Abstract
Single-use bioprocessing bags and bioreactors gained significant importance in the industry as they offer a number of advantages over traditional stainless steel solutions. However, there is continued concern that the plastic materials might release potentially toxic substances negatively impacting cell growth and product titers, or even compromise drug safety when using single-use bags for intermediate or drug substance storage. In this study, we have focused on the in vitro detection of potentially cytotoxic leachables originating from the recently developed new polyethylene (PE) multilayer film called S80. This new film was developed to guarantee biocompatibility for multiple bioprocess applications, for example, storage of process fluids, mixing, and cell culture bioreactors. For this purpose, we examined a protein-free cell culture medium that had been used to extract leachables from freshly gamma-irradiated sample bags in a standardized cell culture assay. We investigated sample bags from films generated to establish the operating ranges of the film extrusion process. Further, we studied sample bags of different age after gamma-irradiation and finally, we performed extended media extraction trials at cold room conditions using sample bags. In contrast to a nonoptimized film formulation, our data demonstrate no cytotoxic effect of the S80 polymer film formulation under any of the investigated conditions. The S80 film formulation is based on an optimized PE polymer composition and additive package. Full traceability alongside specifications and controls of all critical raw materials, and process controls of the manufacturing process, that is, film extrusion and gamma-irradiation, have been established to ensure lot-to-lot consistency. © 2014 American Institute of Chemical Engineers Biotechnol. Prog., 30:1171–1176, 2014
Keywords: single-use systems, disposables, leachables, cell-based assay
Introduction
Single-use bags have gained rapid acceptance in biopharmaceutical manufacturing worldwide and offer a cost-effective alternative to traditional reusable stainless steel systems. Meanwhile, disposable bag technology is well established for cell culture media, buffer, and intermediate storage but most recently also in cell culture single-use bioreactors used for the production of therapeutic antibodies, proteins, and vaccines.
However, one of the most cited disadvantages is the risk for release of potentially toxic or inhibitory substances. These leachables are chemical compounds that originate from the plastic materials used to fabricate the bags or are generated during gamma-irradiation and storage of the polymer products. When in contact with the process fluid (culture medium or culture broth with cells) these compounds might be released from the film into the cell culture medium. The release of cytotoxic leachables under process conditions is particularly undesirable because these chemicals can adversely affect growth and viability of the production cells and, as a consequence, the product titer and potentially even quality.1
What is commonly called plastic material is composed of polymers and additives. Actually, additives are used for many reasons, for example, to facilitate the polymer processing and to increase the stability of the polymer to oxidation2 and gamma-irradiation. Very few polymers can be processed or used without additives, or if possible, they are only stable for a very limited time. Typical additives used in films designed for single-use bioprocess containers are slipping (or slip) agents and antioxidants. The former are added to the polymer to provide surface lubrication to the film during and after extrusion and to prevent single-use container walls to block against each other, and thus, limit the stress on the film during bag-filling processes. Common slip agents are amides of fatty acids such as oleamide, erucamide, and stearamide. Antioxidants are added to the formulation to prevent degradation of the polymer through reaction with atmospheric oxygen. Oxygen is captured by the antioxidant and consumed in a chemical reaction, reducing the interaction of oxygen with the polymer chains, and thus, limiting the risk of polymer chain breakage.
