Abstract
Carboranyl-containing chlorins have emerged as promising dual sensitizers for use in both photodynamic therapy (PDT) and boron neutron capture therapy (BNCT), by virtue of their known tumor affinity, low cytotoxicity in dark conditions, and their strong absorptions in the red region of the optical spectrum. Tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC) is a new synthetic carboranyl-containing chlorin of high boron content (24% by weight). To evaluate TPFC’s applicability as sensitizer for both PDT and BNCT, we performed an in vitro and in vivo study using F98 rat glioma cells and F98 rat glioma-bearing brain tumor models. For the in vivo BNCT study, we used boronophenylalanine (BPA), which is currently used in clinical BNCT studies, via intravenous administration (i.v.) and/or used TPFC via convection-enhanced delivery (CED), a method for local drug infusion directly into the brain. In the in vitro PDT study, the cell surviving fraction following laser irradiation (9 J/cm2) was 0.035 whereas in the in vitro BNCT study, the cell surviving fraction following neutron irradiation (thermal neutron = 1.73 × 1012 n/cm2) was 0.04. In the in vivo BNCT study, the median survival time following concomitant administration of BPA (i.v.) and TPFC (CED) was 42 days (95% confidence interval; 37–43 days).
Keywords: absorption, blood–brain barrier, BNCT, boron-containing chlorin, CED, cell culture, CNS, osmotic pumps, PDT, TPFC
INTRODUCTION
The prognosis of patients with malignant glioma, especially glioblastoma (GB), is poor. The median survival of GB patients is less than 2 years after the initial diagnosis,1 with most recurrence occurring at the site of the original tumor. Therefore, more aggressive local therapies are necessary to eradicate unresectable tumor cells that invade adjacent normal brain tissue. Two adjuvant therapies with the potential to destroy these unresectable tumor cells are photodynamic therapy (PDT)2,3 and boron neutron capture therapy (BNCT).4–7 PDT and BNCT are two binary modalities for malignant glioma treatment that rely on the selective accumulation of a sensitizer within tumor tissue, followed by its activation upon irradiation with either red light (in PDT) or low-energy neutrons (in BNCT). The combination of PDT and BNCT using a single drug has several advantages, including increased therapeutic effect due to the targeting of different cellular components and/or mechanisms of tumor cell destruction.
Boronated porphyrins and their derivatives, in particular dihydroporphyrins or chlorins, are promising dual PDT and BNCT sensitizers because they usually have low dark toxicity, preferentially accumulate within tumor tissues and persist there for a considerable amount of time.8–10 Moreover, their strong absorption of light wavelengths in the red region (~650 nm), which is characterized by the power of deeper penetration into most human tissues,11 makes them ideal candidates for in vivo phototherapeutic applications;12 on the other hand, their high boron content enhances the efficiency of their interaction with thermal neutrons. In 2008, a new carboranyl-containing chlorin, designated TPFC [tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC)], containing four boron clusters (24% boron by weight), was reported by our collaborator, Vicente and coworkers.13 This report showed that TPFC was found to be non-toxic in the dark but showed extensive photosensitizing ability both in vitro and in vivo despite its relatively low singlet oxygen quantum yield. In particular, TPFC exhibited significant photosensitizing activity against highly pigmented melanotic melanoma tumors in mice.
In our study, we evaluated the potential of TPFC as a dual sensitizer for both PDT and BNCT using F98 rat glioma cells and F98 rat glioma-bearing brain tumor models. Although several carboranyl-containing chlorins and their derivatives have been reported as dual sensitizers for both PDT and BNCT,13–15 in most of these studies, in vivo investigations were not conducted. Additionally, there were no reports of in vivo neutron irradiation study using a carboranyl-containing chlorin administered by convection-enhanced delivery (CED), a method for local drug infusion directly into the brain. This study is a first report of an in vivo neutron irradiation study using a new carboranyl-containing chlorin (TPFC) administered by CED.
MATERIALS AND METHODS
Boron Compounds
The TPFC was prepared as previously described.13 TPFC was dissolved in dimethylsulfoxide (DMSO); the final DMSO concentrations never exceeded 1%. Boronophenylalanine (BPA) (L-isomer), which is currently used in clinical BNCT studies, was kindly supplied by the Stella Chemifa Corporation (Osaka, Japan) and was prepared as a fructose complex.16
Cell Culture
F98 rat glioma cells produce infiltrating tumors in the brains of Fischer rats.17 The tumors have been shown to be refractory to a number of treatment modalities, including radiation therapy.18 Based on their in vivo histology, the F98 rat glioma cells have been characterized as anaplastic or undifferentiated glioma.19 In the present study, F98 rat glioma cells were kindly obtained from Dr. Barth (Department of Pathology, Ohio State University, Columbus, Ohio). They were routinely cultivated in our laboratory in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and penicillin at 37°C in an atmosphere of 5% CO2. All the materials for the culture medium were purchased from Gibco Invitrogen (Grand Island, New York).
In Vitro Cellular Uptake Study
In order to investigate the ability of TPFC for delivery of boron intracellularly, a cellular uptake was conducted, in which the cells were exposed to 10 μg 10B/mL of TPFC or BPA for 2.5, 6, 12, and 18 h. First, F98 rat glioma cells were seeded in 100 mm dishes (BD Falcon™, Franklin Lakes, New Jersey), and the culture medium was exchanged for boron compounds-containing (TPFC, BPA) culture medium just before confluence. In this study, three 100 mm dishes for each designated time were used. After the completion of exposure, boron compounds-containing culture medium was removed, and then the cells were washed twice with 4°C phosphate-buffered saline (PBS) and detached with trypsin-ethylenediamine tetraacetic acid solution. Medium was then added, and the cells countered and sedimented (centrifugation: 214.2 G-force for 5 min). Cells were digested overnight with 1 N nitric acid solution, and boron uptake was determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) using an iCAP6000 emission spectrometer (Hitachi High-Technologies, Tokyo, Japan). PBS and trypsin-ethylenediamine tetraacetic acid solution were purchased from Gibco Invitrogen, and the nitric acid solution was purchased from Wako Pure Chemical Industries (Osaka, Japan).
