Abstract
Jatropha gossypiifolia L. (Euphorbiaceae), popularly known as cotton-leaf physicnut, is a milky shrub notable for its medicinal properties. The present study aimed to evaluate the toxic, cytotoxic and genotoxic effects of the latex of J. gossypiifolia, using Allium cepa L. as test system. Seeds of A. cepa were exposed to five concentrations of the latex (1.25; 2.5; 5; 10 and 20 mL/L) in order to evaluate parameters of toxicity (evaluation of root growth), cytotoxicity (mitotic index frequency) and genotoxicity (frequency of chromosome alterations). The latex showed a significant decrease in root mean growth value as well as mitotic index for the tested concentrations, except for 1.25 mL/L, when compared to results from the negative control. The 1.25, 2.5 and 5 mL/L concentrations induced significant chromo-some adherences, C-metaphases and/or chromosome bridges, as genotoxic effects. The significant frequency of chromosome bridges also indicated mutagenic potential for chromosomes of J. gossypiifolia as discussed in the paper. Considering that the latex is used in popular therapies, and that the test system A. cepa presents good correlation with tests carried out in mammals, it can be pointed out that its use for medicinal purposes may be harmful to human health especially if ingested.
Keywords: Allium cepa test, medicinal plant, plant latex, genotoxicity evaluation
Introduction
For thousands of years, several plants have been used in popular medicine. Despite being considered the main source of antimutagenics and antioxidants (Çelik and Aslantürk, 2010) some of their phytochemicals may cause adverse reactions or have potential of interacting with other medications, generating toxic, cytotoxic, mutagenic and genotoxic effects (Pawlowski et al., 2012; Ping et al., 2012; Ray et al., 2013). However, for many of them there is little information available regarding the potential health risks at short, mid- or long term (Nunes et al., 2012).
Jatropha gossypiifolia L. (Euphorbiaceae), commonly known as bellyache bush, black physicnut or cotton-leaf physicnut, is a shrub that contains a characteristic latex largely used for medicinal purposes, though in an empirical way (Cordeiro and Secco, 2014). The leaves are used in natura or in compresses, and are considered to have anti-malarian (Jansen et al., 2010), insecticidal (Valencia et al., 2006), anti-inflammatory (Oliveira et al., 2010) and antimicrobial (Dhale and Birari, 2010; Gaikwad et al., 2012) properties. The root and stem have cytotoxic (Nazeema and Girija, 2013), anti-malarian, leishmanicidal, antimicrobial, insecticidal, molluscicidal (reviewed by Sabandar et al., 2013) and anti-inflammatory (Bhagat et al., 2013) properties. The seeds and fruits are used against influenza, and also as laxative (Sabandar et al., 2013), sedative, analgesic or anti-diarrheal agents (Apu et al., 2013). The latex, in turn, is bactericidal (Gaikwad et al., 2012) and molluscicidal (Matos, 2004). In Brazil, the topical application of latex in natura is employed against wounds and bites of venomous animals (Stasi and Hiruma-Lima, 2002), and its ingestion in diluted form is used for treatment of diarrhea by indigenous peoples (Curto, 1993). In India, the solution of latex with mustard oil (Brassica campestris) and clove oil (Syzygium aromaticum) is applied onto painful gum or teeth (Punjaji, 2012), whereas in the south of Nigeria it is used to reduce nosebleed and wounded skin (Oduola et al., 2007).
Despite its medicinal properties, the latex of J. gossypiifolia in natura, in direct contact with the skin, may produce caustic and irritating effects (Parente and Rosa, 2001). Moreover, the aqueous extract of latex was shown to be toxic for fish as well as upon intraperitoneal administration to house mice, presenting significant reduction of acetylcholinesterase (AChE) inhibition in the first (Singh and Singh, 2012; Pratap and Singh, 2013) and seizures in the latter (Singh and Singh, 2012). The inhibition of AChE prevents the degradation of choline neurotransmitters, such as acetylcholine, and prolongs the signal transmission through synapses. Consequently, it may lead to lethal paralysis by hyperstimulation of the nervous system (Stansley, 1993). The toxic effect may be mainly associated to cyclic peptides of the latex (Horsten et al., 1996; Auvin-Guette et al., 1997; Pratap and Singh, 2013), which show cytotoxic activity, as related for other Jatropha species (reviewed by Sabandar et al., 2013). Therefore, complementary studies are still necessary for the latex of J. gossypiifolia to be safely employed for medicinal purposes, starting with the evaluation of its toxic potential.