Hammond et al.3 identified the first leachable compound from a gamma-irradiated polymer film, which negatively impacts cell growth even at very low concentrations (0.1 mg/L). The identified substance bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP) is a degradation product of the trisarylphosphite processing stabilizer tris(2,4-di-tert-butylphenyl)phosphite (TBPP, supplied under the trade name Irgafos 168 and other denominations as an antioxidant additive) present in many polyethylene (PE)-based polymer films. Meanwhile, it could be demonstrated that the poor cell growth observed in certain bags is due to bDtBPP leaching alone.4
Our group has developed a new PE multilayer film, designed to reproducibly provide a low leachable profile, enhanced robustness and consistent cell growth performance. In an early stage of the film development, we implemented a standardized cell culture test. This test is based on the recently published guideline from the DECHEMA temporary working group (TAK) “single-use technology in biopharmaceutical manufacturing” for early identification of critical films for Chinese hamster ovary (CHO) cell lines in chemically defined culture media.5 The cell culture testing enabled us to identify potentially unsatisfactory film formulations early in the resin selection phase. Furthermore, this biological test was also beneficial as our internal data suggest that the recommended cytotoxicity testing (e.g., United States Pharmacopeia [USP] part 87, using a L929 mouse fibroblasts cell line and 10% serum containing growth media) is not necessarily predictive of the cell growth behavior of cell lines and media typically used in biopharmaceutical production processes.
This cell-based assay was already applied in a previous study6 showing that a film structure containing a nonoptimized additive package of primary and secondary additives failed the biological test while the S80 film (originally called film S), containing an optimized amount of additives necessary for processing, did not show any deviation from the reference. It was also shown that CHO cells grown in chemically defined culture medium show the highest sensitivity to leachables among the examined cell lines and cell culture media combinations.
In an earlier unpublished study, we could demonstrate, that media extracted from a gamma-irradiated film containing a nonoptimized additive package can negatively influence cell growth in vitro, whereas media extracted from the same film but using ethylene oxide (ETO) sterilization had no impact on cell growth (data not shown). This is in accordance with the results from Hammond et al.,3 who showed that gamma-irradiation is generating the toxic component. Although ETO sterilization did not generate any toxic leachables, the necessary subsequent venting of single-use bags is not straightforward and does not lent itself to large scale production of sterile bioprocessing bags. Therefore, gamma-sterilization is the method of choice to produce sterile bioprocessing bags, although it requires careful control of polymer and antioxidant degradation.
In this present study, the impact of extrusion process parameters on cell growth behavior was assessed within a defined window. An assessment of the various extrusion process parameters and their likely impact on quality attributes of the film was performed with extrusion experts. The influencing factor during film extrusion is the amount of heat transferred to process the polymer materials. The oxidation of additives and the quantity of degradation products generated to protect the polymer chains is directly proportional to heat transfer. This heat transfer is mainly linked to three critical extrusion process parameters, that is, the melting temperature of resins (extrusion temperature), the cooling temperature of the film (chill roll temperature) and the quantity of material extruded per hour (output).
Further, the impact of storage time of bags, empty before use or stored after filling, was addressed: we investigated the cell growth performance of our selected film formulation in an accelerated aging study of dry bags and in extraction studies with protein-free cell culture medium at cold room conditions.
Material and Methods
S80 film structure
The S80 multilayer film is a 400 µm thick PE multilayer film manufactured in a coextrusion process. The layers of this film comprises an outer layer, a gas barrier interlayer, and an inner product contact layer (Figure 1). The polymers have been selected to combine strength, flexibility, weldability, gas barrier properties, and biocompatibility and to ensure excellent and reproducible cell growth behavior through an optimized antioxidant package. No slipping agents have been added, instead nontoxic mechanical antiblocking based on silicon dioxide is used,7 which creates a slightly rough surface to counteract stickiness. The antioxidant package of the S80 film was optimized by minimizing the concentration of TBPP,7 a pharmacopeia-listed trisarylphosphite-based process stabilizer,7 commonly used in PE films and known to degrade into the reported growth inhibitory substance bDtBPP.3,4,7 In contrast, the TBPP concentration of the nonoptimized film formulation (NC) is approximately 30-fold higher in the contact layer than in S80. We have deliberately decided to use only pharmacopeia-listed additives as this facilitates the toxicological assessment of the extractable and leachable profile of the final bag product in contact with process intermediates.
Figure 1.
