Abstract
BACKGROUND
Intraoperative cholangiography (IOC) is the current gold standard for biliary imaging during laparoscopic cholecystectomy (LC). However, utilization of IOC remains low. Near Infrared Fluorescence Cholangiography (NIRF-C) is a novel, noninvasive method for real-time, intraoperative biliary mapping. Our aims were to assess the safety and efficacy of NIRF-C for identification of biliary anatomy during LC.
METHODS
Patients were administered indocyanine green (ICG) prior to surgery. NIRF-C was used to identify extrahepatic biliary structures before, and after partial and complete dissection of Calot's triangle. Routine IOC was performed in each case. Identification of biliary structures using NIRF-C and IOC, and time required to complete each procedure were collected.
RESULTS
Eighty-two patients underwent elective LC with NIRF-C and IOC. Mean age and BMI were 42.6±13.7 years and 31.5±8.2 kg/m2, respectively. ICG was administered 73.8±26.4 minutes prior to incision. NIRF-C was significantly faster than IOC (1.9±1.7 vs. 11.8±5.3 minutes, p<0.001). IOC was unobtainable in 20 (24.4%) patients while NIRF-C did not visualize biliary structures in 4 (4.9%) patients. After complete dissection, the rates of visualization of the cystic duct, common bile duct, and common hepatic duct using NIRF-C were 95.1%, 76.8%, and 69.5%, respectively, compared to 72.0%, 75.6%, and 74.3% for IOC. In 20 patients where IOC could not be obtained, NIRF-C successfully identified biliary structures in 80% of the cases. Higher BMI was not a deterrent to visualization of anatomy with NIRF-C. No adverse events were observed with NIRF-C.
CONCLUSIONS
NIRF-C is a safe and effective alternative to IOC for imaging extrahepatic biliary structures during LC. This technique should be evaluated further under a variety of acute and chronic gallbladder inflammatory conditions to determine its usefulness in biliary ductal identification.
Keywords: Near infrared imaging, Fluorescence cholangiography, Biliary mapping, laparoscopic cholecystectomy, Intraoperative cholangiogram, Indocyanine green
INTRODUCTION
Laparoscopic cholecystectomy (LC) is one of the most common elective surgical procedures in the United States, with over 750,000 cases performed annually [1]. Bile duct injury (BDI) is the main cause of morbidity for this procedure and the incidence has remained steady at 0.4% to 0.7% [2-5]. In 70 to 80% of the cases, BDI occurs due to misidentification of biliary anatomy [6, 7]. Surveys show that regardless of training level, 34% to 49% of surgeons will experience a BDI during their career [8, 9].
Currently, intraoperative cholangiography (IOC) is the gold standard method for intraoperative identification of biliary anatomy. However, IOC is underutilized due to significantly increased operating time, added cost, inherent risk of BDI associated with IOC, radiation exposure, and the need for additional equipment and personnel [10, 11]. Near Infrared Fluorescence Cholangiography (NIRF-C) is a novel imaging modality, which offers a rapid, noninvasive, real-time mapping of the extrahepatic biliary system without exposure to ionizing radiation. It requires preoperative intravenous injection of indocyanine green (ICG), a fluorescent dye that is exclusively excreted in the bile [12]. ICG has a half-life of 2 hours [12]. When excited with a near infrared laser, ICG emits light at a peak wavelength of approximately 800 nm [13], which is displayed on the monitor for real-time interpretation. NIRF-C does not require cannulation of the cystic duct or the use of fluoroscopy, making it an attractive alternative to IOC.
At present, there is limited data examining the safety and efficacy of NIRF-C in a North American population. As fluorescence penetration of soft tissues is a major barrier to this technique, it is paramount to determine the effectiveness of NIRF-C in a population with higher average body mass index (BMI), compared to previously studied patient populations [14, 15]. Primary aims of this study were to evaluate the safety, feasibility, and efficacy of NIRF-C in the identification of extrahepatic biliary anatomy during LC. Secondary goal was to compare the efficacy of NIRF-C to that of IOC.
MATERIALS AND METHODS
Patients
Patients were prospectively enrolled during surgical consultation for biliary disease. Inclusion criteria included age 18 years or greater, history of symptomatic biliary disease (chronic cholecystitis, symptomatic cholelithiasis, biliary dyskinesia), and elective surgery. Patients were excluded in cases of acute cholecystitis, cholangitis, conversion to an open procedure, pregnancy, breast-feeding, cirrhosis, or known allergy to iodine or shellfish. All cases were performed at The Ohio State University Wexner Medical Center by the same surgical team. The study was approved by the Institutional Review Board and informed consent was obtained from all patients prior to a study-related intervention. This trial was registered with ClincalTrials.gov (NCT02070640).