Whereas the toxicity tests in animals lead to their death, alternative analysis should be considered. In this sense, genotoxicity tests using the Allium cepa test system showed a good correlation with the test system of mammals (Rank and Nielsen, 1994), indicating its use as an alternative for monitoring the genotoxic potential of chemical compounds (Fachinetto et al., 2007). Additionally, A. cepa stands out among other plants due to presenting large chromosomes and in few number (2n = 16) in its cells (Fiskesjo, 1985). Moreover, the A. cepa test system has high sensitivity in detecting chemical and environmental agents (Leme and Marin-Morales, 2009). This system is easy to use and presents itself as a suitable bioindicator for the first screening of genotoxicity, thanks to its low cost, reliability and concordance with other genotoxicity tests. This way it contributes in the preliminary genotoxicity assessment of compounds for medicinal purposes (Bagatini et al., 2007). Thus, the present work aimed at analyzing the toxic, cytotoxic and genotoxic effects of the latex from J. gossypiifolia by means of the A. cepa test system.
Material and Methods
Biological material
Latex of J. gossypiifolia was collected from an adult plant in Teresina (PI, Brazil) in January 2013. Herbarium specimens containing leaves, flowers and fruits were stored in the Afrânio Fernandes at the State University of Piauí Herbarium (UESPI – Teresina, Brazil); voucher specimen number: HAF 03111. The seeds of A. cepa (cv. Vale Ouro IPA – 11) used in the bioassays were kindly provided by the Agronomic Institute of Pernambuco (IPA, Recife, Brazil).
The latex of J. gossypiifolia was extracted following removal of the leaf petioles using pruning shears, at 8 to 9 am. The latex was immediately stored in Falcon tubes wrapped in aluminum foil in order to reduce the oxidation process. The latex was then transported, in cooling box containing ice, to the Laboratory of Plant Genetics and Bio-technology (Genetics Department, UFPE) where it was diluted in distilled water to yield five different latex concentrations (1.25; 2.5; 5; 10 and 20 mL/L) to be used at A. cepa assay.
Allium cepa assay
One hundred seeds of A. cepa (cv. Vale Ouro IPA -11) were germinated in Petri dishes containing filter paper moistened with distilled water, at room temperature. When the rootlets reached about 1 cm in length, they were transferred to the five cited latex concentrations (one dish for each concentration) for 24 h. Distilled water was used as negative control (NC); MMS (methyl methanesulfonate 4 × 10−4 M), a drug with clastogenic activity, and the herbicide Trifluralin (0.84 ppm of the active agent), a substance with aneugenic activity (Fernandes et al., 2007), were used as positive controls. When rootlets reached about 1.5 cm length, the material was fixed in Carnoy (ethanol:acetic acid 3:1, v/v) for 6–8 h, at room temperature, and stored at −20 °C until slide preparation.
For slide preparation, the root tips were washed three times in distilled water, for 5 min each time, and hydrolyzed at 60 °C for 10 min in HCl 1 N. After hydrolysis, the root tips were again washed in distilled water and transferred to amber glass bottles containing Schiffs reagent, in which they remained for 2 h in the dark. After this time, the root tips were washed until complete removal of the re-agent, transferred onto slides, squashed with one drop of 2% acetic carmine, and mounted with Entellan®.
Latex toxicity was evaluated according to the mean root length variation (in centimeters) of 30 roots per treatment. The experimental unit consisted of one individual root (one root per specimen). Cytotoxicity and genotoxicity were evaluated by scoring 5,000 meristematic cells (experimental unit: slide with 500 cells, with a total of 10 analyzed slides per treatment) under light microscope (400 ×). The assessed aspects were: (1) mitotic index (cytotoxicity) and (2) chromosome alteration index (genotoxicity). The last one includes alterations resulting from aneugenic effects (e.g. C-metaphases, metaphase with chromosome adherences, loss chromosomes, multipolar anaphases, binucleate cells, polyploid metaphases, and other alterations) or clastogenic effects (e.g. chromosome fragments in meta-phase or anaphase, chromosome bridges and other alterations). Micronuclei can be result from either aneugenic or clastogenic effects.
Photographs of the best cells were taken in a Leica conventional microscope (DM 500) (1000 ×), using a digital camera (Nikon 14.0 Megapixels). Images were adjusted for brightness and contrast, in gray tones using Adobe Photoshop CS6.
Statistical Analysis
The toxicity values were expressed as averages, whereas the values of cytotoxicity and genotoxicity were expressed as frequencies. Statistical analysis to evaluate data distribution with regard to normality was carried out by Lilliefors test (D) in the program Assistat 7.7 (Silva and Azevedo, 2002), while homogeneity of variance was evaluated by the Cochran test in the program BioEstat 5.3 (Ayres et al., 2007). Data which did neither present normal distribution nor homogeneity were analyzed by the non-parametric test of Kruskal-Wallis, followed by the a posteriori Student-Newman-Keuls test (p < 0.05) in the program BioEstat 5.3 (Ayres et al., 2007). The data on cytotoxicity were the only presenting normal distribution and homogeneity of variance, and were analyzed by the parametric test of Scott-Knott (p < 0.05) in the program Assistat 7.7 (Silva and Azevedo, 2002).