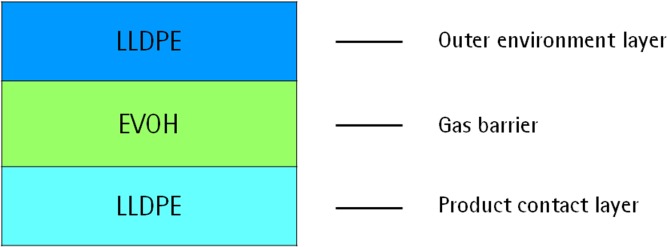
Structure of the multilayer film S80.LLDPE (linear low-density polyethylene) and EVOH (ethylene-vinyl alcohol) are used for the three layers.
Single-use sample bags
For the present study, two-dimensional (2D) pillow-like 0.8 L (total volume) bags were made. Three freshly gamma-irradiated (50 kGy) bags were examined for each of the parameter settings used in the multivariate experimental design of the film extrusion process parameters and a standardized extraction and cell culture test was performed.
Standardized cell culture test
Extraction of leachables was performed using ActiCHO, a protein, hydrolysate, and animal-derived component free cell culture medium (Cellca, Germany) in the 0.8 L sample bags. In this set up, a worst-case surface area to liquid volume ratio of 3 cm2/mL (Figure 2) was applied. The incubation (200 mL) was conducted for 3 days under sterile conditions at 37°C without agitation. As reference, a Duran® glass bottle (Schott AG, Germany) was used. The extract was transferred to six-well plates (ThinCert™, Greiner Bio-one, Germany) for subsequent growth studies.
Figure 2.

Flow chart of the bag extraction with media.S: surface, V: volume.
The human IgG1 secreting CHO cell line CHO-DG44 (Cellca, Germany) was selected as a model CHO cell line. An inoculum cell density of 0.2 × 106 cells/mL was applied. Each experimental condition was examined in triplicates in a CO2 incubator (CERTOMAT CTplus, Sartorius, Germany) over a period of 3 days. The cell culture assay parameters are summarized in Table1. Viable cell count and viability were measured daily using the NucleoCounter (Chemometec, Denmark).
Table 1.
Cultivation Parameters Selected for Biological Compatibility Assay
| Parameter | Set Point |
|---|---|
| Shaking rate | 160 rpm |
| Orbital diameter | 50 mm |
| Temperature | 36.8°C |
| pCO2 | 7.5% |
| Humidity | 80% |
| Volume | 10 mL/well |
Cytotoxicity of bis(2,3-di-tert-butylphenyl)phosphate (bDtBPP)
Dilutions of bDtBPP (kindly provided by Amgen, USA) were prepared in ActiCHO medium according to published spike concentrations.3 Thereafter, CHO-DG44 cells were inoculated into the bDtBPP ActiCHO dilutions, ranging from 0.026 to 0.84 mg/L bDtBPP. The cells were grown for a single-passage as described above.
Multivariate experimental design study on critical film extrusion process parameters
Additive content in the film formulation is indeed a key criterion for cell growth compatibility, but not the only one. As demonstrated by Hammond et al.,3 one of the most harmful moieties is not a pristine additive, but a by-product created by two consequent manufacturing steps: film extrusion and gamma-irradiation. It was, therefore, essential to assess the impact of the variation of extrusion parameters on cell growth behavior within a defined process window. The influencing factor for oxidation in the extruder is the quantity of heat transferred to the processed materials. The higher the amount of heat, the higher will be the oxidation of additives and the higher will be the quantity of by-products generated from the additives. The amount of heat transferred is linked to three critical extrusion process parameters, that is, the temperature used for melting of resins (extrusion temperature), the cooling temperature of the film (chill roll temperature), and the quantity of material extruded per hour (output). To define a process window in which the resulting film is proven not to release any harmful substance, including bDtBPP, a 23 full factorial experimental design consisting of eight runs (combinations of the high and low values of the three variables; Figure 3) were conducted. In summary, 11 extrusions were performed, three representing the center point (CP, Run 1a, 1b, and 1c) and eight variations of the extrusion process parameter (Run 2–9).
Figure 3.

