -- | -- |
| 1,608* | 15 years | 74.5 | 62.9 | 1.18 | 1.11–1.27 | 1.00 | 0.78–1.29 | -- | -- | |
| 1,014§ | 5 years | 32.0 | 25.3 | 1.26 | 1.03–1.54 | 0.93 | 0.68–1.27 | -- | -- | |
| 1,014§ | 15 years | 55.0 | 44.5 | 1.24 | 1.09–1.40 | 0.95 | 0.71–1.27 | -- | -- | |
| Hosmane, 2009* | 98 | 5.5 years | -- | -- | -- | -- | -- | -- | 5.88 | 1.15–30.12 |
| Schreiner, 2001§ | 4,900 | 5.9 years | 17.1 | 21.7 | 0.79 | 0.69–0.90 | 0.65 | 0.55–0.75 | -- | -- |
| Anabitarte, 2009† | 978 | 3.2 years‡‡ | -- | -- | 1.5 | 0.8–2.7 | -- | -- | -- | NS |
| Hellermann, 2005** | 791 | 6.6 years‡‡ | -- | -- | 1.04 | 0.88–1.22 | 0.76 | p<0.05 | 0.72 | 0.61–0.87 |
| Setoguchi, 2008* | 1,625 | 2.2 years‡‡ | 73.6 | 79.5 | 0.82 | 0.72–0.93 | -- | -- | 0.71 | 0.62–0.83 |
| Bufe, 2010 | 500* | 5.6 years‡‡ | 17.7 | 12.8 | 1.39 | 0.88–2.21 | -- | -- | 0.77 | 0.40–1.48 |
| 450§ | 5.6 years‡‡ | 9.7 | 6.9 | 1.43 | 0.72–2.83 | -- | -- | -- | -- | |
| Karp, 2007† | 38,543 | 7 years | 18.2 | 13.8 | 1.31 | 1.26–1.38 | -- | -- | -- | -- |
| Reynolds, 2012†† | 2,201 | 7 years | 18.2 | 14.5 | 1.50 | 1.08–2.08 | 1.10 | 0.78–1.54 | 1.06 | 0.75–1.50 |
| Abdulla, 2001§ | 6,676 | 8 years | -- | -- | -- | -- | -- | -- | -- | NS |
| Grundtvig, 2012 | 2,281* | 7 years | 61.3 | 47.1 | 1.30 | 1.21–1.41 | -- | -- | -- | -- |
| 2,013† | 7 years | 54.7 | 41.0 | 1.46 | 1.31–1.64 | -- | -- | 0.82 | 0.70–0.96 | |
| Iribarren, 2005* | 30,324 | 3.5 years‡‡ | 23.9 | 19.1 | 1.25 | 1.20–1.31 | -- | -- | -- | -- |
| Van Jaarsveld, 2006 | 198* | 8 years | -- | -- | -- | -- | -- | -- | 0.53 | 0.32–0.91 |
| 141§ | 8 years | -- | -- | 0.61 | 0.27–1.25 | 0.31 | 0.13–0.78 | |||
| Martin, 1983† | 666 | 9 years | -- | -- | 0.91 | NS | -- | -- | 0.73 | 0.54–0.98 |
| Galatius-Jensen, 1996‡ | 2,386 | 10 years | 60.9 | 58.7 | 1.04 | 0.97–1.12 | 0.90 | 0.80–1.01 | -- | -- |
| He, 1994§ | 745 | 10 years | 51.1 | 35.9 | 1.55 | 1.17–2.05 | 1.17 | 0.88–1.56 | 1.15 | 0.86–1.54 |
| Langorgen, 2009* | 9,407 | 10 years | 74.6 | 64.0 | 1.17 | 1.13–1.20 | 1.17 | 1.05–1.29 | -- | -- |
| Murabito, 1993* | 385 | 10 years | 69.2 | 52.8 | 1.82 | 1.37–2.38 | 1.20 | 0.90–1.61 | 1.33 | 0.93–1.89 |
| Welin, 2000|| | 275 | 10 years | 35.6 | 22.2 | 1.88 | 1.07–3.30 | -- | -- | 1.85 | 0.99–3.47 |