In Vitro PDT Study
F98 rat glioma cells were incubated in culture media without TPFC and with two different doses of TPFC (2.5 and 5 μg 10B/mL) for 18 h in 150 cm2 flasks (TPPeq\0\ac(o,R), Trasadingen, Switzerland). Following incubation, the cells were retrieved from the flasks, seeded onto 60 mm dishes (BD Falcon™) with 104 cells each and irradiated with visible light of 405 nm from a diode laser (Ball Semiconductor, Frisco, Texas). The cells were evenly irradiated at powers of 0, 4.5, 9, and 18 J/cm2. Following laser irradiation, the cells were seeded onto dishes, each with the same pre-determined number of cells, iteratively. All cells were cultured for 7 days, then fixed in methanol and stained with tripan blue. Colonies composed of more than 50 cells were counted and assessed by calculating the cell surviving fraction (= colony-forming assays).
In Vitro BNCT Study
F98 rat glioma cells were transferred to the atomic reactor (KUR; Kyoto University Research Reactor) and incubated with the only culture medium (= control) and 10 μg 10B/mL of TPFC or BPA-containing culture medium for 24 h. The cells were washed twice with PBS, detached with trypsin-ethylenediamine tetraacetic acid solution, and centrifuged at 1500 rpm for 5 min. The culture medium was then added to 1 mL of the cell suspension at a density of 1 × 106 cells/mL. Both types of cells were irradiated at KUR with reactor thermal neutron beams of 9.63 × 108 (n/cm2·s) for 0, 10, 20, 30 min at 1.0 MW reactor power. After neutron irradiation, colony-forming assays were performed as described above.
Tumor Model
All male Fischer rats (200–250 mg, F344 NSlc; Japan SLC, Shizuoka, Japan) were anesthetized with an intraperitoneal injection of Nembutal (50 mg/kg) and placed in a stereotactic frame (Model 900; David Kopf Instruments, Tujunga, California). A midline scalp incision was made and the bregma was identified. A 1 mm burr hole was made in the right frontal region of the skull and a 22-gauge needle attached to a 25 μL syringe was inserted into the caudate nucleus using the same stereotactic coordinates, with the needle tip inserted 5 mm into the dura. An injection of 105 cells in 10 μL of serum free medium was administered at a rate of 1 μL/min. After the infusion, the needle was left in place for 3 min and the burr hole was then covered with bone wax and the scalp was sutured.
Convection-Enhanced Delivery
Convection-enhanced delivery, a method for local drug infusion directly into the brain,20 enables the distribution of any drugs homogeneously in the brain, keeping high concentration at the target site without mechanical damage to the surrounding normal tissue. CED depends on the bulk flow in the interstitial space produced by continuous slow infusion into the brain under low positive pressure. Using this method, it is theoretically possible to deliver drugs to regions where tumor cells invade microscopically with little accumulation in the blood and other organs. Moreover, because it does not depend on the molecular weight of the infused agent, this method allows the selection of many kinds of therapeutic agents and/or carriers, such as liposomes, dendrimers, and nanotubes.21–25 Clinical trials of targeted toxins with epidermal growth factor receptor, transferrin receptor, interleukin-13 receptor, and interleukin-4 receptor are reported in the leterature.22,26–29
In Vivo Biodistribution Study
For the biodistribution study, 10 days after tumor implantation, F98 rat glioma-bearing brain tumor models were generally anesthetized, placed in a stereotactic frame as described above, shaved and washed the skin over the rat’s scalp and back. The midline scalp incision and a skull burr hole that were made when tumor models were made were re-opened and 5 mm-long cannula (ALZET Brain Infusion Kit 2; DURECT Corporation, Cupertino, California) were inserted into the burr hole through the skull. A mid-scapular incision was made, by spreading the subcutaneous tissue of the back to create a pocket for the infusion pump (ALZET Osmotic Pumps 2001D; DURECT Corporation), to be inserted. This infusion pump can administer compounds to animals over 24 h at a rate of 8 μL/h. After the insertion of the infusion pump, a catheter leading to the brain cannula was attached to the subcutaneous pocket. Finally, the midline scalp incision and the mid-scapular incision were closed. The total amounts of TPFC administered via CED to each group of rats were 0.125, 0.25, and 0.5 mg per rat. We then assayed the 10B concentration of tumor, blood, normal brain, liver, spleen, kidney, heart, lung, skin, and muscle by ICP-AES. In this study, we sampled normal tissues from both hemispheres and defined the normal tissue from the tumor-bearing hemisphere as ipsilateral normal brain and the normal tissue from the other hemisphere as contralateral normal brain. The normal brain of the ipsilateral side, at least a couple of millimeters away from the tumor border, was sampled with approximately 1 g for 10B content measurement. Similarly, the normal brain of the contralateral side, symmetric relative to the ipsilateral sampling, was also sampled for the boron measurement. Normal brain for the tumor-to-normal brain ratio (= T/N) determinations used normal brain from the contralateral side.
In Vivo BNCT Study
For the therapy study, neutron irradiation was performed in an atomic reactor (KUR) 14 days after the implantation of 103 F98 rat glioma cells. The radiation field delivered to the tissue during irradiation was composed mainly of thermal neutrons (<0.53 eV), gamma photons, and heavy-charged particles (4He, 7Li, 1H, and 14C) from the 10B (n, alpha)7Li capture reaction and the 14N(n, p)14C reaction with normal tissue nitrogen. The fast neutron level (>10,000 eV) was very low and therefore ignored. The thermal neutron flux was measured using 3 mm long gold wires placed on the surface of tumors. The average of the thermal neutron flux was 6.08 × 108 (n/cm2/s) and the thermal neutron fluence became 2.19 × 1012 n/cm2 for 60 min irradiation. The equivalent dose was calculated using the equations below.30
Equivalent dose (Gy-Eq) = DB × CBEB + DN × RBEN + Dγ × hours
Gy-Eq refers to a biologically equivalent X-ray dose that can give radiation effects totally to the tissue, DB (boron dose) = 7.43 × 10−14 × boron concentration (μg10B/g) × Φ thermal neutron fluence (Gy),DN (nitrogen dose) = 6.78 × 10−14 × nitrogen concentration (weight%) ×Φ thermal neutron fluence (Gy), Dγ (gamma-ray dose) = 0.83 Gy/h, RBEN is the RBE (relative biological effectiveness) for DN and it equals 2.5, CBEB is the CBE (compound biological effectiveness) for DB and, in the case of BPA, this value is 3.8 for the tumor tissue and 1.35 for the normal brain.31–33 The nitrogen concentration (%) is estimated as 4% and the RBE for Dγis 1.0.