Graphical representation of the full factorial design of experiments with three variables (extruder temperature, chill roll temperature, and output) at low (L), high (H), and middle (M) settings.Batch numbers 2–9 and the center point (CP) with the three runs 1a, 1b, and 1c are specified.
Shelf-life study (aging study)
Aging of single-use sample bags was performed post gamma-irradiation at 50 kGy either through natural aging at room temperature or accelerated aging specified in ASTM F1980 (Standard Guide for Accelerated Aging of Sterile Medical Device Packages).8 Briefly, accelerated aging was conducted at 40°C and 75% humidity, simulating 1, 3, 6, 12, 24, and 36 months of storage, respectively, and was performed in duplicate. Natural aged bags were examined weekly over 5 weeks. After aging, leachable extraction and standardized cell culture tests were performed as described above.
Extended media extraction trials
Extended media extraction trials were conducted in duplicates with cell culture medium filled, freshly gamma-irradiated (50 kGy) 0.8 L sample bags at 4–8°C and warmed up to 37°C in glass bottles, directly prior use. The impact of individual media on CHO cell growth were verified over 6 months as described above.
Results and Discussion
Sensitivity of CHO-DG44 cell line for bDtBPP
The suitability of CHO-DG44 cells to detect toxic leachables from single-use bioprocess containers was validated. For this purpose, the cytotoxic impact of bis(2,3-di-tert-butylphenyl)phosphate (bDtBPP)3 was evaluated.
The effect of different bDtBPP concentrations on viable cell density and cell viability are shown in Figure 4. Whereas the cell viability was only slightly reduced at the highest investigated bDtBPP concentration of 0.84 mg/L, the impact on cell proliferation was strongly dose-dependent. The bDtBPP specific half-maximal effective concentration inhibiting 50% of cell growth (EC50) is approximately 0.3 mg/L for the used CHO-DG44 cell line.
Figure 4.

Dose dependency of bDtBPP-cytotoxicity. CHO-DG44 cells were used to examine cell growth (solid line) and viability (dashed line) in the presence of different bDtBPP concentrations (mg/L) for up to 3 days. Cell growth is expressed normalized to untreated cells (%).
When bDtBPP was identified as migrating leachable compound from gamma-irradiated single-use containers, the toxic impact on nine CHO cell lines was examined.3 All investigated cell lines differed from each other with regard to sensitivity against bDtBPP. EC50 values for cell growth ranged from 0.12 to 0.73 mg/L. Only two of the nine investigated CHO lines were slightly more sensitive than the CHO-DG44 used in this study, one CHO cell line was comparable, but all other CHO lines were less sensitive to the toxic substance. It should be noted that in contrast to the published bDtBPP spiking study3 where three consecutive cell passages were performed only one cell passage was evaluate in this study. Nevertheless, the suitability of the used CHO-DG44 cell line for detecting toxic leachables could be demonstrated.
Multivariate experimental design study on critical film extrusion process parameters
A design of experiment (DoE) was used to examine the impact of critical extrusion process parameters and to define the design space of the film extrusion. Interacting parameters comprised the extrusion temperature, the chill roll temperature, and the quantity of extruded film produced per unit time as the latter is related to the residence time of polymers in the screw and the amount of heat transferred to the melted polymer material, which could impact material properties and the antioxidant degradation profile, for example, creation of by-products.
The influence of the variation of film extrusion parameters on CHO-DG44 cell growth is shown in Figure 5. CHO-DG44 cell growth (Figure 5) and cell viability (data not shown) showed no difference compared to reference for all freshly gamma-irradiated sample bags made of films generated in the DoE. Since, all other factors were kept constant including resin and additive formulation used for film extrusion, we concluded that extrusion parameters within the investigated design space do not influence the biological performance of the S80 PE film.
Figure 5.