| Johansson, 1983† | 1,521 | 5 years | 20.0 | 19.0 | 1.07 | 0.81–1.38 | -- | -- | -- | -- |
| Marragut, 1993 | 1,216* | 5 years‡‡ | 48.7 | 28.3 | 1.72 | 1.44–2.05 | -- | -- | -- | -- |
| 1,061§ | 5 years‡‡ | 35.7 | 19.2 | 1.86 | 1.45–2.39 | -- | -- | 1.27 | 0.90–1.78 | |
| Nicolau, 2004 | 686* | 5.1 years‡‡ | 45.6 | 40.4 | 1.13 | 0.91–1.40 | -- | -- | -- | NS |
| 618‡ | 5.6 years‡‡ | 35.2 | 34.9 | 1.01 | 0.76–1.33 | -- | -- | -- | NS | |
| Benderly, 1997† | 4,808 | 12 years | 65.3 | 51.8 | 1.26 | 1.19–1.33 | 1.12 | 1.02–1.22 | 1.20 | 1.08–1.33 |
| Lawesson, 2012* | 54,146 | 4.6 years‡‡ | 45.5 | 32.3 | 1.59 | 1.55–1.64 | 0.98 | 0.95–1.01 | 0.92 | 0.89–0.96 |
| Chan, 1989† | 75 | 14 years | -- | -- | -- | NS | -- | -- | -- | -- |
| Goldberg, 1993† | 3,148 | 14 years | -- | -- | >1.0 | p<0.05 | 0.91 | 0.79–1.05 | 0.83 | 0.72–0.97 |
| Nauta, 2012* | 14,434 | 20 years | 71.0 | 65.0 | 1.10 | 1.00–1.20 | -- | -- | 0.77 | 0.66–0.90 |
| Isaksson, 2011* | 7,466 | 7.1 years‡‡ | 35.7 | 36.3 | 0.98 | 0.91–1.06 | 1.02 | 0.93–1.11 | -- | -- |
Long-term mortality calculated from admission
Long-term mortality calculated from discharge
Long-term mortality calculated for 15-day survivors
Long-term mortality calculated for 30-day survivors
Long-term mortality calculated for 3-month survivors
Long-term mortality calculated for 4-month survivors
Long-term mortality calculated from onset of heart failure.
Long-term mortality calculated from randomization (median 8 days after MI).
Mean/median length of follow-up when subjects were recruited and followed for varying lengths of time.
Abbreviations: CI, confidence interval; RR, risk ratio.
Similar results were observed for studies examining survival beyond ten years. Eleven studies (73%) reported significantly higher mortality for women, and four studies (27%) found no difference. No studies reported higher mortality for men in unadjusted analyses.
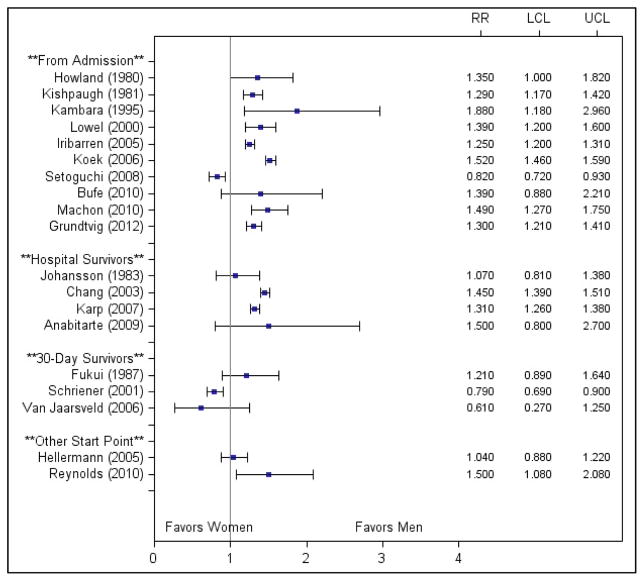
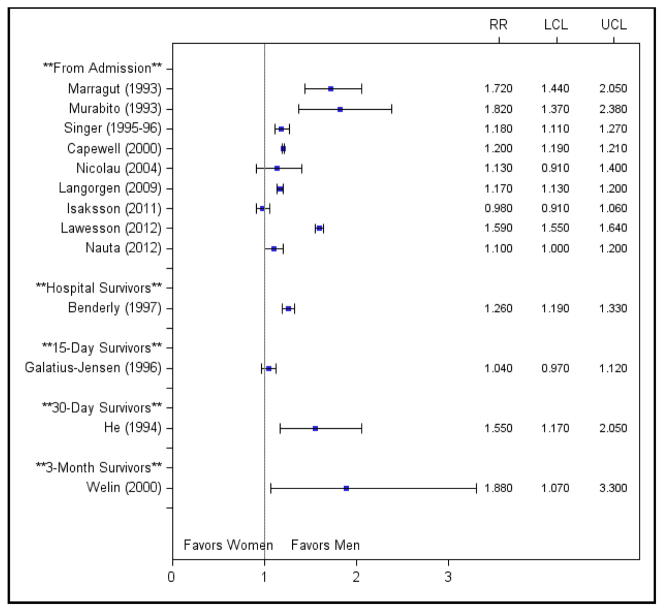
To determine whether study results varied by start point of follow-up, we constructed forest plots of the unadjusted risk ratios stratified by these variables (Figures 2 and 3). No consistent patterns were observed by start point of follow-up.
Figure 2.