Fischer rats were transported to the KUR, randomized at 14 days after implantation, and divided into five experimental groups composed of 6–10 animals each. These groups included untreated control and irradiated control animals without boron compounds. The other three groups were composed of TPFC administered by CED, BPA administered by intravenous injection, and a combination of TPFC and BPA group. Two and a half hours after intravenous administration of BPA (250 mg/kg b.w.) and/or twelve hours after termination of CED using TPFC (0.25 mg/rat), neutron irradiation was initiated. All irradiated rats were anesthetized and put in a whole body stabilizer with their heads placed out of the shield. The heads were irradiated at a reactor power of 1.0 MW for 1 h. After neutron irradiation, the neutron irradiated animals and the neutron unirradiated animals remained at KUR for observation. We checked the survival time of all rats.
In Vitro/In Vivo Fluorescence Microscopy
For the in vitro fluorescence microscopy study, F98 rat glioma cells were seeded in a two-well chamber mounted on glass slides with a cover (Thermo Fisher Scientific Inc., Rochester, NY), and the culture medium without TPFC was exchanged for TPFC-containing culture medium just before confluence. The cells were exposed to 20 μg 10B/mL of TPFC for 24 h. After exposure, the glass slides were washed with 4°C PBS and the two-well chamber was removed. The nuclei specific Hoechest dye (Hoechst 33342; Lonza) was added (10 μg/mL) and the glass slides mounted onto cover glasses using DPX Mountant for histology (Fluka Biochemika 44581, Germany). The two-well chamber slides were observed using an inverted fluorescence microscope system (BZ-8000; KEYENCE, Japan).
The in vivo fluorescence microscopy study was conducted twenty four hours after termination of CED administration of TPFC to F98 rat glioma-bearing brain tumor model. The rats were euthanized and strip preparations of the brains were made. Similar to the in vitro fluorescence microscopy study, the strip preparations of brain were observed using an inverted fluorescence microscope system (BZ-8000; KEYENCE).
Statistical Analysis
Values are presented as means ± SD. Statistical analysis was performed by the Kaplan Meier method using JMP® Pro 10 (SAS Institute Inc., Cary, North Carolina).
RESULTS
In Vitro Cellular Uptake Study
The ICP-AES measured cellular boron concentrations obtained in the in vitro cellular delivery study using TPFC were 40.8 ± 1.8, 47.4 ± 2.4, 65.4 ± 4.7, and 67.4 ± 1.9 ng10B/106 cells after 2.5, 6, 12, or 18 h of exposure, respectively. On the other hand, the values obtained using BPA under the same conditions were 17.9 ± 1.4, 17.6 ± 1.6, 25.8 ± 1.3, and 36.7 ± 3.5 ng10B/106 cells after 2.5, 6, 12, or 18 h of exposure, respectively. These results are shown in Figure 1.
Figure 1.
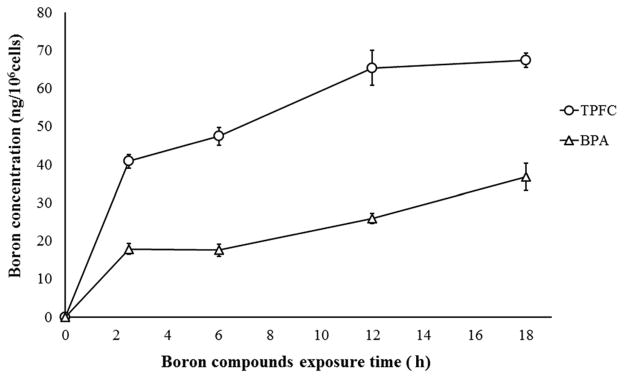
TPFC’s cellular uptake of boron (ng10B/106 cells) was about twice as high as BPA’s cellular uptake of boron on each time.
In Vitro PDT Study
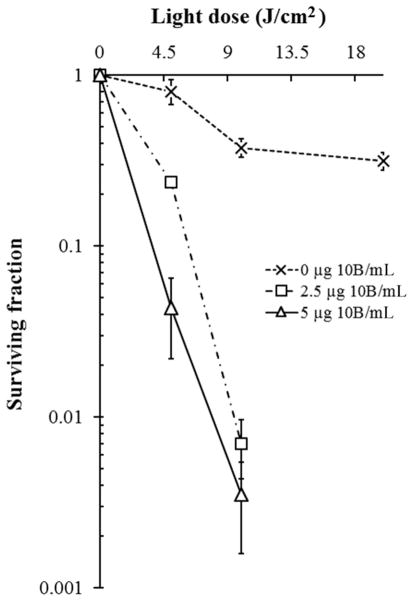
The cytotoxicity of TPFC determined by laser irradiation using a colony-forming assay showed the surviving fraction of cells following exposure to TPFC (0, 2.5, and 5 μg 10B/mL for 18 h) and laser irradiation to be: 0.238 ± 0.012, 0.007 ± 0.003, and unmeasurable value (for TPFC = 2.5 μg 10B/mL for 18 h), and 0.043 ± 0.021, 0.004 ± 0.002, and unmeasurable value (for TPFC = 5 μg 10B/mL for 18 h), using 4.5, 9, and 18 J/cm2 light dose, respectively. The unmeasurable value means no colonies on dishes (<0.000) owing to the high photocytotoxicity of TPFC under the experimental conditions. The surviving fractions for the laser-only control (without TPFC) were 0.805 ± 0.135, 0.377 ± 0.046, and 0.315 ± 0.036, using 4.5, 9, and 18 J/cm2 light dose, respectively. These results are shown in Figure 2.
Figure 2.
Colony-forming assay using F98 rat glioma cells exposed to 0 (control), 2.5, and 5 μg 10B/mL of TPFC and irradiated with light doses of 0, 4.5, 9, and 18 J/cm2, respectively. The most efficient PDT-induced tumoricidal effect was achieved when the cells were irradiated with 9 J/cm2, 18 h after exposure to 5 μg 10B/mL of TPFC (less than 0.05).