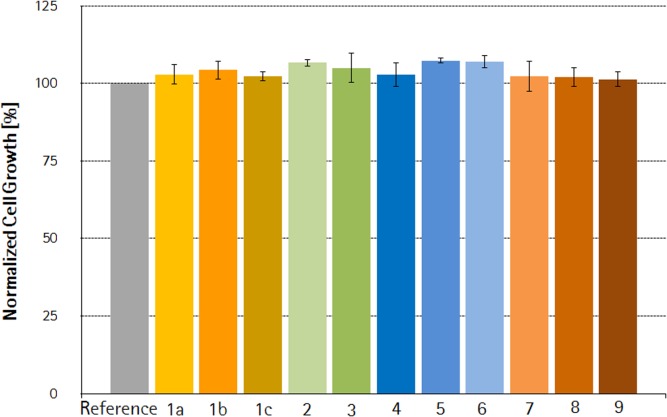
Influence of critical S80 film extrusion process parameters on cell growth.Viable cell densities were examined with medium used for film extraction and presented as percent cell growth normalized to reference, +/− SEM (standard error of mean). Column numbers correlate with run numbers shown in Figure 2, whereby 1a–1c defining three independent extrusions of the CP.
Shelf-life study of dry stored sample bags
The impact of polymer aging on biological performance of the S80 film formulation was examined using sample bags made of film extruded at CP conditions. After gamma-irradiation, the bags were stored under accelerated aging conditions as specified in the ASTM F19808 simulating 1, 3, 6, 12, 24, and 36 months of shelf life at ambient temperature.
As shown in Figure 6, accelerated aging of gamma-irradiated S80 bags did not affect cell proliferation during the entire study. Cell growth in all S80 bag medium extracts was comparable to reference during the entire 337 days of the accelerated aging study performed at 40°C and 75% humidity (equivalent to 36 months of ambient aging). In contrast, a remarkable growth inhibition was observed using extracts obtained from a PE film containing a nonoptimized additive package (NC) and a bag from another vendor. Notably, a 40% reduction of cell proliferation was detected with NC extracts directly after irradiation. However, already 1 month later, cell growth was nearly comparable to reference. Notwithstanding that the NC film contains a nonoptimized additive package, cells grew better in extracts of NC bags than in those of another vendor suggesting a higher/nonoptimized additive content in the investigated vendor bags. Cell proliferation was completely suppressed up to 1 month and increased to 83% after 3 months postirradiation in the other vendor bags. This observation supports the hypothesis that bDtBPP degrades over time4 into nontoxic derivatives depending on the TBPP quantity used in the film formulation.
Figure 6.
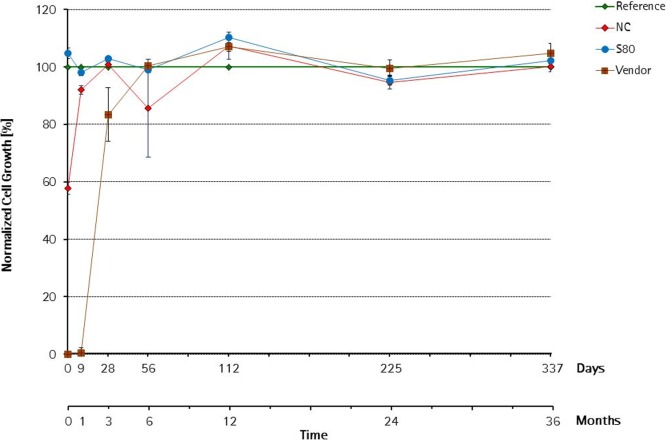
Influence of shelf-life of gamma-irradiated bags stored under accelerated aging conditions.Cell growth using media extracts was performed over 337 days at 40°C equivalent to 36 months real-time aging and expressed as % of reference.
Accelerated aging studies are commonly carried out to simulate the impact of polymer aging within a relatively short time, however, the results may differ from natural aging. To verify the correlation between accelerated vs. natural aging under environmental conditions, S80 and NC bags were naturally aged over a critical period of 5 weeks. Similar to the results obtained from the accelerated aging, no negative impact on cell growth was observed with S80 medium extracts. Already 1 month after gamma-irradiation, cell growth almost recovered to reference for the NC extracts (data not shown). Thus, results achieved with naturally aged film formulations correlate well with accelerated aging results within the tested timeframe.