Unadjusted risk ratios for studies examining 5 to 10 year mortality stratified by start point of follow-up. Abbreviations: LCL, lower confidence limit; RR, risk ratio; UCL, upper confidence limit.
Figure 3.
Unadjusted risk ratios for studies examining 10 or more year mortality stratified by start point of follow-up. Abbreviations: LCL, lower confidence limit; RR, risk ratio; UCL, upper confidence limit.
Age-Adjusted Mortality
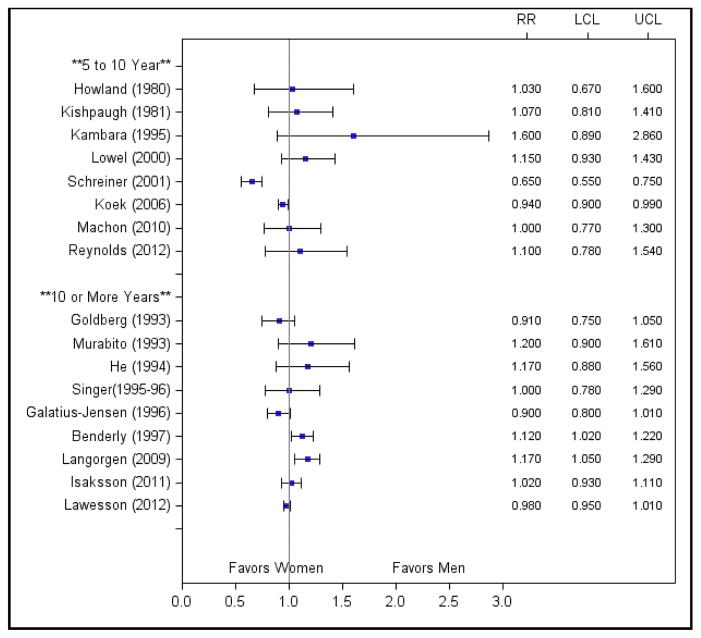
Thirteen of the 39 studies reported analyses adjusted for age only, and age-adjusted Mantel-Haenszel odds ratios could be calculated for an additional six studies. Age adjustment was performed in a variety of ways, including logistic or proportional hazards regression, Mantel-Haenszel statistics, and direct standardization.
Age adjustment attenuated the association between female sex and increased long-term mortality (Figure 4). Most studies found nonsignificant differences in age-adjusted mortality between men and women, and one study even found a reversal in risk after adjustment for age.36 Only two studies continued to report a significantly higher age-adjusted risk of mortality for women, which may be due to inadequate adjustment for the effect of age.6, 18 Benderly et al categorized age into 5-year increments, and Langorgen et al adjusted for age using only two age strata (<60 and ≥60).
Figure 4.
Age-adjusted risk ratios for studies stratified by length of follow-up. Abbreviations: LCL, lower confidence limit; RR, risk ratio; UCL, upper confidence limit.
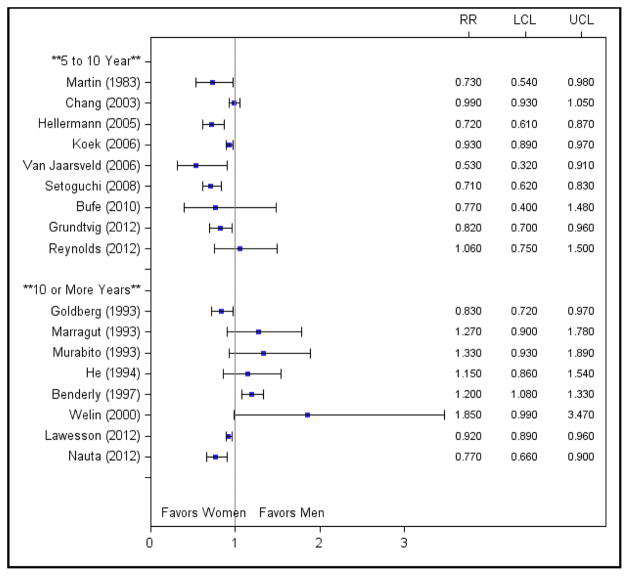
Multivariate-Adjusted Mortality
Twenty-two studies reported multivariate risk estimates (Figure 5); nearly all of which found a reduction in the sex differences after adjustment. Eleven studies reported nonsignificant female-to-male relative risks after adjustment; two reported significantly higher risk for women; and the remaining nine reported lower risk for women. Interestingly, risk ratios for female sex actually switched directions after adjustment in five of these studies.31, 32, 36, 37, 40 In all five, female sex was significantly associated with increased mortality in unadjusted analyses but became protective after multivariate adjustment.
Figure 5.
Multivariate-adjusted risk ratios for studies stratified by length of follow-up. Abbreviations: LCL, lower confidence limit; RR, risk ratio; UCL, upper confidence limit.