Results
The tests of toxicity and cytotoxicity carried out with seeds of A. cepa exposed to the different concentrations of latex from J. gossypiifolia showed a significant reduction in root mean growth and in mitotic index (MI) values of all tested latex concentrations, when compared to NC, except for 1.25 mL/L. In these analyses, the reduction was partially or entirely dose-dependent for root mean growth and in mitotic index, respectively (Table 1).
Table 1. Values of mean length for the root tips of Allium cepa and mitotic indexes in meristematic cells, observed 24 h after Jatropha gossypiifolia latex treatment at different concentrations.
| Latex treatment (mL/L) | Mean root length (cm) | Mitotic index (%) |
|---|---|---|
| Distilled water (NC) 1 | 3.32 ± 0.90 | 51.57 ± 2.69a 5 |
| 1.25 | 3.21 ± 0.89 | 51.77 ± 6.63a |
| 2.5 | 2.43 ± 0.64**4 | 44.33 ± 5.53b |
| 5 | 2.11 ± 0.64** | 36.70 ± 5.27c |
| 10 | 2.28 ± 0.85** | 36.32 ± 5.43c |
| 20 | 1.87 ± 0.62** | 22.02 ± 8.00d |
| MMS (4 × 10−4 M) 2 | 2.78 ± 0.63 | 36.67 ± 7.11c |
| Trifluralin (0.84 ppm) 3 | 2.38 ± 0.69** | 23.00 ± 2.76d |
NC: negative control.
MMS (Methyl methanesulfonate): positive control.
Trifluralin: positive control.
Significant in the Kruskal-Wallis test with a posteriori Student-Newman-Keuls test
p < 0.05;
p < 0.01
Scott-Knott test (p < 0.05; averages followed by the same lowercase letter are not significantly different). The results refer to analysis of 5,000 cells per treatment.
With regards to chromosome alterations (CA), the increase in the total indexes (Table 2; Figure 1) was highly significant when compared with NC results, except for the 10 and 20 mL/L concentrations. From these results, we may infer that J. gossypiifolia latex has a genotoxic action. On the other hand, the nemployed empirically in the popular medicine of maon-significant CA frequencies found for the higher latex concentrations (10 and 20 mL/L) may result from MI reduction with these treatments.
Table 2. Frequency of chromosome alterations in meristematic cells of Allium cepa root tips, observed 24 h after Jatropha gossypiifolia latex treatment at different concentrations.
| Chromosome alteration | Negative control | Latex treatment (mL/L) | Positive control | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Distilled water | 1.25 | 2.5 | 5 | 10 | 20 | MMS 1 (4 × 10 −4 M) | Trifluralin (0.84 ppm) | |
| Metaphases with chromosome adherences | 0.66 ±0.32 | 1.46 ±0.47**2 | 1.29 ±0.68 * | 1.28 ±0.24 ** | 0.86 ±0.35 | 0.76 ±0.34 | 0.11 ±0.18 | 3.25 ±1.86 ** |
| C-metaphases | 0.02 ±0.06 | 0.11 ±0.15 | 0.19 ±0.18 * | 0.37 ±0.25 ** | 0.11 ±0.15 | 0.09 ±0.16 | 0.07 ±0.10 | 0.39 ±0.55 * |
| Chromosome losses | 0.02 ±0.06 | 0.15 ±0.17 | 0.15 ±0.19 | 0.06 ±0.12 | 0.14 ±0.11 | 0.13 ±0.17 | 0.00 ±0.00 | 0.08 ±0.10 |
| Binucleated cells | 0.07 ±0.10 | 0.04 ±0.12 | 0.00 ±0.00 | 0.00 ±0.00 | 0.07 ±0.14 | 0.00 ±0.00 | 0.00 ±0.00 | 0.94 ±1.29 |
| Polyploid cells | 0.04 ±0.12 | 0.04 ±0.08 | 0.00 ±0.00 | 0.00 ±0.00 | 0.06 ±0.10 | 0.02 ±0.06 | 0.00 ±0.00 | 0.30 ±0.39 |
| Multipolar anaphases | 0.00 ±0.00 | 0.02 ±0.06 | 0.00 ±0.00 | 0.02 ±0.06 | 0.00 ±0.00 | 0.00 ±0.00 | 0.00 ±0.00 | 0.30 ±0.44 |
| Lobulated nucleus | 0.00 ±0.00 | 0.00 ±0.00 | 0.06 ±0.18 | 0.02 ±0.06 | 0.02 ±0.06 | 0.00 ±0.00 | 0.02 ±0.06 | 5.28 ±3.85 ** |
| Nuclear buds | 0.07 ±0.13 | 0.22 ±0.14 | 0.11 ±0.24 | 0.31 ±0.27 | 0.14 ±0.19 | 0.17 ±0.24 | 0.13 ±0.15 | 1.24 ±0.64 ** |
| Micronucleus | 0.35 ±0.31 | 0.66 ±0.53 | 0.77 ±0.56 | 0.93 ±0.93 | 0.57 ±0.32 | 0.46 ±0.58 | 4.55 ±2.55 ** | 2.18 ±1.27 ** |
| Chromosome fragments | 0.02 ±0.00 | 0.02 ±0.06 | 0.02 ±0.06 | 0.07 ±0.17 | 0.02 ±0.06 | 0.02 ±0.06 | 0.17 ±0.06 ** | 0.04 ±0.08 |
| Chromosome bridges | 0.00 ±0.00 | 0.27 ±0.20 ** | 0.09 ±0.13 | 0.22 ±0.24 * | 0.27 ±0.26 ** | 0.04 ±0.08 | 0.00 ±0.00 | 0.19 ±0.22 ** |
| Total | 1.25 ±0.62 | 2.99 ±1.03 ** | 2.68 ±1.15 * | 3.28 ±1.35 ** | 2.26 ±0.58 | 1.69 ±1.10 | 5.05 ±2.26 ** | 14.19 ±7.10 ** |
MMS (Methyl methanesulfonate).