Indocyanine Green
Two and a half milligrams of ICG (2.5 mg/ml IC-Green®, Akorn Inc., Lake Forest, IL; 2.5 mg/ml ICG, Pulsion Medical Inc., Irving, TX) were injected intravenously, approximately 60 minutes prior to making a surgical incision. Patients were monitored for any signs of an allergic reaction to the dye. An additional 2.5 mg of ICG was administered if fluorescence degradation was noted intraoperatively.
Fluorescence Imaging System
NIRF-C was performed using the Stryker Infrared Fluorescence (IRF) Imaging System (Stryker Endoscopy, San Jose, CA), an investigational endoscopic illumination and imaging system for high definition visible light and near infrared fluorescence imaging. The system consists of an IRF light source, light cable, camera control unit (CCU), 1488 camera head, coupler, 0° and 30° 10 mm laparoscopes, and surgical display unit. The light source, which outputs light within a visible and an infrared spectrum, was used to illuminate the surgical site. Image signals were transmitted from the laparoscope to the CCU for processing, and the final image was displayed on the monitor. A button on the camera head was used to toggle from visible light to near infrared image.
Operative Technique
The surgical procedure for LC was performed according to the critical view of safety technique described by Strasberg [16], which entails visualizing Calot's triangle. NIRF-C was performed at three time points during the case: 1) Following achievement of pneumoperitoneum and exposure of the liver hilum, prior to any dissection, 2) After partial dissection of Calot's triangle, which included dividing adhesions and stripping of anterior fat, but not skeletonizing of structures, and 3) After complete dissection of Calot's triangle. This was performed by visualizing biliary structures in visible light and switch to near infrared mode for identification with NIRF-C. After complete dissection of Calot's triangle, routine IOC was performed, followed by completion of the LC in a standard fashion. The entire procedure was recorded and saved for review.
Intraoperative Cholangiogram
Following the collection of all data relating to NIRF-C, radiographic IOC was performed using a standard technique. The cystic duct was cannulated with a 4-Fr cholangiogram catheter and omnipaque contrast dye (GE Healthcare Inc., Princeton, NJ) was injected. Fluoroscopy was used to visualize biliary anatomy.
Data Collection
Patient demographic data and medical history were collected. Operative outcomes included time from ICG injection to surgical incision, operative time, time required for cholangiography, inability to perform NIRF-C and IOC, and complications. The identification of extrahepatic biliary anatomy (left and right hepatic ducts, common hepatic duct [CHD], cystic duct [CD], and common bile duct [CBD]) at the three time points using NIRF-C, and the time spent on the near infrared camera mode were recorded. The ability to identify biliary anatomy using IOC and the time taken to perform an IOC were also recorded. Intent to treat and per protocol comparison of the rate of identification of biliary structures using NIRF-C and IOC were performed.
Statistical Analysis
The statistical analysis was performed with SAS Statistical Software Version 9.3 (SAS Institute, Inc., Cary, NC). A paired t-test was used to compare the procedure time for NIRF-C and IOC while McNemar's test was used for paired dichotomous data to test the difference in visualization of biliary structures by the two methods. To compare the difference in visualizing biliary structures between patients with BMI greater than 30 kg/m2 to those with BMI less than 30 kg/m2, we used a Chi-squared test or Fisher's exact test, where relevant. Continuous data are expressed as mean ± standard deviation or median (range), while discrete data are expressed as proportions.
RESULTS
Ninety-three patients were consented for elective LC with NIRF-C and IOC between January 2013 and March 2014. Eighty-two patients met inclusion criteria and were included in the study, while 11 patients were excluded due to NIRF-C equipment unavailable or malfunctioned (n = 7), acute cholecystitis (n = 2), conversion to open cholecystectomy (n = 1), and ICG infiltration (n = 1).
Mean age and BMI were 42.6 ± 13.7 years and 31.49 ± 8.2 kg/m2, respectively. Sixty-four (78%) patients were female. Indications for surgery included symptomatic cholelithiasis (n = 49), chronic cholecystitis (n = 18), biliary dyskinesia (n = 9), gallstone pancreatitis (n = 4), choledocholithiasis (n = 1), and gallbladder polyp (n = 1).