Stratified Analyses and Interactions
Age
The twelve studies reporting unadjusted age-stratified analyses are presented in Supplementary Figure S1. No consistent patterns were observed when risk ratios were stratified by age, but there appeared to be a trend towards higher mortality for younger women when compared with men of the same age. For example, Alter et al. found that the hazard of five-year death for women compared with men decreased by 14.2% for every 10-year increase in age.27 Studies examining interactions between sex and age reported mixed results. Two found significant interactions,9, 27 two reported borderline significant interactions,36, 43 and four found no interaction.30, 38, 42, 44 However, it is important to note that three of the studies reporting nonsignificant interactions restricted their samples to patients within certain age ranges, and thus may not have been equipped to detect an age interaction.30, 38, 42
Treatment
Six studies included only patients treated with PCI or presented results stratified by treatment type.7, 9, 28, 34, 36, 42 When only patients undergoing revascularization (PCI or CABG) were examined, five studies reported nonsignificant unadjusted and adjusted risk ratios for the effect of sex.7, 9, 28, 36, 42 Only Hosmane et al reported higher risk for women receiving PCI, which may be due to patient selection given their small sample size (98 patients) and high-risk population (patients with cardiac arrest followed by emergent PCI) studied.34 Only one study reported results for patients receiving medical management only and found significantly higher mortality for women.42
Time Trends
Study recruitment periods varied in length from one to 35 years, and data collected by these studies spanned the years 1951 to 2008. All studies examining temporal trends in post-AMI survival found significant increases in survival for both men and women over their study periods.8, 18, 31, 40
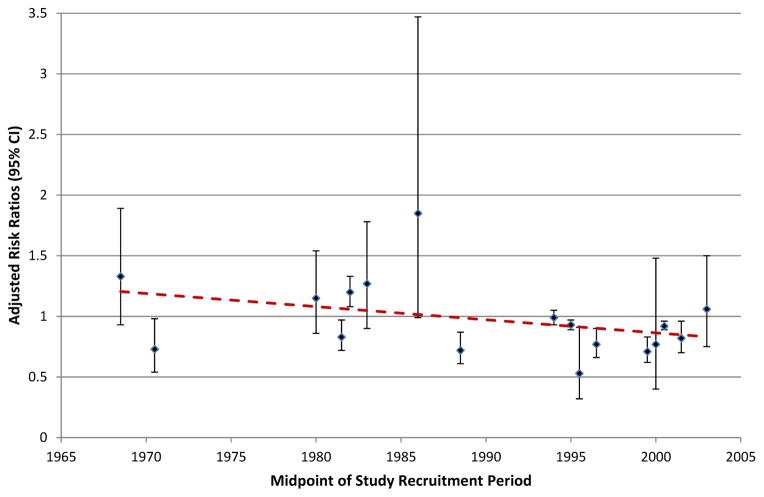
Given recent improvements in AMI management over the last several decades, we evaluated whether the adjusted risk ratios for women versus men also declined over time (Figure 6). Although risk ratios varied considerably over time, there did appear to be a slight downward trend, suggesting these sex differences have attenuated slightly over time.
Figure 6.
Time trends in adjusted risk ratios for women vs. men. Adjusted risk ratios were plotted by mid-point of study recruitment periods.
DISCUSSION
To our knowledge, this is the first systematic review to evaluate the existing literature on sex differences in mortality after AMI with follow-up periods of five years or longer. Our review included 39 studies evaluating ranges in follow-up from five to 23 years. We found significant heterogeneity in study populations, methodology, and risk adjustment, which produced substantial variability in risk estimates across studies. Although this heterogeneity precludes a formal meta-analysis, several important findings can be deduced from review of the individual studies.
In general, most studies reported higher unadjusted mortality for women compared with men at both five and ten years after AMI. Several factors may explain the observed trend towards higher long-term mortality in women, including differences in age, AMI risk factors, clinical presentation, and treatment. On average, women tend to be older than men at the time of AMI, which may place them at greater risk of mortality in both the short and long term. Indeed, many of the differences in mortality between men and women became attenuated after adjustment in age. In addition, age may also act as an effect modifier. Although studies examining interactions between sex and age reported mixed results, studies examining stratified analyses between by age tended to show that higher mortality for younger women but lower mortality for older women when compared with men of the same age. These findings are consistent with previous reports of sex differences in short-term mortality after AMI. For example, Vaccarino et al. reported an 11.1% increase in the odds of hospital death for women compared with men for every five-year increase in age.46
In addition to age, women and men with AMI have different distributions of cardiovascular risk factors and comorbid conditions, which may also explain the observed mortality difference. Consistent with previous literature, we found that studies tended to report a higher prevalence of diabetes, CHF, hypertension, depression, and renal dysfunction in female patients compared with male patients. In addition, most studies reported poorer clinical presentations and higher rates of complications in women, suggesting that women may experience more severe AMIs placing them at higher risk of mortality over the long term. The higher prevalence of cardiovascular risk factors and poorer clinical presentation in women is likely related to their older age at presentation. Because multivariate models varied substantially between studies, it is difficult to determine which factors contributed to the increased mortality in women. However, most studies reported a further reduction in the sex differences after adjustment for covariates other than age.