*Significant in the Kruskal-Wallis test with a posteriori Student-Newman-Keuls test (
p < 0.05;
p < 0.01
The results refer to analysis of 5,000 cells per treatment.
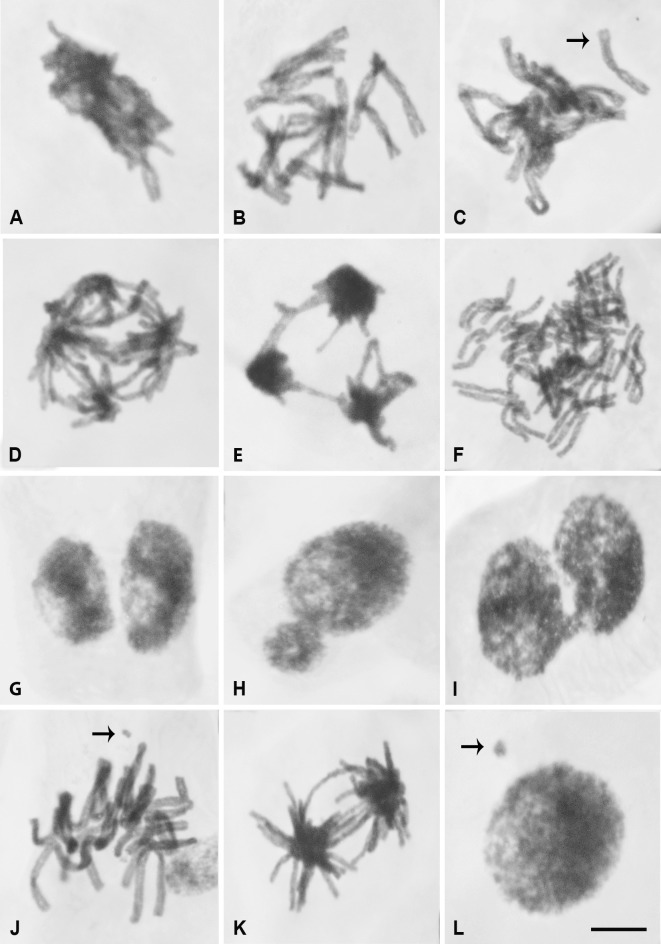
Figure 1. Chromosome alterations after Jatropha gossypiifolia latex treatment of Allium cepa meristematic cells. (A) Chromosomal adherence (1.25 mL/L). (B) C-metaphase (5 mL/L). (C) Metaphase with chromosome loss (2.5 mL/L). (D) Multipolar anaphase with chromosome bridges (5 mL/L). (E) Multipolar telophase with chromosome bridges (5 mL/L). (F) Polyploid metaphase (10 mL/L). (G) Binucleated cell (10 mL/L). (H) Nucleus with nuclear bud (5 mL/L). (I) Lobulated nucleus (2.5 mL/L). (J) Metaphase with chromosome fragment (5 mL/L). (K) Anaphase with chromosome bridge (10 mL/L). (L) Micronucleated cell (1.25 mL/L). Arrows in (C), (J) and (L) indicate chromosome loss, chromosome fragment and micronucleus, respectively. Bar: 10 μm (for all pictures).
When the chromosome alterations were analyzed separately, metaphases with chromosome adherences (Figure 1A) have been found in all the treatments carried out with the latex; nevertheless significant NC related results were only recorded for the three lower concentrations (1.25; 2.5 and 5 mL/L). The presence of C-metaphases (Figure 1B) was significant only for 2.5 and 5 mL/L concentrations (Table 2). Both chromosome adherences and C-metaphases are result from aneugenic mechanisms.
Other non-significant alterations in chromosome segregation during anaphase and telophase were registered, such as chromosome losses (Figure 1C) and multipolarities (Figure 1D–E; Table 2). Additionally, polyploid (Figure 1F) and binucleated cells (Figure 1G), nuclear buds (Figure 1H) and lobulated nuclei (Figure 1I) have been found in all analyzed treatments, although no significant alterations have been observed for any concentration (Table 2).