In Vitro BNCT Study
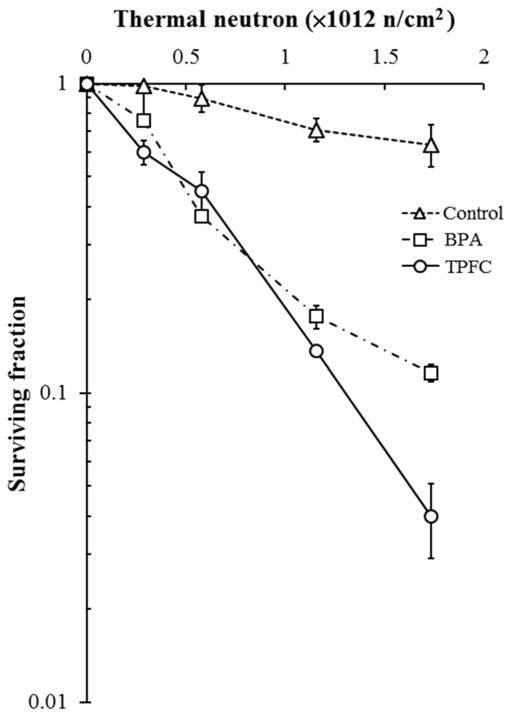
The cytotoxicity of TPFC upon neutron irradiation was also evaluated with a colony forming assay, performed on F98 rat glioma cells 7 days after irradiation with the reactor thermal neutron beam. The results are shown in Figure 3. For the control groups exposed to the neutron beam without boron compounds (BPA or TPFC), the surviving fraction decreased only slightly, with increasing thermal neutron fluence, with determined values of 1, 0.98, 0.90, 0.71, and 0.64 for each neutron fluence of 0, 0.289, 0.578, 1.156, and 1.733 × 1012 n/cm2, respectively. On the other hand, the survival ratios of tumor cells treated with BPA or TPFC decreased exponentially with the increase of neutron fluence. The cytotoxicity of BNCT was especially significant in the TPFC group, with the determined cell surviving fraction following thermal neutron for 30 minutes of 0.04.
Figure 3.
F98 rat glioma cells were exposed to culture medium without boron compound (= control), with 10 μg 10B/mL of BPA or TPFC for 24 h. Then they were irradiated with thermal neutron, respectively. BPA’s cell surviving fraction following thermal neutron for 30 min was 0.12. On the other hand, TPFC’s cell surviving fraction following thermal neutron for 30 min was 0.04.
In Vivo Biodistribution Study
The boron concentrations following CED of TPFC to the F98 rat glioma-bearing brain tumor models at 24 h after termination of CED were also observed at different TPFC doses (0.125, 0.25, and 0.5 mg TPFC/rat). The tumor boron concentrations showed the highest concentrations at a dose of 0.25 mg TPFC/rat; the boron concentrations at a dose of 0.125, 0.25, and 0.5 mg TPFC/rat were 22.69 ± 1.79, 40.35 ± 11.78, and 29.56 ± 10.44 μg 10B/g, respectively. Low concentrations were observed in the ipsilateral brain, the contralateral brain, and the blood at the different TPFC doses: 0.21 ± 0.16, 2.01 ± 1.30, and 0.23 ± 0.35 in the ipsilateral brain, 0.05 ± 0.03, 0.97 ± 0.40, and 0.14 ± 0.23 in the contralateral brain, and 0.82 ± 0.61, 0.46 ± 0.19, and 0.26 ± 0.01 in blood, respectively. Tumor/normal brain ratio and tumor/blood ratio of boron at the different TPFC doses were 454, 42, and 211 for the tumor/normal brain ratio and 28, 88, and 114 for the tumor/blood ratio, respectively.
Table 1 summarizes the biodistribution data (tumor, ipsilateral brain, contralateral brain, and blood) for TPFC administered by CED. We first focused our efforts on the differences corresponding to the time after termination of CED of TPFC up to 48 h. The boron concentrations in F98 tumor nodules were 13.02 ± 0.78, 22.69 ± 1.79, and 9.75 ± 3.96 μg 10B/g at 0, 24, and 48 h after termination of CED of 0.125 mg/rat TPFC, respectively. The boron concentrations in the ipsilateral brain, the contralateral brain, and the blood also increased up to 24 h after termination of CED and decreased at 48 h after termination of CED. The corresponding tumor/normal brain ratios for the three different times were 186, 454, and 975, respectively.
Table 1.
Boron Concentrations in Brain Tumors in Rats Bearing F98 Gliomas to which TPFC was Administered by Convection-Enhanced Delivery
| TPFC Concentration | Time (h)a | Boron concentration (μg/mL)
|
Ratio
|
||||
|---|---|---|---|---|---|---|---|
| Tumor | Ipsilateral Brain | Contralateral Brain | Blood | T/Nb | T/Bc | ||
| 0.125 mg TPFC/rat (N = 11) | O (N = 3) | 13.02 ± 0.78 | 0.09 ± 0.02 | 0.07 ± 0.01 | 1.13 ± 0.20 | 186 | 12 |
| 24 (N = 5) | 22.69 ± 1.79 | 0.21 ± 0.16 | 0.05 ± 0.03 | 0.82 ± 0.61 | 454 | 28 | |
| 48 (N = 3) | 9.75 ± 3.96 | 0.05 ± 0.01 | 0.01 ± 0.01 | 0.33 ± 0.04 | 975 | 30 | |
| 0.25 mg TPFC/rat (N = 3) | 24 | 40.35 ± 11.78 | 2.01 ± 1.30 | 0.97 ± 0.40 | 0.46 ± 0.19 | 42 | 88 |
| 0.5 mg TPFC/rat (N = 3) | 24 | 29.56 ± 10.44 | 0.23 ± 0.35 | 0.14 ± 0.23 | 0.26 ± 0.01 | 211 | 114 |
Hours after termination of convection-enhanced delivery of TPFC.
T/N is tumor/normal brain (ipsilateral brain).
T/B is tumor/blood.
Table 2 summarizes the biodistribution data (liver, spleen, kidney, heart, lung, skin, and muscle) obtained for TPFC administered by CED. The boron concentrations of all organs showed very low dose.
Table 2.
Boron Concentrations in Organs Other than Brains in Rats Bearing F98 Gliomas to which TPFC was Administered by Convection-Enhanced Delivery
| TPFC Concentration | Time (h)a | Boron Concentration (μg/mL)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Liver | Spleen | Kidney | Heart | Lung | Skin | Muscle | ||
| 0.125 mg TPFC/rat (N = 8) | O (N = 3) | 0.20 ± 0.11 | 0.52 ± 0.13 | 0.16 ± 0.08 | 0.11 ± 0.05 | 0.19 ± 0.12 | 0.04 ± 0.06 | 0.01 ± 0.02 |
| 24 (N = 5) | 0.19 ± 0.03 | 0.61 ± 0.42 | 0.24 ± 0.04 | 0.21 ± 0.01 | 0.40 ± 0.11 | 0.08 ± 0.06 | 0.03 ± 0.03 | |
Hours after termination of convection-enhanced delivery of TPFC.