Finally, there may be an interaction between sex and treatment type. Previous reviews and meta-analyses have proposed that sex differences in treatment utilization may contribute to some of the observed differences in mortality.47 Although relatively few studies in this review adjusted for treatment in multivariate analyses, all reported nonsignificant or lower risk of mortality for women after adjustment. Similarly, studies examining analyses stratified by treatment type found nonsignificant risk ratios for women versus men among patients undergoing revascularization (PCI or CABG) but significantly higher mortality for women among patients treated with medical management only. These findings suggest an important sex-by-treatment interaction. Although the mechanisms underlying this interaction are unclear, it may be related to improvements in the standard of care for patients with AMI and increased emphasis on prompt intervention in the PCI era.
Indeed, when we examined time trends, we found a slight downward trend suggesting a reduction in sex differences in long-term mortality over time. Given that men and women appear to have similar mortality risk after PCI or CABG, it is not surprising that sex differences have decreased with the widespread adoption of revascularization procedures as the standard of care. However, larger time trend analyses are needed to confirm this observation.
Our findings are consistent with previous systematic reviews of sex differences in short-term mortality after AMI. Nohria et al. concluded that sex-related mortality differences were less related to intrinsic characteristics of coronary disease in women but instead could be largely explained by differences in age, cardiac risk factors, and use of fibrinolytic therapy and aspirin in women versus men.4 Similarly, Bell and Nappi cited underuse of cardiac procedures and medical therapies in women as an explanation for the worse prognosis in women;47 and Berger and Brown found that the mortality rate after primary angioplasty for AMI was equal for men and women.48 Our study adds to these reviews by identifying factors that contribute to sex differences in long-term prognosis after AMI and exploring potential effect modifiers that place women at particularly high risk. In addition, we consider trends in mortality risk ratios over time, which have not been examined in previous reviews.
We observed significant heterogeneity across studies, which precluded us from conducting a formal meta-analysis and limited our ability to draw uniform inferences from all studies combined. Several studies contained specific treatment, age, or other inclusion criteria, which created large differences in sample characteristics across studies. Covariate selection and definition also varied across studies, and because sex was not the primary focus of several studies, multivariate models may not have adequately controlled for factors associated with sex. Finally, heterogeneity in follow-up assessment may explain some of the discrepancy in long-term risk estimates between studies. Although we stratified results by start point and duration of follow-up, several strata contained only a few studies making it difficult to identify patterns within and across strata.
Inferences from this review may also by limited by potential selection bias in the inclusion of women in these studies. In nearly all studies, women represented the minority with most populations containing less than one-third women. The exclusion of elderly patients in several studies likely explains some of the sex discrepancy in patient inclusion; however, the low percentage of women in these studies may also represent an important diagnosis or hospitalization referral bias. The underrepresentation of women in many of these studies is an important finding in its own right.
The findings in this review have important implications for both clinicians and researchers. We found that differences in age, clinical presentation, and treatment utilization explain much of the disparity in long-term prognosis after AMI between men and women. Future research should aim to clarify how differences in cardiovascular risk factors and clinical presentation contribute to sex differences in long-term mortality. Specifically, research is needed in three areas. First, studies should focus on identifying which patient factors are the strongest confounders in the relationship between sex and mortality in order to identify potential targets for intervention. Second, previous studies have suggested that the prognostic value of certain risk factors may differ by sex. Three studies in this review reported that diabetes, renal insufficiency, and smoking had a stronger effect on mortality in women than in men, whereas respiratory disease and history of CVD were stronger predictors of mortality in men.6, 8, 41 Future studies should aim to understand how these factors affect men and women differently in order to identify opportunities to improve care in women. Finally, novel strategies for managing these behavioral and clinical risk factors in women are needed, both at the time of presentation and post-hospitalization. This review sets the foundation for future studies that aim to characterize patient factors that drive the sex gap in mortality and propose new approaches for tailoring care to the needs of women.
Supplementary Material
Acknowledgments
Funding Sources: HMK is supported by grant 1U01HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: HMK reports being the recipient of research grants from Medtronic, Inc and from Johnson and Johnson, through Yale University, to develop methods of clinical data sharing, and the chair of a cardiac scientific advisory board for UnitedHealth.. No other relevant disclosures are reported.