ICG was administered 73.8 ± 26.4 minutes prior to surgical incision, and the mean operating time was 78.2 ± 30.7 minutes. Three (3.7%) patients had bile leakage during gallbladder dissection. There were no bile duct injuries or other major complications during the study, and no one experienced an allergic reaction to ICG. There were no alteration in patient management due to performing NIRF-C (Table 1).
Table 1.
Patient characteristics and operative indications
| Patient details | Total (n = 82) |
|---|---|
| Age, years | 42.6 ± 13.7 |
| Gender, n (%) | |
| Male | 18 (22.0) |
| Female | 64 (78.0) |
| BMI (kg/m2) | 31.5 ± 8.2 |
| Surgical Indication, n (%) | |
| Cholelithiasis | 49 (59.8) |
| Chronic cholecystitis | 18 (22.0) |
| Biliary dyskinesia | 9 (11.0) |
| Gallstone pancreatitis | 4 (4.9) |
| Choledocholithiasis | 1 (1.2) |
| Gallbladder polyp | 1 (1.2) |
BMI body mass index
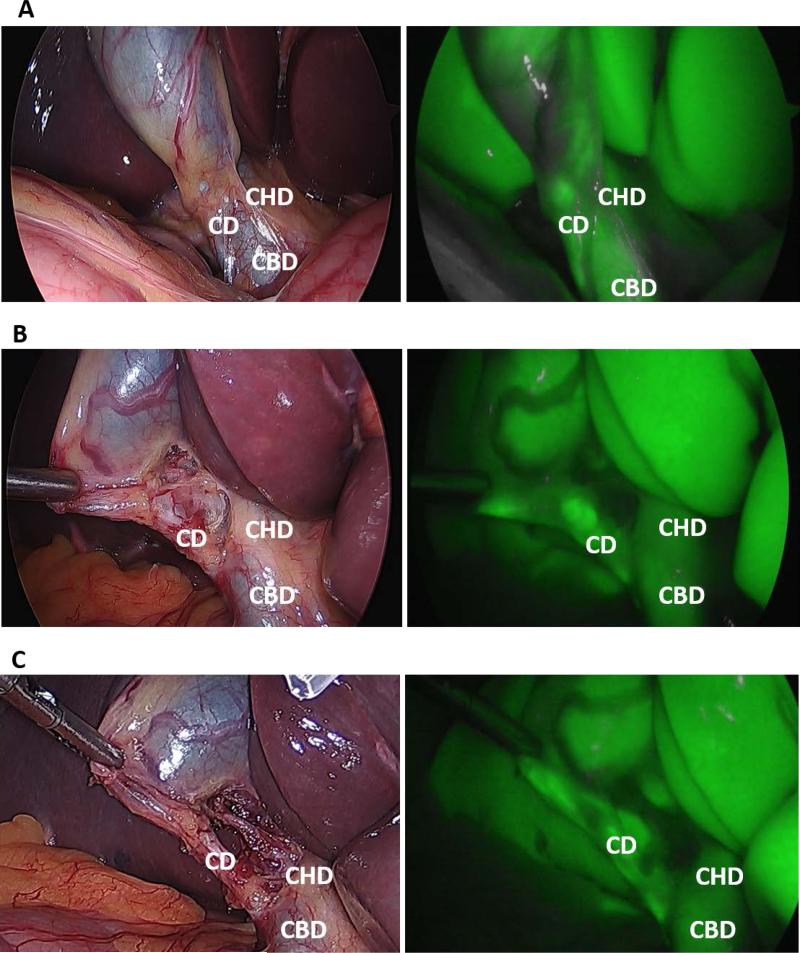
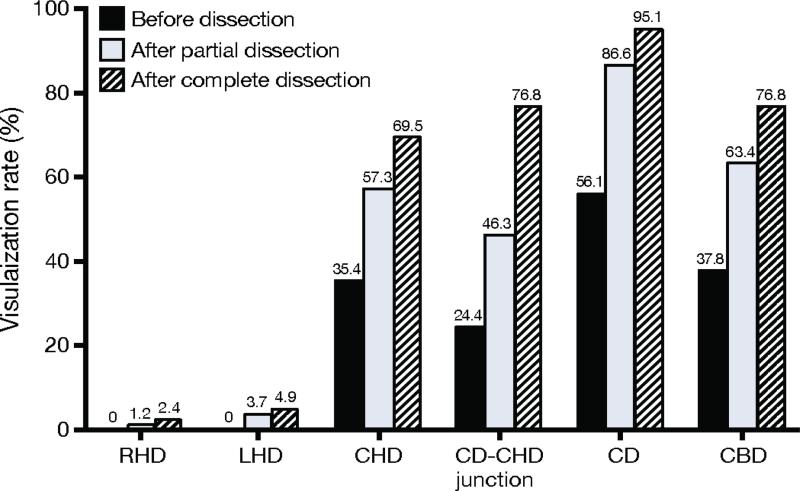
NIRF-C required 1.9 ± 1.7 minutes to complete compared to 11.8 ± 5.3 minutes for IOC (p < 0.001). There was a learning curve effect associated with performing NIRF ± C, with the first 41 cases requiring 2.7 ± 2.0 minutes compared to 1.2 ± 0.7 minutes for the final 41 cases (p < 0.001). An example of the intraoperative NIRF-C image is displayed in Figure 1. The rates of successful visualization of the extrahepatic biliary structures with NIRF-C before, after partial, and after complete dissection of Calot's triangle are shown in Figure 2. NIRF-C successfully visualized the CD in 46 (56.1%) patients before dissection and 78 (95.1%) patients after complete dissection.