Extended media extraction trials
To evaluate potential migration of toxic leachables from single-use polymer bags into cell culture media during normal media storage conditions, a prolonged media extraction trial was conducted.
The influence of medium stored at 2–8°C on CHO cell growth is shown for S80 and NC bag formulations over 6 month (Figure 7). During this time, no negative impact on cell growth was observed, neither with medium stored in S80 sample bags and, interestingly, nor with medium stored in NC bags. Thus, migration of growth inhibiting leachables is suggested to be negligible for media-filled bags under cold room conditions. However, it is important to note that the extracted medium was warmed up in glass bottles prior to the cell growth investigation.
Figure 7.
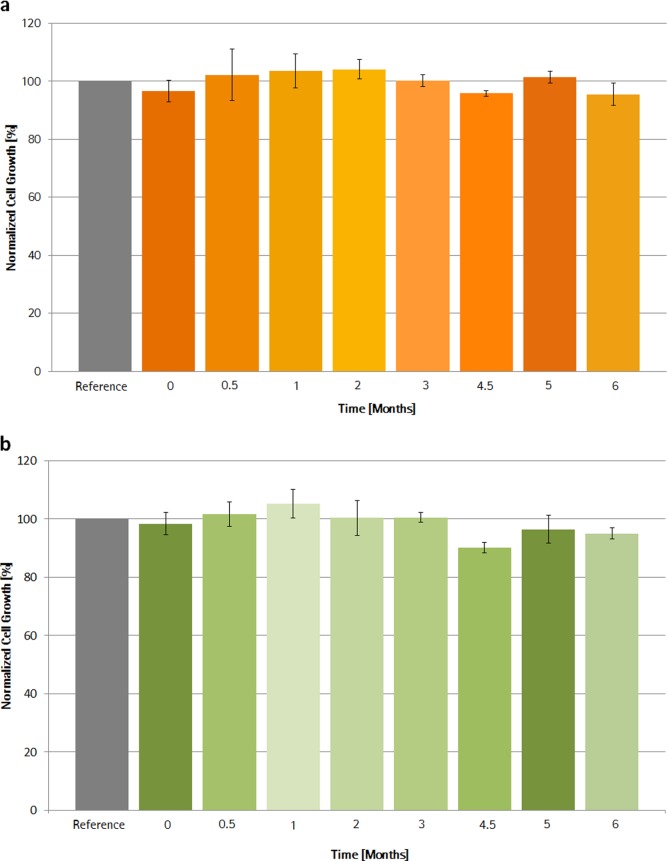
Extended media extraction using S80 sample bags (a) and NC sample bags (b) at cold room conditions (2–8°C).Cell growth is shown over 6 months and expressed normalized to reference, +/− SEM.
A correlation between time of warming up cell culture medium in disposable bags, from cold room conditions to 37°C, and a reduced subsequent cell growth has already been demonstrated.1,9,10 In contrast to data obtained with medium warmed in bags prior to inoculation, the cell growth studies described in this study were performed with extracts that were transferred into glass bottles for warming up. Thus, the lack of cell growth inhibition observed in extracted medium from NC bags suggests amplified migration of leachable compounds at higher temperatures, typically achieved during warm up of the medium in bags. This was also verified by Hammond et al.3
Summary
We developed and implemented an application oriented, standardized CHO cell culture test for detection of potentially cytotoxic leachables early in the development of the new S80 film. The dose–response behavior of the chosen model CHO-DG-44 cell line was verified in a bDtBPP spiking study. The assay was successfully applied during the resin selection phase (data not shown), and further, used to establish the operating ranges of the film extrusion process. During an accelerated aging study, the S80 film formulation demonstrated growth behavior comparable to reference over a 36 months period. No aging effect could be determined neither in the accelerated aging study nor a short 5 weeks real time aging study. Similarly, extended protein-free media extraction trials at cold room conditions did not reveal any negative impact on cell growth. In essence, we could demonstrate the suitability and lot-to-lot consistency of the optimized additive package of the new S80 PE film. Furthermore, the data underpin the importance of cell culture testing to qualify films developed for single-use bioprocessing bags, for example, during resin and additive selection, extrusion process development, and gamma-irradiation to guarantee a robust product.