References
- 1.Fiebach NH, Viscoli CM, Horwitz RI. Differences between women and men in survival after myocardial infarction. Biology or methodology? JAMA. 1990;263:1092–1096. [PubMed] [Google Scholar]
- 2.Kannel WB, Sorlie P, McNamara PM. Prognosis after initial myocardial infarction: The Framingham Study. Am J Cardiol. 1979;44:53–59. doi: 10.1016/0002-9149(79)90250-9. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Douglas PS, Lauer MS, Pasternak RC. Selection of patients for coronary angiography and coronary revascularization early after myocardial infarction: Is there evidence for a gender bias? Ann Intern Med. 1992;116:785–790. doi: 10.7326/0003-4819-116-10-785. [DOI] [PubMed] [Google Scholar]
- 4.Nohria A, Vaccarino V, Krumholz HM. Gender differences in mortality after myocardial infarction. Why women fare worse than men. Cardiol Clinics. 1998;16:45–57. doi: 10.1016/s0733-8651(05)70383-0. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Krumholz HM, Berkman LF, Horwitz RI. Sex differences in mortality after myocardial infarction. Is there evidence for an increased risk for women? Circulation. 1995;91:1861–1871. doi: 10.1161/01.cir.91.6.1861. [DOI] [PubMed] [Google Scholar]
- 6.Benderly M, Behar S, Reicher-Reiss H, Boyko V, Goldbourt U. Long-term prognosis of women after myocardial infarction. SPRINT Study Group. Secondary Prevention Reinfarction Israeli Nifedipine Trial. Am J Epidemiol. 1997;146:153–160. doi: 10.1093/oxfordjournals.aje.a009246. [DOI] [PubMed] [Google Scholar]
- 7.Bufe A, Wolfertz J, Dinh W, Bansemir L, Koehler T, Haltern G, Guelker H, Futh R, Scheffold T, Lankisch M. Gender-based differences in long-term outcome after ST-elevation myocardial infarction in patients treated with percutaneous coronary intervention. J Womens Health (Larchmt) 2010;19:471–475. doi: 10.1089/jwh.2009.1371. [DOI] [PubMed] [Google Scholar]
- 8.Capewell S, Livingston BM, MacIntyre K, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, Evans CJ, McMurray JJ. Trends in case-fatality in 117,718 patients admitted with acute myocardial infarction in scotland. European Heart J. 2000;21:1833–1840. doi: 10.1053/euhj.2000.2318. [DOI] [PubMed] [Google Scholar]
- 9.Chang WC, Kaul P, Westerhout CM, Graham MM, Fu Y, Chowdhury T, Armstrong PW. Impact of sex on long-term mortality from acute myocardial infarction vs. unstable angina. Arch Intern Med. 2003;163:2476–2484. doi: 10.1001/archinte.163.20.2476. [DOI] [PubMed] [Google Scholar]
- 10.Fukui S, Tani A, Hamano Y, Katoh O, Suzuki K, Minamino T. Immediate and long-term prognoses of acute myocardial infarction: Analysis of determinants of prognosis. Jpn Circ J. 1987;51:344–351. doi: 10.1253/jcj.51.344. [DOI] [PubMed] [Google Scholar]
- 11.He J, Klag MJ, Whelton PK, Zhoa Y, Weng X. Short- and long-term prognosis after acute myocardial infarction in Chinese men and women. Am J Epidemiol. 1994;139:693–703. doi: 10.1093/oxfordjournals.aje.a117059. [DOI] [PubMed] [Google Scholar]
- 12.Howland JS, Vaillant HW. Long-term survival of 224 patients with myocardial infarction treated in a community hospital. J Fam Pract. 1980;10:979–983. [PubMed] [Google Scholar]
- 13.Iribarren C, Tolstykh I, Somkin CP, Ackerson LM, Brown TT, Scheffler R, Syme L, Kawachi I. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: A cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165:2105–2113. doi: 10.1001/archinte.165.18.2105. [DOI] [PubMed] [Google Scholar]
- 14.Isaksson RM, Jansson JH, Lundblad D, Naslund U, Zingmark K, Eliasson M. Better long-term survival in young and middle-aged women than in men after a first myocardial infarction between 1985 and 2006. An analysis of 8,630 patients in the northern Sweden MONICA study. BMC Cardiovasc Disord. 2011;11:1. doi: 10.1186/1471-2261-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kambara H, Kinoshita M, Nakagawa M, Kawai C. Gender difference in long-term prognosis after myocardial infarction--clinical characteristics in 1,000 patients. The Kyoto and Shiga Myocardial Infarction (KYSMI) study group. Jpn Circ J. 1995;59:1–10. doi: 10.1253/jcj.59.1. [DOI] [PubMed] [Google Scholar]