Fig. 1.
Intraoperative identification of biliary structures with visible light (VL) and NIRF-C. VL (left) and NIRF-C (right) images obtained (A) following exposure of the gallbladder and before dissection, (B) after partial dissection, and (C) after complete dissection of Calot's triangle. CD cystic duct; CHD common hepatic duct; CBD common bile duct.
Fig. 2.
Identification of biliary structures using NIRF-C during laparoscopic cholecystectomy. RHD right hepatic duct; LHD left hepatic duct; CHD common hepatic duct; CD cystic duct; CBD common bile duct.
The CD was not visualized after final dissection in 4 (4.9%) patients. In 3 of these patients, visualization was impaired due to bile leakage from the gallbladder, and 1 patient had visualization of the liver only. NIRF-C did not identify the right and left hepatic ducts before dissection in any patient. Patients with BMI < 30 kg/m2 had modestly improved identification of biliary structures with NIRF-C compared to those with BMI > 30 kg/m2, though only visualization of the CD-CHD junction was statistically different (p = 0.04; Table 2). The rate of visualization of the CD and CBD in 22 (26.8%) patient with BMI > 35 kg/m2 were 91% and 64%, respectively. The CD was the only structure visualized in the patient with the highest BMI (63 kg/m2).
Table 2.
Identification of biliary structures with NIRF-C after final dissection in obese and non-obese patients
| BMI <30 n = 43 (%) | BMI >30 n = 39 (%) | p value | |
|---|---|---|---|
| CHD | 33 (76.7) | 24 (61.5) | 0.135 |
| CD-CHD junction | 37 (86.1) | 26 (66.7) | 0.038 |
| CD | 42 (97.7) | 36 (92.3) | 0.342 |
| CBD | 35 (81.4) | 28 (71.8) | 0.304 |
NIRF-C near infrared fluorescence cholangiography; BMI body mass index; CHD common hepatic duct; CD cystic duct; CBD common bile duct
IOC was successfully completed in 62 (75.6%) patients and could not be performed in 20 (24.4%) patients, due to technical difficulties (n = 18, 90%) or imaging equipment being unavailable for use after 15 minutes of wait (n = 2, 10%). The intention to treat comparison of the visualization of biliary structures in all patients using NIRF-C and IOC is shown in Table 3. IOC was significantly more likely to image the right and left hepatic ducts (p < 0.001) while NIRF-C visualized the CD at a significantly higher overall rate (95.1% vs 72.0%, p < 0.001). Table 4 shows the per protocol comparison of the successful visualization of biliary structures in the 62 patients who completed both NIRF-C and IOC. There was no difference in the visualization of the CD with either imaging modalities in this group of patients (98.4% vs 95.2%, p = 0.625), while the visualization of other biliary structures were higher with IOC. Of note, the CD was visualized with NIRF-C in 16 of the 20 (80%) patients that did not undergo an IOC due to reasons mentioned above.
Table 3.