Chromosome fragments (Figure 1J) and chromosome bridges (Figure 1K; Table 2), arising from clastogenic effects, were also registered, but only the presence of chromosome bridges was significant for concentrations 1.25, 5 and 10 mL/L. Non-significant micronucleus (Figure 1L) frequencies were also observed.
Discussion
The latex of J. gossypiifolia is broadly used in popular medicine in many countries. However, this plant has phyto-chemicals in its composition such as alkaloids and cyclic peptides, which may be toxic to users (Singh and Singh, 2012; Pratap and Singh, 2013). In the present study, the test system A. cepa was used to evaluate the toxic, cytotoxic and genotoxic effects of different concentrations (1.25; 2.5; 5; 10 and 20 mL/L) of latex from J. gossypiifolia. The concentrations tested were selected based on a usage concentration recommended in popular medicine (approximately 10 mL/L, corresponding to one tablespoon in one liter of water). Apart from the recommended concentration, others were also tested based on results of latex toxicity in fish, which varied from 10 to 21.5 mL/L (Singh and Singh, 2012; Pratap and Singh, 2013), but mainly on antimicrobial activity data, which varied from 1.9 to 4.4 mL/L (Patil et al., 2012) for the latex of J. gossypiifolia and from 0.5 to 10 mL/L for the latex of J. curcas (Arekemase et al., 2011).
The bioassays carried out here revealed dose-dependent toxicity (mean root growth) and cytotoxicity (mitotic index) for all analyzed treatments except for the lowest concentration (1.25 mL/L). These effects may be attributed to various chemical substances present in the latex of J. gossypiifolia, mainly cyclic peptides (CPi), such as cyclogossines A (Horsten et al., 1996) and B (Auvin-Guette et al., 1997), terpenes (Patil et al., 2012) and alkaloids that have cytotoxic activity (Sabandar et al., 2013). Peptides present in other species of the genus Jatropha have also been reported as cytotoxic, such as integerrimides A and B of J. integerrima (Mongkolvisut et al., 2006) and the curcacyclins A and B of J. curcas (Insanu et al., 2012).
Cyclic peptides have a cyclical conformation (Sakai et al., 1996), which facilitates their passing through cell membranes, as well as absent exposure of the C- and N-terminal groups to exopeptidases (Wu et al., 2007). These characteristics may be related to the ease with which these peptides enter and remain inside cells of A. cepa, causing toxic effects, as observed here and in previous studies with latex of J. curcas in Artemia salina as well as in cell culture of human ovarian cancer (Insanu et al., 2012). On the other hand, terpenes, such as diterpenes, are some of the most toxic compounds in Jatropha (Devappa et al., 2011), whereas lipophilic nature (Wink, 2012) facilitates their entry and remaining inside cells of A. cepa, causing toxic effects. Additionally, terpenes in latex may be associated with a reduction in calcium concentration, thus inhibiting protein kinase C (PKC), as observed in leaf ethanol extract of J. gossypiifolia (Silva et al., 1995; Paes et al., 2012), decreasing cell proliferation (Alberts et al., 2010). Similar results were observed with inhibitors of PKC in A. cepa (Blume et al., 2008), Arabidopsis thaliana (Sheremet et al., 2010) and Nicotiana tabacum (Sheremet et al., 2012).
In the current study, most chromosome alterations (CA), such as chromosome adherences and C-metaphases, were considered a result of genotoxic effects, since they represent damage to the genetic material which is not necessarily fixed in the organism (Leme and Marin-Morales, 2009; Mazzeo et al., 2011). When these alterations can be repaired, they are not transmitted to descendant cells (Ventura-Camargo et al., 2011). The presence of chromo-some adherences and C-metaphases confirms the interference of phytochemicals of J. gossypiifolia latex in the assembly, stabilization and/or inactivation of spindle fibers, characterizing an aneugenic activity of the latex.
Aneugenic activities in metaphase may generate other types of cell abnormalities such as multipolar ana-phases, nuclear buds, lobulated nuclei, and polyploid cells (Fernandes et al., 2009). However, these alterations were not significant in the present study, what indicates that chromosome adherences and C-metaphases did not contribute to further alterations, and suggests a reversible mechanism for them (Odeigah et al., 1997).
Chromosome bridges, chromosome fragments and part of micronucleus formation are related to clastogenic activity (Fenech et al., 2011). Chromosome bridges and fragments, for instance, could be the result of chromosome breakage-fusion-bridge cycles, elucidated for the first time by Barbara McClintock in the 1930s in maize chromosomes (reviewed by Jones, 2005). However, chromosome fragments could also be induced by several factors involved in DNA breaks (Fenech, 2000). On the other hand, micronuclei formation could be induced by both aneugenic and clastogenic activities, related to entire chromosomes or chromosome fragments (respectively) not incorporated into the main nucleus during the cell cycle (Fenech et al., 2011). In the present results, from these three clastogenic alterations, only chromosome bridges had a significant increase.