- 16.Karp I, Chen SF, Pilote L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ. 2007;176:333–338. doi: 10.1503/cmaj.060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishpaugh KK, Ford MH, Castle CH, Reading JC. Myocardial infarction: A five-year follow-up of patients. Western J Med. 1981;134:1–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Langorgen J, Igland J, Vollset SE, Averina M, Nordrehaug JE, Tell GS, Irgens LM, Nygard O. Short-term and long-term case fatality in 11 878 patients hospitalized with a first acute myocardial infarction, 1979–2001: The Western Norway cardiovascular registry. Eur J Cardiovasc Prev Rehabil. 2009;16:621–627. doi: 10.1097/HJR.0b013e32832e096b. [DOI] [PubMed] [Google Scholar]
- 19.Lowel H, Koenig W, Engel S, Hormann A, Keil U. The impact of diabetes mellitus on survival after myocardial infarction: Can it be modified by drug treatment? Results of a population-based myocardial infarction register follow-up study. Diabetologia. 2000;43:218–226. doi: 10.1007/s001250050032. [DOI] [PubMed] [Google Scholar]
- 20.Machon M, Basterretxea M, Martinez-Camblor P, Aldasoro E, Maria San Vicente J, Larranaga N. Sex differences in relative survival and prognostic factors in patients with a first acute myocardial infarction in Guipuzcoa, Spain. Rev Esp Cardiol. 2010;63:649–659. doi: 10.1016/s1885-5857(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 21.Marrugat J, Anto JM, Sala J, Masia R. Influence of gender in acute and long-term cardiac mortality after a first myocardial infarction. Regicor investigators. J Clin Epidemiol. 1994;47:111–118. doi: 10.1016/0895-4356(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 22.Nicolau JC, Auxiliadora Ferraz M, Nogueira PR, Coimbra Garzon SA, Serrano CV, Jr, Ramires JA. The role of gender in the long-term prognosis of patients with myocardial infarction submitted to fibrinolytic treatment. Ann Epidemiol. 2004;14:17–23. doi: 10.1016/s1047-2797(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 23.Schreiner PJ, Niemela M, Miettinen H, Mahonen M, Ketonen M, Immonen-Raiha P, Lehto S, Vuorenmaa T, Palomaki P, Mustaniemi H, Kaarsalo E, Arstila M, Torppa J, Puska P, Tuomilehto J, Pyorala K, Salomaa V. Gender differences in recurrent coronary events; the FINMONICA MI register. Eur Heart J. 2001;22:762–768. doi: 10.1053/euhj.2000.2501. [DOI] [PubMed] [Google Scholar]
- 24.Singer RB. Comparative mortality by sex and age in residents of Rochester, Minnesota with acute myocardial infarction during 1960–1979 (sudden deaths included) J Insur Med. 1995;27:235–240. [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Abdulla J, Brendorp B, Torp-Pedersen C, Kober L. Does the electrocardiographic presence of q waves influence the survival of patients with acute myocardial infarction? Eur Heart J. 2001;22:1008–1014. doi: 10.1053/euhj.2000.2426. [DOI] [PubMed] [Google Scholar]
- 27.Alter DA, Naylor CD, Austin PC, Tu JV. Biology or bias: Practice patterns and long-term outcomes for men and women with acute myocardial infarction. J Am Coll Cardiol. 2002;39:1909–1916. doi: 10.1016/s0735-1097(02)01892-2. [DOI] [PubMed] [Google Scholar]
- 28.Anabitarte P, Kurz DJ, Stettler I, Naegeli B, Bertel O, Frielingsdorf J, Maurer D, Straumann E. Long-term survival and functional outcome of unselected patients undergoing percutaneous coronary intervention for acute myocardial infarction. Swiss Med Wkly. 139:636–641. doi: 10.4414/smw.2009.12505. [DOI] [PubMed] [Google Scholar]
- 29.Chan NS, Hughes M, Irvine NA, Kenmure AC. Long-term prognosis after resuscitation from primary ventricular fibrillation complicating acute transmural myocardial infarction in the north east of Scotland. Scott Med J. 1989;34:430–433. doi: 10.1177/003693308903400206. [DOI] [PubMed] [Google Scholar]
- 30.Galatius-Jensen S, Launbjerg J, Mortensen LS, Hansen JF. Sex related differences in short and long-term prognosis after acute myocardial infarction: 10 year follow up of 3073 patients in database of first Danish Verapamil Infarction trial. BMJ. 1996;313:137–140. doi: 10.1136/bmj.313.7050.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg RJ, Gorak EJ, Yarzebski J, Hosmer DW, Jr, Dalen P, Gore JM, Alpert JS, Dalen JE. A communitywide perspective of sex differences and temporal trends in the incidence and survival rates after acute myocardial infarction and out-of-hospital deaths caused by coronary heart disease. Circulation. 1993;87:1947–1953. doi: 10.1161/01.cir.87.6.1947. [DOI] [PubMed] [Google Scholar]