Intent to treat comparison of successful identification of biliary structures after final dissection using NIRF-C and IOC (n = 82)
| NIRF-C (%) | IOCa (%) | p value | |
|---|---|---|---|
| Procedure time (mins) | 1.9 ± 1.7b | 11.8 ± 5.3 | <0.001 |
| RHD | 2.4 | 64.6 | <0.001 |
| LHD | 4.9 | 64.6 | <0.001 |
| CHD | 69.5 | 74.3 | 0.608 |
| CD-CHD junction | 76.8 | 72.0 | 0.572 |
| CD | 95.1 | 72.0 | <0.001 |
| CBD | 76.8 | 75.6 | 0.999 |
NIRF-C near infrared fluorescence cholangiography; IOC intraoperative cholangiogram; RHD right hepatic duct; LHD left hepatic duct; CHD common hepatic duct; CD cystic duct; CBD common bile duct
Data includes cases where IOC was unobtainable
Cumulative time for performing NIRF-C at three time points
Table 4.
Per protocol comparison of successful identification of biliary structures after final dissection using NIRF-C and IOC (n = 62)
| NIRF-C (%) | IOC (%) | p value | |
|---|---|---|---|
| RHD | 1.6 | 85.5 | <0.001 |
| LHD | 4.8 | 85.5 | <0.001 |
| CHD | 69.4 | 98.4 | <0.001 |
| CD-CHD junction | 79.0 | 95.2 | 0.013 |
| CD | 98.4 | 95.2 | 0.625 |
| CBD | 82.3 | 100.0 | 0.001 |
NIRF-C near infrared fluorescence cholangiography; IOC intraoperative cholangiogram; RHD right hepatic duct; LHD left hepatic duct; CHD common hepatic duct; CD cystic duct; CBD common bile duct
DISCUSSION
The aims of this study were to determine the safety, feasibility, and efficacy of NIRF-C in the identification of extrahepatic biliary structures in a U.S. patient population. Our results show that NIRF-C is safe and able to identify the CD in 95.1% of patients after complete dissection of Calot's triangle. There was a trend towards improved visualization of biliary structures with NIRF-C in patients with BMI < 30 kg/m2 compared to those with BMI > 30 kg/m2, though this was only significant for visualization of the CD-CHD junction. In all patients, the CD was visualized at a significantly higher rate with NIRF-C than with IOC. In the 62 patients who underwent both imaging procedures, NIRF-C was completed almost 10 minutes faster than IOC. Our study represents the largest North American experience to utilize NIRF-C in LC and the only one to compare the visualization achieved with NIRF-C to that of IOC.
The incidence of BDI during LC increased to more than 0.7% in the early 1990s, attributable in part to surgeons’ learning curve with the new minimally invasive technique [5]. Although the overall incidence of BDI remains low, the rate is unacceptable considering the consequences of such an injury. BDI decreases patient quality of life and imposes a significant burden on the health care system [17]. The treatment cost for CBD injury is estimated to approach $70,000 per patient, and could be much higher with delayed diagnosis [18]. In addition, BDI is among the leading sources of medical malpractice claims and the sixth most expensive claim to litigate [19, 20].
While evidence supports the relationship between IOC and the decreased incidence and early detection of BDI [1, 21, 22], the use of routine versus selective IOC remains controversial. It has been estimated that greater than 30,000 patients would be needed to conduct a sufficiently powered randomized control trial to assess the true relationship between IOC and BDI [19]. Flum et al. recommended routine IOC based on cost effectiveness in terms of dollar per life-year saved from BDI [23]. On the contrary, opponents of routine IOC contend that it results in higher complication rates, cost, and rates of additional postsurgical procedures, without a proven reduction in BDI rates or mortality [24]. Also, limitations including radiation exposure, increased operative time, and technical difficulties with the procedure are real concerns for many surgeons.
NIRF-C has shown promising results in identifying biliary structures without the limitations associated with IOC. In 2009, Ishizawa et al. first used NIRF-C during open cholecystectomy; the cystic duct was identified in 9/10 patients [25]. They expanded this technique to LC in 52 patients [14]. The CD was identified in all patients and the CDCHD junction in 96% of patients before dissection of Calot's triangle. In another study, Schols et al. observed that the CD and CBD could be identified significantly earlier with NIRF-C than with traditional optics [15]. Other case series have shown NIRF-C to be feasible, safe, and to shorten operative time during laparoscopic and robotic cholecystectomy [26-28].
In addition to its own safety profile, NIRF-C might improve the safety of LC. NIRF-C can delineate landmarks before any dissection is performed, which can potentially decrease incidence of BDI that may occur prior to the performance of an IOC. Additionally, NIRF-C can eliminate the inherent risk of BDI associated with the IOC. Though we performed NIRF-C at three time points in this study, it should be noted that NIRF-C can be performed at any time during the case, especially when the anatomy is in question. Repetitive real-time images obtained by pressing a button on the camera make serial biliary imaging during dissection logistically more feasible than with static IOC. The safety benefit of NIRF-C is also apparent in the ability to identify anatomy in cases where IOC cannot be obtained. We were able to visualize the CD with NIRF-C in 80% of such cases.