The clastogenic alterations triggered by the latex indicate a chromosome mutagenic potential. This type of alteration represents damage to the genetic material at chromosome level that could not be repaired by the cell, being possibly transmitted to descendant cells (Grant, 1978). Furthermore, J. gossypiifolia latex caused toxicity and cytotoxicity at the tested concentrations, except for 1.25 mL/L. It also generated significant alterations in the assembly and stabilization of the mitotic spindle fibers, resulting in disturbance of the cell cycle and chromosome alterations for all concentrations. Based on these last results, it can be inferred that the mechanism of action of the latex also has aneugenic nature.
Although J. gossypiifolia is considered an important potential plant for the generation of pharmacological and/or biotechnological products (Félix-Silva et al., 2014), overall, the tests performed indicated toxicity, cytotoxicity and genotoxicity of J. gossypiifolia latex, including the concentrations acknowledged as antimicrobial (1.9 and 4.4 mL/L) (Patil et al., 2012). Considering that the latex of J. gossypiifolia is employed empirically in the popular medicine of many countries, and that the A. cepa test system presents good correlation with the tests carried out in mammals (Rank and Nielsen, 1994), it should be pointed out that it may potentially harm the human health especially if ingested. In addition, other studies with this plant material are necessary in order to elucidate the mechanisms of action of its bioactive compounds, and to reduce its toxicity while keeping its therapeutic action, for further use of isolated latex compounds in the pharmaceutical industry.
Acknowledgments
We are grateful to the Federal University of Pernambuco (UFPE) and the State University of Piauí (UESPI) for providing the installations and infrastructure for realization of the present study; to Dr. Francisco Soares Filho, for taxonomic identification of the botanic material (J. gossypiifolia); and to Dr. Fátima de Oliveira Pires for technical help with microscopy. This work was supported by fellowship within the Post-Graduation Promotion Program (PROF) from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil), and research grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and FACEPE (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco).
Footnotes
Associate Editor: Carlos F.M. Menck
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Biologia Molecular da Célula. 5ª edition. Artes Médicas Sul LTDA; São Paulo: 2010. 1396 [Google Scholar]
- Apu AS, Hossain F, Rizwan F, Bhuyan SH, Matin M, Jamaluddin ATM. Study of pharmacological activities of metanol extract of Jatropha gossypifolia fruits. J Basic Clin Pharm. 2013;4:20–24. doi: 10.4103/0976-0105.109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arekemase MO, Kayode RMO, Ajiboye AE. Antimicrobial activity and phytochemical analysis of Jatropha Curcas plant against some selected microorganisms. Int J Biol. 2011;3:52–59. [Google Scholar]
- Auvin-Guette C, Baraguey C, Blond A, Pousset JL, Bodo B. Cyclogossine B, a cyclic octapeptide from Jatropha gossypifolia . J Nat Prod. 1997;60:1155–1157. [Google Scholar]
- Ayres M, Ayres JRM, Ayres DL, Santos AS. BioEstat 5.3 - Aplicações Estatísticas nas Áreas das Ciências Biomédicas. Sociedade Civil Mamirauá; Belém: 2007. 364 [Google Scholar]
- Bagatini DM, Silva ACF, Tedesco SB. Uso do sistema teste de Allium cepa como bioindicador de genotoxicidade de infusões de plantas medicinais. Rev Bras Farmacogn. 2007;17:444–447. [Google Scholar]
- Bhagat R, Misar AV, Ambavade SD, Kulkarni DK. HPTLC analysis and anti-inflammatory activity of Jatropha gossypifolia L. root in mice and wistar rats. IJPR. 2013;3:13–17. [Google Scholar]
- Blume Y, Yemets A, Sulimenko V, Sulimenko T, Chan J, Lloyd C, Draber P. Evidence of tyrosine phosphorylation of plant tubulin. Planta. 2008;229:143–150. doi: 10.1007/s00425-008-0816-z. [DOI] [PubMed] [Google Scholar]
- Çelik TA, Aslantürk OS. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J Biomed Biotechnol. 2010;2010:1–8. doi: 10.1155/2010/189252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto LA. Índio: Manual de Saúde. Aldo Lo Curto; Canzo: 1993. 208 [Google Scholar]
- Devappa RK, Makkar HPS, Becker K. Jatropha diterpenes: A review. J Am Oil Chem Soc. 2011;88:301–322. [Google Scholar]
- Dhale DA, Birari R. Preliminary screening of antimicrobial and phytochemical studies of Jatropha gossypifolia L. Rec Res Sci Tech. 2010;2:24–28. [Google Scholar]