- 32.Grundtvig M, Hagen TP, Amrud ES, Reikvam A. Mortality after myocardial infarction: Impact of gender and smoking status. Euro J Epidemiol. 2011;26:385–393. doi: 10.1007/s10654-011-9557-6. [DOI] [PubMed] [Google Scholar]
- 33.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: Clinical presentation and survival. Eur J Heart Fail. 2005;7:119–125. doi: 10.1016/j.ejheart.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Hosmane VR, Mustafa NG, Reddy VK, Reese CLt, DiSabatino A, Kolm P, Hopkins JT, Weintraub WS, Rahman E. Survival and neurologic recovery in patients with ST-segment elevation myocardial infarction resuscitated from cardiac arrest. J Am Coll Cardiol. 2009;53:409–415. doi: 10.1016/j.jacc.2008.08.076. [DOI] [PubMed] [Google Scholar]
- 35.Johansson S, Bergstrand R, Ulvenstam G, Vedin A, Wilhelmsson C, Wedel H, Wilhelmsen L, Aberg A. Sex differences in preinfarction characteristics and longterm survival among patients with myocardial infarction. Am J Epidemiol. 1984;119:610–623. doi: 10.1093/oxfordjournals.aje.a113778. [DOI] [PubMed] [Google Scholar]
- 36.Koek HL, de Bruin A, Gast F, Gevers E, Kardaun JW, Reitsma JB, Grobbee DE, Bots ML. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am J Cardiol. 2006;98:993–999. doi: 10.1016/j.amjcard.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Lawesson SS, Alfredsson J, Fredrikson M, Swahn E. A gender perspective on short- and long term mortality in ST-elevation myocardial infarction - A report from the SWEDEHEART register. Int J Cardiol. 2013;168:1041–7. doi: 10.1016/j.ijcard.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 38.Martin CA, Thompson PL, Armstrong BK, Hobbs MS, de Klerk N. Long-term prognosis after recovery from myocardial infarction: A nine year follow-up of the Perth Coronary Register. Circulation. 1983;68:961–969. doi: 10.1161/01.cir.68.5.961. [DOI] [PubMed] [Google Scholar]
- 39.Murabito JM, Evans JC, Larson MG, Levy D. Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation. 1993;88:2548–2555. doi: 10.1161/01.cir.88.6.2548. [DOI] [PubMed] [Google Scholar]
- 40.Nauta ST, Deckers JW, van Domburg RT, Akkerhuis KM. Sex-related trends in mortality in hospitalized men and women after myocardial infarction between 1985 and 2008: Equal benefit for women and men. Circulation. 2012;126:2184–2189. doi: 10.1161/CIRCULATIONAHA.112.113811. [DOI] [PubMed] [Google Scholar]
- 41.Perers E, Caidahl K, Herlitz J, Karlsson T, Hartford M. Impact of diagnosis and sex on long-term prognosis in acute coronary syndromes. Am Heart J. 2007;154:482–488. doi: 10.1016/j.ahj.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds HR, Forman SA, Tamis-Holland JE, Steg PG, Mark DB, Pearte CA, Carvalho AC, Sopko G, Liu L, Lamas GA, Kruk M, Loboz-Grudzien K, Ruzyllo W, Hochman JS. Relationship of female sex to outcomes after myocardial infarction with persistent total occlusion of the infarct artery: analysis of the Occluded Artery Trial (OAT) Am Heart J. 2012;163:462–9. doi: 10.1016/j.ahj.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setoguchi S, Solomon DH, Levin R, Winkelmayer WC. Gender differences in the management and prognosis of myocardial infarction among patients > or = 65 years of age. Am J Cardiol. 2008;101:1531–1536. doi: 10.1016/j.amjcard.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 44.van Jaarsveld CH, Ranchor AV, Kempen GI, Coyne JC, van Veldhuisen DJ, Ormel J, Sanderman R. Gender-specific risk factors for mortality associated with incident coronary heart disease--a prospective community-based study. Prev Med. 2006;43:361–367. doi: 10.1016/j.ypmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Welin C, Lappas G, Wilhelmsen L. Independent importance of psychosocial factors for prognosis after myocardial infarction. J Intern Med. 2000;247:629–639. doi: 10.1046/j.1365-2796.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- 46.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 participants. New Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 47.Bell DM, Nappi J. Myocardial infarction in women: A critical appraisal of gender differences in outcomes. Pharmacotherapy. 2000;20:1034–1044. doi: 10.1592/phco.20.13.1034.35034. [DOI] [PubMed] [Google Scholar]
- 48.Berger JS, Brown DL. Impact of gender on mortality following primary angioplasty for acute myocardial infarction. Prog Cardiovasc Dis. 2004;46:297–304. doi: 10.1016/j.pcad.2003.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.