NIRF-C has been suggested to be less expensive to perform compared to IOC, and its use could translate into potential cost saving for patients compared to the application of routine IOC. In an analysis of the 2001 National Inpatient Survey database, Livingston et al. reported an additional hospital charge of $739 for performing an IOC during LC [29]. A recently published study by Dip et al. compared the variable cost of using an IOC to that of using fluorescence cholangiography (FC) [30]. They reported an average cost of $778 for IOC, compared to $14.10 for FC (p < 0.001). In addition to being less expensive to perform, NIRF-C can save costs by reducing the time spent in the operating room. The ability to use this novel technique to guide dissection may result in earlier identification of structures, ultimately translating into shorter operative time and reduced cost. Though hospitals desiring to perform NIRF-C would incur the cost of purchasing the infrared camera system, this represents a recoverable capital investment.
A significant limitation of NIRF-C is the inability to penetrate tissues thicker than 5-10 mm [31]. This may limit its effectiveness in the setting of acute inflammation, such as in patients with acute cholecystitis or cholangitis. It might also limit its effectiveness in obese patients. We observed this limited tissue penetration in our study as patients with severe chronic inflammatory changes had poor visualization of biliary structures. Visualization did improve after meticulous dissection and repeated imaging may prove useful in this setting. Our study shows that obesity is not a limitation to using NIRF-C; however, there was a trend toward improved visualization of biliary structures in non-obese patients.
Iatrogenic gallbladder perforation with leakage of bile during retraction can also limit the efficacy of NIRF-C. This proved true in three cases in which biliary structures could not be identified due to leakage of ICG-containing bile. Additionally, NIRF-C may not identify stones in the CD or CBD, and the sensitivity for visualizing larger stones is unknown. It also lacks the capacity to intervene when stones or debris are encountered. As a result, IOC remains the gold standard for identifying choledocholithiasis and should be performed in this setting. There is also utility in learning and maintaining the skills required to perform IOC. Nonetheless, NIRF-C demonstrates promise and further improvements may enhance visualization of intrahepatic bile ducts and bile duct stones.
NIRF-C is associated with minimal risk of complications, although anaphylactic reaction to ICG has been reported to occur at an incidence of 3/1000 (0.003%), especially at doses higher than 0.5 mg/kg [32]. As ICG is excreted by hepatocytes unaltered into the bile, excretion and detection with the near infrared camera might be compromised in patients with cirrhosis, NASH, or fatty liver disease [33]. As a result, patients with hepatic cirrhosis were excluded from the current study. ICG use is also contraindicated in patients with iodide allergy. We did not observe any allergic reactions or complications from ICG use in our study population and none have been reported in recently published ICG-related studies [25-28].
A potential logistical concern with the use of NIRF-C is the preoperative administration of ICG. In our study, ICG was administered by a surgical research fellow approximately one hour prior to surgery. This regimen might not be feasible in small or busy surgical practices with no additional qualified personnel to administer the dye. To mitigate this concern, Schols et al. injected ICG in the OR after induction of general anesthesia and showed adequate visualization of biliary structures [15]. Also, we were able to adequately visualize the CD and CBD in two patients who received ICG 158 minutes and 162 minutes prior to incision, indicating that ICG administration can be performed over a wide range of time.
This study is limited by the relatively low rate of IOC completion. Success rates of performing IOC have been reported at up to 90% [34]; our success rate was 76%. This lower completion rate could introduce bias in comparing the efficacy of NIRF-C to that of IOC. For example, the rate of visualization of the CD with IOC was 72.0% on intent to treat analysis, while it was 95.2% on per protocol analysis.
CONCLUSIONS
In summary, NIRF-C is safe, feasible, and a noninvasive alternative to IOC for imaging extrahepatic biliary structures during LC. Currently, NIRF-C does not replace IOC for identification of CBD stones; however, it allows identification of biliary structures regardless of patient's BMI and can be valuable in instances where IOC cannot be performed. Further studies with larger numbers are needed to assess the efficacy of NIRF-C in comparison to IOC, and to evaluate its effectiveness in the setting of acute inflammation and in identifying CBD stones.