- Fachinetto JM, Bagatini MD, Durigon J, Silva ACF, Tedesco SB. Efeito anti-proliferativo das infusões de Achyrocline satureioides DC (Asteraceae) sobre o ciclo celular de Allium cepa . Rev Bras Farmacogn. 2007;17:49–54. [Google Scholar]
- Félix-Silva J, Giordani RB, Silva AA, Jr, Zucolotto SM, Pedrosa MFF. Jatropha gossypiifolia L. (Euphorbiaceae): A review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant. Evid Based Complement Alternat Med. 2014;2014:1–32. doi: 10.1155/2014/369204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa YH, Eastmond DA, Tucker JD, Thomas P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–132. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol. 2007;88:252–259. [Google Scholar]
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. Origin of nuclear and chromosomal alterations derived from the action of an aneugenic agent Trifluralin herbicide. Ecotox Environ Safe. 2009;72:1680–1686. doi: 10.1016/j.ecoenv.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Fiskesjo G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Gaikwad RS, Kakde RB, Kulkarni AU, Gaikwad DR, Panchal VH. In vitro antimicrobial activity of crude extracts of Jatropha species. Curr Bot. 2012;3:09–15. [Google Scholar]
- Grant WF. Chromosome aberrations in plants as a monitoring system. Environ Health Persp. 1978;27:37–43. doi: 10.1289/ehp.782737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsten SF, Berg AJVD, Bosch JJKVD, Leeflang BR, Labadie RP. Cyclogossine A: A novel cyclic heptapeptide isolated from the latex of Jatropha gossypifolia . Planta Med. 1996;62:46–50. doi: 10.1055/s-2006-957795. [DOI] [PubMed] [Google Scholar]
- Insanu M, Anggadiredja J, Kayser O. Curcacycline A and B - New pharmacological insights to an old drug. IJARNP. 2012;5:26–34. [Google Scholar]
- Jansen O, Angenot L, Tits M, Nicolas JP, Mol DP, Nikiema JB, Frederich M. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnopharmacol. 2010;130:143–150. doi: 10.1016/j.jep.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Jones RN. McClintock’s controlling elements: The full story. Cytogenet Genome Res. 2005;109:90–103. doi: 10.1159/000082387. [DOI] [PubMed] [Google Scholar]
- Leme DM, Marin-Morales MA. Chromosome aberration and micronucleus frequencies in Allium cepa cells exposed to petroleum polluted water - A case study. Mutat Res. 2008;650:80–86. doi: 10.1016/j.mrgentox.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Leme DM, Marin-Morales MA. Allium cepa test in environmental monitoring: A review on its application. Mutat Res. 2009;7934:1–11. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Matos FJA. Constituintes químicos ativos e propriedades biológicas de plantas medicinais brasileiras. Editora UFC; Fortaleza: 2004. 448 [Google Scholar]
- Mazzeo DEC, Fernandes TCC, Marin-Morales MA. Cellular damages in the Allium cepa test system, caused by BTEX mixture prior and after biodegradation process. Chemosphere. 2011;85:13–18. doi: 10.1016/j.chemosphere.2011.06.056. [DOI] [PubMed] [Google Scholar]
- Mongkolvisut W, Sutthivaiyakit S, Leutbecher H, Mika S, Klaiber I, Moller W, Rosner H, Beifuss U, Conrad J. Integerrimides A and B, cyclic heptapeptides from the latex of Jatropha integerrima . J Nat Prod. 2006;69:1435–1441. doi: 10.1021/np0602012. [DOI] [PubMed] [Google Scholar]
- Nazeema TH, Girija S. Characterisation of the active antiproliferative principles of Jatropha curcus and Jatropha gossippifolia on Hela cell lines. Int J Pharm Pharm Sci. 2013;5:346–355. [Google Scholar]
- Nunes LG, Gontijo DC, Souza CJA, Fietto LG, Carvalho AF, Leite JPV. The mutagenic, DNA-damaging and anti-oxidative properties of bark and leaf extracts from Coutarea hexandra (Jacq.) K. Schum. Environ Toxicol Pharmacol. 2012;3:297–303. doi: 10.1016/j.etap.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Odeigah PGC, Nurudeen O, Amund OO. Genotoxicity of oil field wastewater in Nigeria. Hereditas. 1997;126:161–167. [Google Scholar]
- Oduola T, Popoola GB, Avwioro OG, Oduola TA, Ademosun AA, Lawal MO. Use of Jatropha gossypifolia latex as a haemostatic agent: How safe is it? J Med Plants Res. 2007;1:14–17. [Google Scholar]
- Oliveira FCS, Barros RFM, Neto MJM. Plantas medicinais utilizadas em comunidades rurais de Oeiras, semi-árido piauiense. Ver Bras Pl Med. 2010;12:282–301. [Google Scholar]
- Paes AMA, Camara AL, Freire SMF, Borges MOR. Relaxant effect of Jatropha gossypiifolia L. on uterine smooth muscle. International Journal of phytomedicine. 2012;4:310–313. [Google Scholar]
- Parente CET, Rosa MMT. Plantas comercializadas como medicinais no Município de Barra do Piraí, RJ. Rodriguésia. 2001;52:47–59. [Google Scholar]