In a recently conducted study, performed by the DECHEMA temporary working group (TAK) “single-use technology in biopharmaceutical manufacturing,” the same test procedure was applied to investigate a range of different single-use bags in an interlaboratory study using different CHO cell lines and media.5 The growth test was performed in shake flasks instead of the six-well plates used in this study. Interestingly, only 4 of 10 examined bags, supplied by multiple vendors, passed the test without deviation to reference (medium extracted from borosilicate bottles). Notably, the S80 film successfully passed this test verifying the results obtained in this study.
Acknowledgments
The authors thank Amgen Inc. (USA) for providing bDtBPP. The authors also acknowledge Lucie Delaunay, Hanni Sun, Oliver Scheibe, and Tanja Frick for excellent technical assistance.
Literature Cited
- 1.Wood J, Mahajan E, Shiratori M. Strategy for selecting disposable bags for cell culture media applications based on a root-cause investigation. Biotechnol Prog. 2013;29:1535–1549. doi: 10.1002/btpr.1802. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez RJ, Selke SEM, Culter JD. Plastics Packaging: Properties, Processing, Applications, and Regulations. Hanser Publishers; 2000. , Munich; [Google Scholar]
- 3.Hammond M, Nunn H, Rogers G, Lee H, Marghitoiu A-L, Perez L, Nashed-Samuel Y, Anderson C, Vandiver M, Kline S. Identification of a leachable compound detrimental to cell growth in single-use bioprocess containers. PDA J Pharm Sci Technol. 2013;67:123–134. doi: 10.5731/pdajpst.2013.00905. [DOI] [PubMed] [Google Scholar]
- 4.Hammond M, Marghitoiu L, Lee H, Lourdes P, Rogers G, Nashed-Samuel Y, Nunn H, Kline S. A cytotoxic leachable compound from single-use bioprocess equipment that causes poor cell growth performance. Biotechnol Prog. 2014;30:332–337. doi: 10.1002/btpr.1869. doi: 10.1002/btpr.1869. [DOI] [PubMed] [Google Scholar]
- 5.Eibl R, Steiger N, Fritz C, Eisenkrätzer D, Bär J, Müller D, Eibl D. Recommendation for leachable studies—standardized cell culture test for the early identification of critical films for CHO cell lines in chemically defined culture media. Dechema Biotechnologie. 2014 http://biotech.dechema.de/Publikationen.html. Last accessed on 27 May, 2014. [Google Scholar]
- 6.Steiger N, Imseng N, Schirmaier C, Husemann U, Jurkiewicz E, Greller G, Eibl R. Poster Presentation, BioTech 2013 Conference, Single-Use Technology in Biopharmaceutical Manufacturing. Switzerland: 2013. Influence of bag materials on cell growth of permanent animal cell lines and primary human mesenchymal stem cells; p. 163. In:, Wädenswill, Book of abstracts: [Google Scholar]
- 7. PCT patent application numbers: PCT/EP2014/050954, PCT/EP2014/050958 and PCT/EP2014/050962.
- 8. “ASTM F1980 −07” Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices. ASTM International—Standards Worldwide. http://www.astm.org/Standards/F1980.htm. Last accessed on 27 May, 2014.
- 9.Horvath B. A generic growth test method for improving quality control of disposables in industry cell culture. BioPharm Int. 2013;23:34–41. [Google Scholar]
- 10.Fritz C, Eisenkrätzer D. Oral Presentation, BioTech 2013 Conference, Single-Use Technology in Biopharmaceutical Manufacturing. Switzerland: 2013. Leachable test of bag materials. In:, Wädenswil;, Book of abstracts: 56–57. [Google Scholar]