ACKNOWLEDGEMENTS
Special thanks to Rebecca Dettorre, MA, CCRC and Andrew Suzo, BS, clinical research coordinators, for assisting with patient consent and data collection. We would like to thank the Foundation for Surgical Fellowship for support of Dr. Chaudhry. We would like to acknowledge protocol development assistance we received from the Center for Clinical and Translational Science (CCTS) at The Ohio State University, NIH Award UL1TR001070.
Funding Sources: This study was funded by a grant from SAGES and an in-kind device grant from Stryker.
Footnotes
DISCLOSURES
Dr. Melvin is a consultant for Stryker. Dr. Narula obtained a grant in the form of an in-kind investigational device loan from Stryker in order to conduct this study. Adel-Rasoul, and Drs. Osayi, Wendling, Drosdeck, Chaudhry, Perry, Noria, Mikami, Needleman, Muscarella, and Hazey have no conflicts of interest or financial ties to disclose.
References
- 1.Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639–1644. doi: 10.1001/jama.289.13.1639. [DOI] [PubMed] [Google Scholar]
- 2.Waage A, Nilsson M. Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish Inpatient Registry. Arch Surg. 2006;141:1207–1213. doi: 10.1001/archsurg.141.12.1207. [DOI] [PubMed] [Google Scholar]
- 3.Nuzzo G, Giuliante F, Giovannini I, Ardito F, D'Acapito F, Vellone M, Murazio M, Capelli G. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56 591 cholecystectomies. Arch Surg. 2005;140:986–992. doi: 10.1001/archsurg.140.10.986. [DOI] [PubMed] [Google Scholar]
- 4.Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 2003;290:2168–2173. doi: 10.1001/jama.290.16.2168. [DOI] [PubMed] [Google Scholar]
- 5.Adamsen S, Hansen OH, Funch-Jensen P, Schulze S, Stage JG, Wara P. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg. 1997;184:571–578. [PubMed] [Google Scholar]
- 6.Olsen D. Bile duct injuries during laparoscopic cholecystectomy. Surg Endosc. 1997;11:133–138. doi: 10.1007/s004649900315. [DOI] [PubMed] [Google Scholar]
- 7.Hugh TB. New strategies to prevent laparoscopic bile duct injury--surgeons can learn from pilots. Surgery. 2002;132:826–835. doi: 10.1067/msy.2002.127681. [DOI] [PubMed] [Google Scholar]
- 8.Archer SB, Brown DW, Smith CD, Branum GD, Hunter JG. Bile duct injury during laparoscopic cholecystectomy: results of a national survey. Ann Surg. 2001;234:549–558. doi: 10.1097/00000658-200110000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francoeur JR, Wiseman K, Buczkowski AK, Chung SW, Scudamore CH. Surgeons' anonymous response after bile duct injury during cholecystectomy. Am J Surg. 2003;185:468–475. doi: 10.1016/s0002-9610(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 10.Khan OA, Balaji S, Branagan G, Bennett DH, Davies N. Randomized clinical trial of routine on-table cholangiography during laparoscopic cholecystectomy. Br J Surg. 2011;98:362–367. doi: 10.1002/bjs.7356. [DOI] [PubMed] [Google Scholar]
- 11.Buddingh KT, Nieuwenhuijs VB, van Buuren L, Hulscher JB, de Jong JS, van Dam GM. Intraoperative assessment of biliary anatomy for prevention of bile duct injury: a review of current and future patient safety interventions. Surg Endosc. 2011;25:2449–2461. doi: 10.1007/s00464-011-1639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mordon S, Devoisselle JM, Soulie-Begu S, Desmettre T. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res. 1998;55:146–152. doi: 10.1006/mvre.1998.2068. [DOI] [PubMed] [Google Scholar]
- 14.Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369–1377. doi: 10.1002/bjs.7125. [DOI] [PubMed] [Google Scholar]
- 15.Schols RM, Bouvy ND, Masclee AA, van Dam RM, Dejong CH, Stassen LP. Fluorescence cholangiography during laparoscopic cholecystectomy: a feasibility study on early biliary tract delineation. Surg Endosc. 2013;27:1530–1536. doi: 10.1007/s00464-012-2635-3. [DOI] [PubMed] [Google Scholar]