- Patil SV, Borase HP, Patil CD, Salunke BK. Bio-synthesis of silver nanoparticles using latex from few Euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol. 2012;167:776–790. doi: 10.1007/s12010-012-9710-z. [DOI] [PubMed] [Google Scholar]
- Pawlowski Â, Kaltchuk-Santos E, Zini CA, Caramão EB, Sudhakar GLGS, Gowda R, Venu NG. Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): Mitodepressive and aneugenic inducers in onion and lettuce root meristems. S Afr J Bot. 2012;80:96–103. [Google Scholar]
- Ping KY, Darah I, Yusuf UK, Yeng C, Sasidharan S. Genotoxicity of Euphorbia hirta: An Allium cepa Assay. Molecules. 2012;17:7782–7791. doi: 10.3390/molecules17077782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap B, Singh A. Piscicidal and anti AChE activity of medicinal plant Jatropha gossypifolia (Family-Euphorbiaceae) World J Fish and Marine Sci. 2013;5:367–372. [Google Scholar]
- Punjaji SA. Traditional oral healthcare practices in pathardi areas of Ahmednagar district, Maharashtra, India. Environ Pharmacol Life Sci. 2012;1:84–88. [Google Scholar]
- Rank J, Nielsen MH. Evaluation of the Allium ana-phase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat Res. 1994;312:17–24. doi: 10.1016/0165-1161(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Ray S, Kundu LM, Goswami S, Roy GC, Chatterjee S, Dutta S, Chaudhuri A, Chakrabarti CS. Metaphase arrest and delay in cell cycle kinetics of root apical meristems and mouse bone marrow cells treated with leaf aqueous extract of Clerodendrum viscosum Vent. Cell Prolif. 2013;46:109–117. doi: 10.1111/cpr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabandar CW, Ahmat N, Jaafar FM, Sahidin I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry. 2013;85:7–29. doi: 10.1016/j.phytochem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Sakai R, Rinehart KL, Kishore V, Kundu B, Faircloth G, Gloer JB, Carney JR, Namikoshi M, Sun F, Hughes RG, et al. Structure and activity relationships of the didemnins1,2. J Med Chem. 1996;39:2819–2834. doi: 10.1021/jm960048g. [DOI] [PubMed] [Google Scholar]
- Sheremet YA, Emets AI, Azmib A, Vissenberg K, Verbelen JP, Blume YB. Effect of serine/threonine protein kinases and protein phosphatases inhibitors on mitosis progression in a synchronized tobacco BY-2 culture. Cytol Genet. 2012;46:89–95. [Google Scholar]
- Sheremet YA, Yemets AI, Vissenberg K, Verbelen JP, Blume YB. Effects of inhibitors of serine/threonine protein kinases on Arabidopsis thaliana root morphology and microtubule organization in its cells. Cell Tissue Biol. 2010;4:399–409. [PubMed] [Google Scholar]
- Silva AM, Brum RL, Calixto JB. The relaxant action of jatrophone in rat portal vein. A comparison with protein kinase C inhibitors. Life Sci. 1995;57:863–871. doi: 10.1016/0024-3205(95)02019-f. [DOI] [PubMed] [Google Scholar]
- Silva FAS, Azevedo CAV. Versão do programa computacional Assistat para o sistema operacional Windows. Revista Brasileira de Produtos Agroindustriais. 2002;4:71–78. [Google Scholar]
- Singh P, Singh A. Acute toxic effects of medicinal plant Jatropha gossypifolia on non- target fish and mice. Wudpecker J Agric Res. 2012;1:433–438. [Google Scholar]
- Stansley W. Field results using cholinesterase reactivation techniques to diagnose acute anticholinesterase poisoning in birds and fish. Arch Environ Com Tox. 1993;25:315–321. [Google Scholar]
- Stasi DLC, Hiruma-Lima CA. Plantas Medicinais na Amazônia e na Mata Atlântica. 2 edition. Editora da UNESP; São Paulo: 2002. 604 [Google Scholar]
- Valencia A, Frérot B, Guénego H, Múnera DF, Sá MFGD, Calatayud PA. Effect of Jatropha gossypiifolia leaf extracts on three lepidoptera species. Rev Colomb Entomol. 2006;32:45–48. [Google Scholar]
- Ventura-Camargo BC, Maltempi PPP, Marin-Morales MA. The use of the cytogenetic to identify mechanisms of action of an azo dye in Allium Cepa meristematic cells. J Environment Analytic Toxicol. 2011;1:1–12. [Google Scholar]
- Wink M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules. 2012;17:12771–12791. doi: 10.3390/molecules171112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Lu Y, Zheng QT, Tan NH, Li CM, Zhou J. Study on the spatial structure of annomuricatin A, a cyclohexa-peptide from the seeds of Annona muricata . J Mol Struct. 2007;827:145–148. [Google Scholar]
Internet Resources
- Cordeiro I, Secco R. Jatropha in Lista de Espécies da Flora do Brasil. 2014 Jardim Botânico do Rio de Janeiro. http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB17581 (Accessed on March 20, 2014). [Google Scholar]