In Vivo BNCT Study
Median survival times of control groups (untreated and irradiated groups), a TPFC administered by CED group, a BPA administered by intravenous injection group and a combination of TPFC and BPA group were 25 days [95% confidence interval (CI); 22–28 days], 26 days (95% CI; 26–33 days), 31 days (95% CI; 27–42 days), 36 days (95% CI; 30–44 days), and 42 days (95% CI; 37–43 days), respectively. There is no over-lap in 95% CI between TPFC group and untreated group/ irradiated group. There is over-lap in 95% CI between a TPFC group and a BPA group and a combination group. These results are shown in Figure 4.
Figure 4.
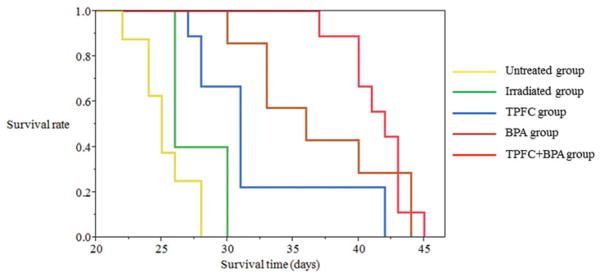
Median survival times of untreated group, irradiated group, TPFC group, BPA group, and TPFC + BPA group were 25 days (95% CI; 22–28 days), 26 days (95% CI; 26–30 days), 31 days (95% CI; 27–42 days), 36 days (95% CI; 30–44 days), and 42 days (95% CI; 37–43 days), respectively (yellow, green, blue, brown, and red line indicated untreated, irradiated, TPFC, BPA, and TPFC and BPA group, respectively.). It was significantly between TPFC group and TPFC + BPA group (p value = 0.007 by Log-rank test and 0.004 by Wilcoxon test). However, it was no significantly between BPA group and TPFC + BPA group (p value = 0.350 by Log-rank test and 0.133 by Wilcoxon test). And it was no significantly between TPFC group and BPA group (p value = 0.110 by Log-rank test and 0.063 by Wilcoxon test).
In Vitro/In Vivo Fluorescence Microscopy
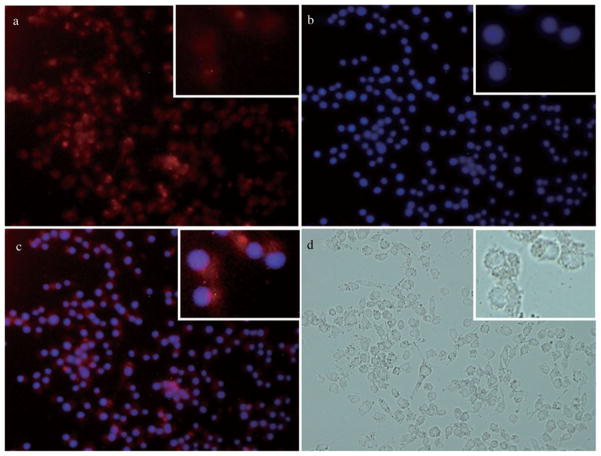
In vitro fluorescence microscopy showed the intracellular chlorin fluorescence (Fig. 5a) and the images observed from the co-localization experiment using the nucleus-specific Hochest dye (Fig. 5b). These results show that TPFC was taken up into the cells and also localized in the nuclei (Fig. 5c). In vivo fluorescence microscopy also showed fluorescence of tumor, as shown in Figure 6.
Figure 5.
(a) Fluorescence of chlorin (TPFC), (b) nuclear fluorescence by the Hoechst dye (excitation wavelength was 340–380 nm), (c) merged image (magnification of all images: ×400), and (d) bright field image.
Figure 6.
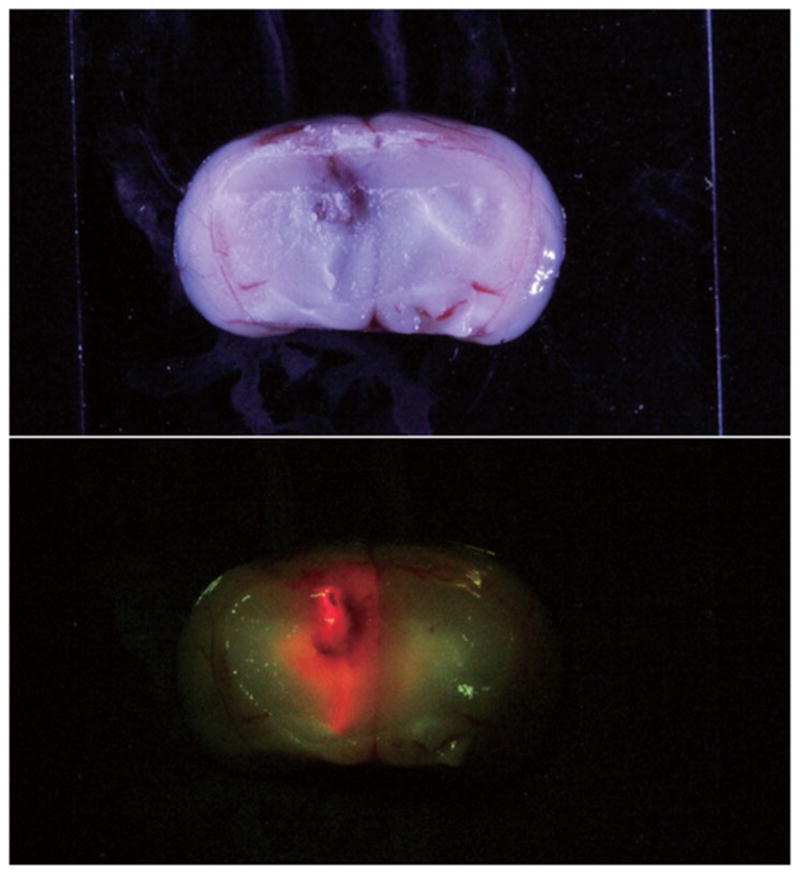
In vivo fluorescence microscopy also showed fluorescence of tumor. Red fluorescence indicated the tumor and green fluorescence indicated the normal brain.