- 16.Vettoretto N, Saronni C, Harbi A, Balestra L, Taglietti L, Giovanetti M. Critical view of safety during laparoscopic cholecystectomy. JSLS. 2011;15:322–325. doi: 10.4293/108680811X13071180407474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerma D, Rauws EA, Keulemans YC, Bergman JJ, Obertop H, Huibregtse K, Gouma DJ. Impaired quality of life 5 years after bile duct injury during laparoscopic cholecystectomy: a prospective analysis. Ann Surg. 2001;234:750–757. doi: 10.1097/00000658-200112000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon RM, Brock G, Buell JF. A novel classification system to address financial impact and referral decisions for bile duct injury in laparoscopic cholecystectomy. HPB Surg. 2011;2011:371245. doi: 10.1155/2011/371245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massarweh NN, Flum DR. Role of intraoperative cholangiography in avoiding bile duct injury. J Am Coll Surg. 2007;204:656–664. doi: 10.1016/j.jamcollsurg.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Kern KA. An overview of 711 general surgery liability cases. The anatomy of surgical malpractice claims. Bull Am Coll Surg. 1995;80:34–49. [PubMed] [Google Scholar]
- 21.Flum DR, Koepsell T, Heagerty P, Sinanan M, Dellinger EP. Common bile duct injury during laparoscopic cholecystectomy and the use of intraoperative cholangiography: adverse outcome or preventable error? Arch Surg. 2001;136:1287–1292. doi: 10.1001/archsurg.136.11.1287. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher DR, Hobbs MS, Tan P, Valinsky LJ, Hockey RL, Pikora TJ, Knuiman MW, Sheiner HJ, Edis A. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229:449–457. doi: 10.1097/00000658-199904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flum DR, Flowers C, Veenstra DL. A cost-effectiveness analysis of intraoperative cholangiography in the prevention of bile duct injury during laparoscopic cholecystectomy. J Am Coll Surg. 2003;196:385–393. doi: 10.1016/S1072-7515(02)01806-9. [DOI] [PubMed] [Google Scholar]
- 24.Ragulin-Coyne E, Witkowski ER, Chau Z, Ng SC, Santry HP, Callery MP, Shah SA, Tseng JF. Is routine intraoperative cholangiogram necessary in the twenty-first century? A national view. J Gastrointest Surg. 2013;17:434–442. doi: 10.1007/s11605-012-2119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizawa T, Tamura S, Masuda K, Aoki T, Hasegawa K, Imamura H, Beck Y, Kokudo N. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:e1–4. doi: 10.1016/j.jamcollsurg.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Buchs NC, Hagen ME, Pugin F, Volonte F, Bucher P, Schiffer E, Morel P. Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot. 2012;8(4):436–440. doi: 10.1002/rcs.1437. [DOI] [PubMed] [Google Scholar]
- 27.Spinoglio G, Priora F, Bianchi PP, Lucido FS, Licciardello A, Maglione V, Grosso F, Quarati R, Ravazzoni F, Lenti LM. Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc. 2013;27:2156–2162. doi: 10.1007/s00464-012-2733-2. [DOI] [PubMed] [Google Scholar]
- 28.Buchs NC, Pugin F, Azagury DE, Jung M, Volonte F, Hagen ME, Morel P. Real-time near-infrared fluorescent cholangiography could shorten operative time during robotic single-site cholecystectomy. Surg Endosc. 2013;27:3897–3901. doi: 10.1007/s00464-013-3005-5. [DOI] [PubMed] [Google Scholar]
- 29.Livingston EH, Miller JA, Coan B, Rege RV. Costs and utilization of intraoperative cholangiography. J Gastrointest Surg. 2007;11:1162–1167. doi: 10.1007/s11605-007-0209-9. [DOI] [PubMed] [Google Scholar]
- 30.Dip FD, Asbun D, Rosales-Velderrain A, Menzo EL, Simpfendorfer CH, Szomstein S, Rosenthal RJ. Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2014 Jan 11; doi: 10.1007/s00464-013-3394-5. [Epub ahead of print]. Doi: 10.1007/s00464-013-3394-52014. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speich R, Saesseli B, Hoffmann U, Neftel KA, Reichen J. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med. 1988;109:345–346. doi: 10.7326/0003-4819-109-4-345_2. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuhashi N, Kimura F, Shimizu H, Imamaki M, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, et al. Usefulness of intraoperative fluorescence imaging to evaluate local anatomy in hepatobiliary surgery. J Hepatobiliary Pancreat Surg. 2008;15:508–514. doi: 10.1007/s00534-007-1307-5. [DOI] [PubMed] [Google Scholar]
- 34.Debru E, Dawson A, Leibman S, Richardson M, Glen L, Hollinshead J, Falk GL. Does routine intraoperative cholangiography prevent bile duct transection? Surg Endosc. 2005;19:589–593. doi: 10.1007/s00464-004-8711-6. [DOI] [PubMed] [Google Scholar]