DISCUSSION
PDT is a localized therapy that relies on the specific uptake of a photosensitizer in the tumor relative to the surrounding normal tissue, followed by laser irradiation for activation of the photosensitizer.12 The photoactivation of the sensitizer causes oxidative damage to a variety of cellular targets via the release of singlet oxygen and other reactive oxygen species, with subsequent tumor necrosis. To date, the clinical trials with PDT employed as an adjuvant treatment for human gliomas have used the poorly defined heterogeneous porphyrin mixture hematoporphyrin derivative (HpD) or its more enriched commercial preparation, Photofrin,2 which does not contain boron. These compounds have several draw backs. The most significant are cutaneous photosensitivity lasting up to 8 weeks following treatment,34–36 they are a complex mixture of porphyrins which makes accurate study difficult,37 and the wavelength of light absorption is not optimal for tissue penetration.38,39 Chlorin photosensitizers show great promise for use in PDT.40–45 They have enhanced molar extinction and electronic absorption peaks at longer wavelengths than HpD, allowing for both increased tissue penetration and enhanced tumor photodestruction.40–42 Furthermore, chlorins do not appear to cause severe cutaneous photosensitization after 24 h.41–43 In 2008, a new carboranyl-containing chlorin (TPFC) was reported.13 As TPFC is a boron-containing chlorin of high boron content (24% by weight), it is a promising dual sensitizer for the PDT and BNCT of gliomas.
In a similar fashion to PDT, BNCT is a targeted chemo-radiation therapy that significantly increases the therapeutic ratio relative to conventional radiotherapeutic modalities. In BNCT, a 10B-labeled compound delivers therapeutic concentrations of 10B (~30 μg 10B/g tumor) to the target tumor, with high tumor-to-blood and tumor-to-normal-tissue ratios and low cytotoxicity.4,5 Subsequently the tumor is irradiated with epithermal neutrons that become thermalized at a certain depth within the tissues. The short range (<10 μm) of the α and 7Li high linear energy transfer (high-LET) particles released from the 10B(n,α)7Li neutron capture reaction makes the tumor microdistribution of 10B critically important in BNCT. As the high-LET particles are highly cytotoxic, their killing effect depends on the site of generation. These characteristics contribute to the tumor selectivity and strong tumoricidal activity of BNCT, with negligible damage to normal tissue. Therefore, if sufficient quantities of boron can be selectively delivered to tumor tissues, BNCT could be an ideal tumor-selective particle beam irradiation local therapy for malignant gliomas. Clinically, BPA and/or sodium borocaptate (BSH) are currently available for BNCT as boron delivery agents. BPA is a boronated derivative of an essential amino acid (L-phenylalanine) that is actively taken up by tumor cells, presumably via the amino acid transport mechanism. BSH, on the other hand, is believed to preferentially accumulate within tumor tissue via a partially destroyed or leaky blood brain barrier. We have used both of these boron delivery agents in combination in clinical BNCT studies, and have previously reported on the survival benefit from BNCT for newly diagnosed GB patients6 as well as for recurrent malignant glioma patients.7 However, the present results using BPA and/or BSH are far from satisfactory, and the use of more effective boron delivery agents should provide enhanced clinical outcomes for BNCT. The so-called third-generation of boron delivery agents,5 including boronated porphyrin derivatives, carboranyl-containing chlorins, molecular-targeted agents (e.g., to EGFR), and liposome-linked boron delivery agents could potentially greatly increase the efficacy of BNCT. Among these boron delivery agents, carboranyl-containing chlorin TPFC is particularly promising because of its high boron content compared with other reported boron-containing chlorins, and also because TPFC has shown high efficacy as photosensitizer for PDT.13
In this study, our results show the efficacy of TPFC in both the in vitro PDT study (Fig. 2) and in the in vitro BNCT study (Fig. 3), TPFC was highly phototoxic and showed equal or superior performance compared with BPA upon neutron irradiation (Fig. 3). However in vivo TPFC did not show as high antitumor effect than BPA following neutron irradiation (Fig. 4). Although there are no great differences between the TPFC, BPA, and the combination of TPFC and BPA groups, the later combination of TPFC and BPA group showed the tendency to extend the survival time. It is possible that if PDT was followed by BNCT, greater survival times could have been observed. In the fluorescence microscopy experiment, TPFC was shown to be taken up by F98 rat glioma cells in vitro and by F98 rat glioma tumor in vivo (Figs. 5 and 6). However, the sites of localization of TPFC might be different following different routes of administration. Our results suggest that TPFC has promise as dual sensitizer for both PDT and BNCT of gliomas. Additionally, TPFC could also be used intraoperatively for photodynamic diagnosis (PDD) and fluorescence-guided resection of brain tumors.
Pre-operative administration of a carboranyl-containing chlorin has a number of advantages in the clinical setting. As shown in Figure 6, carboranyl-containing chlorins could be useful in PDD and in fluorescence-guided resection of brain tumors during surgery. Using fluorescence-guided resection of such tumors during surgery, the resection rate can be augmented, with expected further improvements in patient prognosis.46 In addition, carboranyl-containing chlorins can be used with intra-operative PDT and post-operative BNCT. Although the initial results with commonly used photosensitizers for PDT such as Photofrin (or its unpurified form HpD) were encouraging, treatment failures did occur, mainly due to the limited penetration of light of 630 nm wavelength (=Photofrin and HpD) into the brain. Penetration of light of 630 nm, recorded by Muller and Wilson,47,48 for tumor, brain infiltrated by tumor and normal brain is 2.9 ± 1.5, 2.4 ± 1.2, and 1.5 ± 0.4 mm, respectively. However, TPFC has a large molar coefficient at 652 nm (ε ~ 30,000 in DMSO),13 which is characterized by the power of deep penetration into most human tissues. Nevertheless PDT alone will probably not be sufficient for malignant glioma treatment. In cases with deep lesions, PDT alone may be inadequate to achieve complete tumor treatment, and it would be preferable in such cases to use BNCT as a supplementary treatment, using a boron-containing photosensitizer such as TPFC. Fairchild et al.49 reported that thermal and epithermal neutrons are transported to a depth of approximately 10 cm, and BNCT has been shown to be adequate for the treatment of deep lesions. As boron-containing chlorins can be effective for BNCT as boron delivery agents while retaining their photosensitizer ability, the limited penetration of light can be overcome using a combination of BNCT and PDT for the treatment of human gliomas.
CONCLUSIONS
The present study demonstrates that TPFC is a promising dual sensitizer for PDT and BNCT of F98 rat gliomas. In in vitro studies, TPFC is shown to be extremely phototoxic and to localize near the cell nuclei. In addition, both in vitro and in vivo, TPFC was efficient at treating F98 rat gliomas, comparable to known BPA. Interestingly, an in vivo study using a combination of TPFC and BPA showed the greatest rat survival times (mean value of 42 days), compared to TPFC alone (mean of 31 days) and BPA alone (mean of 36 days). Additionally, TPFC could be used in PDD and fluorescence-guided resection, as it is highly fluorescent.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) (20340549) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT) to S.K. and by the United States National Institutes of Health, grant number R01 CA 098902 to M.G.H.V. We thank Dr. Barth (Department of Pathology, the Ohio State University) for providing the F98 rat glioma cells.
Abbreviations used
- BNCT
boron neutron capture therapy
- BPA
boronophenylalanine
- BSH
sodium borocaptate
- CBE
compound biological effectiveness
- CED
convection-enhanced delivery
- CI
confidence interval
- DMSO
dimethylsulfoxide
- GB
glioblastoma
- HpD
hematoporphyrin derivative
- KUR
Kyoto University Research Reactor
- LET
linear energy transfer
- PBS
phosphate-buffered saline
- PDD
photodynamic diagnosis
- PDT
photodynamic therapy
- RBE
relative biological effectiveness
- TPFC
tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin
References
- 1.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. Changing paradigms—An update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11(2):165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 2.Origitano TC, Caron MJ, Reichman OH. Photodynamic therapy for intracranial neoplasms. Literature review and institutional experience. Mol Chem Neuropathol. 1994;21(2–3):337–352. doi: 10.1007/BF02815360. [DOI] [PubMed] [Google Scholar]
- 3.Kostron H, Obwegeser A, Jakober R. Photodynamic therapy in neurosurgery: A review. J Photochem Photobiol B. 1996;36(2):157–168. doi: 10.1016/s1011-1344(96)07364-2. [DOI] [PubMed] [Google Scholar]
- 4.Barth RF, Soloway AH, Fairchild RG, Brugger RM. Boron neutron capture therapy for cancer. Realities and prospects. Cancer. 1992;70(12):2995–3007. doi: 10.1002/1097-0142(19921215)70:12<2995::aid-cncr2820701243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: Current status and future prospects. Clin Cancer Res. 2005;11(11):3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 6.Kawabata S, Miyatake S, Kuroiwa T, Yokoyama K, Doi A, Iida K, Miyata S, Nonoguchi N, Michiue H, Takahashi M, Inomata T, Imahori Y, Kirihata M, Sakurai Y, Maruhashi A, Kumada H, Ono K. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res. 2009;50(1):51–60. doi: 10.1269/jrr.08043. [DOI] [PubMed] [Google Scholar]
- 7.Miyatake S, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, Kumada H, Suzuki M, Maruhashi A, Kirihata M, Ono K. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol. 2009;91(2):199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 8.Fabris C, Jori G, Giuntini F, Roncucci G. Photosensitizing properties of a boronated phthalocyanine: Studies at the molecular and cellular level. J Photochem Photobiol B. 2001;64(1):1–7. doi: 10.1016/s1011-1344(01)00184-1. [DOI] [PubMed] [Google Scholar]
- 9.Friso E, Roncucci G, Dei D, Soncin M, Fabris C, Chiti G, Colautti P, Esposito J, De Nardo L, Riccardo Rossi C, Nitti D, Giuntini F, Borsetto L, Jori G. A novel 10B-enriched carboranyl-containing phthalocyanine as a radio- and photo-sensitising agent for boron neutron capture therapy and photodynamic therapy of tumours: In vitro and in vivo studies. Photochem Photobiol Sci. 2006;5(1):39–50. doi: 10.1039/b506364g. [DOI] [PubMed] [Google Scholar]
- 10.Gottumukkala V, Ongayi O, Baker DG, Lomax LG, Vicente MG. Synthesis, cellular uptake and animal toxicity of a tetra(carboranylphenyl)-tetrabenzoporphyrin. Bioorg Med Chem. 2006;14(6):1871–1879. doi: 10.1016/j.bmc.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy JC, Marcus SL, Pottier RH. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Mechanisms and clinical results. J Clin Laser Med Surg. 1996;14(5):289–304. doi: 10.1089/clm.1996.14.289. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao E, Friso E, Miotto G, Jori G, Soncin M, Fabris C, Sibrian-Vazquez M, Vicente MG. Synthesis and biological investigations of tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC) Org Biomol Chem. 2008;6(20):3732–3740. doi: 10.1039/b807836j. [DOI] [PubMed] [Google Scholar]
- 14.Ol’shevskaya VA, Nikitina RG, Savchenko AN, Malshakova MV, Vinogradov AM, Golovina GV, Belykh DV, Kutchin AV, Kaplan MA, Kalinin VN, Kuzmin VA, Shtil AA. Novel boronated chlorin e6-based photosensitizers: Synthesis, binding to albumin and antitumour efficacy. Bioorg Med Chem. 2009;17(3):1297–1306. doi: 10.1016/j.bmc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Luguya R, Jensen TJ, Smith KM, Vicente MG. Synthesis and cellular studies of a carboranylchlorin for the PDT and BNCT of tumors. Bioorg Med Chem. 2006;14(17):5890–5897. doi: 10.1016/j.bmc.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Coderre JA, Button TM, Micca PL, Fisher CD, Nawrocky MM, Liu HB. Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalanine-fructose complex. Int J Radiat Oncol Biol Phys. 1994;30(3):643–652. doi: 10.1016/0360-3016(92)90951-d. [DOI] [PubMed] [Google Scholar]
- 17.Barth RF. Rat brain tumor models in experimental neurooncology: The 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36(1):91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 18.Biston MC, Joubert A, Adam JF, Elleaume H, Bohic S, Charvet AM, Esteve F, Foray N, Balosso J. Cure of Fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004;64(7):2317–2323. doi: 10.1158/0008-5472.can-03-3600. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Allen N, Clendenon NR, Ko LW. An improved rat brain-tumor model. J Neurosurg. 1980;53(6):808–815. doi: 10.3171/jns.1980.53.6.0808. [DOI] [PubMed] [Google Scholar]
- 20.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q, Kullberg EB, Gedda L. Trastuzumab-conjugated boron-containing liposomes for tumor-cell targeting; development and cellular studies. Int J Oncol. 2003;23(4):1159–1165. [PubMed] [Google Scholar]
- 22.Wu G, Barth RF, Yang W, Kawabata S, Zhang L, Green-Church K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol Cancer Ther. 2006;5(1):52–59. doi: 10.1158/1535-7163.MCT-05-0325. [DOI] [PubMed] [Google Scholar]
- 23.Mukundan S, Jr, Ghaghada KB, Badea CT, Kao CY, Hedlund LW, Provenzale JM, Johnson GA, Chen E, Bellamkonda RV, Annapragada A. A liposomal nanoscale contrast agent for preclinical CT in mice. AJR Am J Roentgenol. 2006;186(2):300–307. doi: 10.2214/AJR.05.0523. [DOI] [PubMed] [Google Scholar]
- 24.Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Roberts B, Larson RF, Yamashita Y, Krauze M, Noble CO, Drummond D, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: Laboratory investigation. J Neurosurg. 2008;108(5):989–998. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]
- 26.Yang W, Barth RF, Adams DM, Ciesielski MJ, Fenstermaker RA, Shukla S, Tjarks W, Caligiuri MA. Convection-enhanced delivery of boronated epidermal growth factor for molecular targeting of EGF receptor-positive gliomas. Cancer Res. 2002;62(22):6552–6558. [PubMed] [Google Scholar]
- 27.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 28.Murad GJ, Walbridge S, Morrison PF, Garmestani K, Degen JW, Brechbiel MW, Oldfield EH, Lonser RR. Real-time, image-guided, convection-enhanced delivery of interleukin 13 bound to pseudomonas exotoxin. Clin Cancer Res. 2006;12(10):3145–3151. doi: 10.1158/1078-0432.CCR-05-2583. [DOI] [PubMed] [Google Scholar]
- 29.Vogelbaum MA. Convection enhanced delivery for the treatment of malignant gliomas: Symposium review. J Neurooncol. 2005;73(1):57–69. doi: 10.1007/s11060-004-2243-8. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Masunaga SI, Kinashi Y, Takagaki M, Sakurai Y, Kobayashi T, Ono K. The effects of boron neutron capture therapy on liver tumors and normal hepatocytes in mice. Jpn J Cancer Res. 2000;91(10):1058–1064. doi: 10.1111/j.1349-7006.2000.tb00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151(1):1–18. [PubMed] [Google Scholar]
- 32.Morris GM, Coderre JA, Hopewell JW, Micca PL, Nawrocky MM, Liu HB, Bywaters A. Response of the central nervous system to boron neutron capture irradiation: Evaluation using rat spinal cord model. Radiother Oncol. 1994;32(3):249–255. doi: 10.1016/0167-8140(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 33.Morris GM, Coderre JA, Hopewell JW, Micca PL, Rezvani M. Response of rat skin to boron neutron capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother Oncol. 1994;32(2):144–153. doi: 10.1016/0167-8140(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WG, Smith KM, McCullough JL, Berns MW. Skin photosensitivity and photodestruction of several potential photodynamic sensitizers. Photochem Photobiol. 1989;49(4):431–438. doi: 10.1111/j.1751-1097.1989.tb09191.x. [DOI] [PubMed] [Google Scholar]
- 35.McCaughan JS., Jr Photodynamic therapy of skin and esophageal cancers. Cancer Invest. 1990;8(3–4):407–416. doi: 10.3109/07357909009012058. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan RB, Carruth JA, McKenzie AL, Williams SR. Photodynamic therapy in the treatment of malignant tumours of the skin and head and neck. Eur J Surg Oncol. 1989;15(5):400–406. [PubMed] [Google Scholar]
- 37.Moan J. Porphyrin photosensitization and phototherapy. Photochem Photobiol. 1986;43(6):681–690. doi: 10.1111/j.1751-1097.1986.tb05647.x. [DOI] [PubMed] [Google Scholar]
- 38.Gomer CJ, Rucker N, Ferrario A, Wong S. Properties and applications of photodynamic therapy. Radiat Res. 1989;120(1):1–18. [PubMed] [Google Scholar]
- 39.Profio AE, Doiron DR. Transport of light in tissue in photodynamic therapy. Photochem Photobiol. 1987;46(5):591–599. doi: 10.1111/j.1751-1097.1987.tb04819.x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts WG, Shiau FY, Nelson JS, Smith KM, Berns MW. In vitro characterization of monoaspartyl chlorin e6 and diaspartyl chlorin e6 for photodynamic therapy. J Natl Cancer Inst. 1988;80(5):330–336. doi: 10.1093/jnci/80.5.330. [DOI] [PubMed] [Google Scholar]
- 41.Nelson JS, Roberts WG, Berns MW. In vivo studies on the utilization of mono-L-aspartyl chlorin (NPe6) for photodynamic therapy. Cancer Res. 1987;47(17):4681–4685. [PubMed] [Google Scholar]
- 42.Aizawa K, Okunaka T, Ohtani T, Kawabe H, Yasunaka Y, O’Hata S, Ohtomo N, Nishimiya K, Konaka C, Kato H. Localization of mono-L-aspartyl chlorin e6 (NPe6) in mouse tissues. Photochem Photobiol. 1987;46(5):789–793. doi: 10.1111/j.1751-1097.1987.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 43.Gomer CJ, Ferrario A. Tissue distribution and photosensitizing properties of mono-L-aspartyl chlorin e6 in a mouse tumor model. Cancer Res. 1990;50(13):3985–3990. [PubMed] [Google Scholar]
- 44.Roberts WG, Berns MW. In vitro photosensitization I. Cellular uptake and subcellular localization of mono-L-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin II. Lasers Surg Med. 1989;9(2):90–101. doi: 10.1002/lsm.1900090203. [DOI] [PubMed] [Google Scholar]
- 45.Pandey RK, Bellnier DA, Smith KM, Dougherty TJ. Chlorin and porphyrin derivatives as potential photosensitizers in photodynamic therapy. Photochem Photobiol. 1991;53(1):65–72. doi: 10.1111/j.1751-1097.1991.tb08468.x. [DOI] [PubMed] [Google Scholar]
- 46.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 47.Muller PJ, Wilson BC. An update on the penetration depth of 630 nm light in normal and malignant human brain tissue in vivo. Phys Med Biol. 1986;31(11):1295–1297. doi: 10.1088/0031-9155/31/11/012. [DOI] [PubMed] [Google Scholar]
- 48.Muller PJ, Wilson BC. Photodynamic therapy of malignant primary brain tumours: Clinical effects, post-operative ICP, and light penetration of the brain. Photochem Photobiol. 1987;46(5):929–935. doi: 10.1111/j.1751-1097.1987.tb04871.x. [DOI] [PubMed] [Google Scholar]
- 49.Fairchild RG, Saraf SK, Kalef-Ezra J, Laster BH. Comparison of measured parameters from a 24-keV and a broad spectrum epithermal neutron beam for neutron capture therapy: An identification of consequential parameters. Med Phys. 1990;17(6):1045–1052. doi: 10.1118/1.596455. [DOI] [PubMed] [Google Scholar]