Abstract
Background
Marmoset wasting syndrome (MWS) is one of the leading causes of morbidity and mortality in captive marmosets, and thus far no reliable treatment has been found. Glucocorticoids are used widely to treat inflammatory conditions of the GI tract such as human and feline inflammatory bowel disease, which, such as MWS, are histologically characterized by chronic lymphoplasmacytic inflammation in the intestines. Budesonide is a glucocorticoid with few reported side effects due to the majority of it being metabolized into inactive compounds by the liver before entering the systemic circulation.
Method
Eleven marmosets presented with antemortem signs consistent with MWS and were treated with oral prednisone or budesonide for 8 weeks.
Results
The marmosets in our study demonstrated a significant increase in both weight and albumin levels (relative to pre-treatment values) after glucocorticoid therapy.
Conclusions
Glucocorticoids are an effective therapy to ameliorate the clinical signs associated with MWS with minimal side effects.
Keywords: callitrichid, corticosteroids, inflammatory bowel disease, monkey
Introduction
Marmoset wasting syndrome (MWS) is one of the leading causes of morbidity and mortality in captive marmosets. Its prevalence has been reported to be as high as 60% in captive colonies 21, but these ranges may be misleading because one of the challenges surrounding MWS is that the same label has been applied inconsistently and to a dizzying array of disease processes. The only clinical sign consistently attributed to MWS is chronic weight loss, with concurrent alopecia and diarrhea also commonly reported, but paresis and paralysis have also been attributed to MWS (Table1). Associated clinical pathology findings have included increases in calprotectin and decreases in hematocrit, whereas others have failed to find such changes and instead revealed decreases in albumin (Table1). Histologically, many report a lymphoplasmacytic infiltration of the intestines, but even here, there is disagreement, with at least one report finding that neutrophils are the predominant white blood cell in the intestines.
Table 1.
This is a list of published studies that operationalized marmoset wasting syndrome and used it to define a study population
| Citations | Clinical signs | Clinical pathology | Significant histological findings |
|---|---|---|---|
| Beglinger et al. 6 | Weight loss, emaciation, progressing to muscular weakness, and hindlimb paralysis | Anemia, ↑ AST, ↑ ALP, ↑ ALT | Pancreatitis with Trichospirura eggs |
| Brack & Rothe 7 | Diarrhea, cachexia, and hindlimb paresis to paralysis | N/A | Pancreatic and renal fibrosis, lymphocytic inflammation and tubular degeneration of kidneys, and hepatic hemosiderosis |
| Kuehnel et al. 18 | Chronic diarrhea, weight loss, poor fur condition, and tail alopecia | N/A | N/A |
| Baxter et al. 5 | Weight <325 g, weight loss >0.05% peak weight per day | Albumin <3.5 g/dl | Chronic mild inflammation of 2 or more intestinal segments or chronic moderate or severe inflammation in one intestinal segment |
| Nakashima et al. 23 | Persistent high-viscosity diarrhea for at least 2 weeks | Fecal occult blood positive, ↓ hematocrit, ↑calprotectin, higher WBC than control group | Neutrophilic infiltrate in colon and apoptosis |
| Logan & Khan 20 | Weight loss, muscle atrophy, and tail alopecia | Macrocytic normochromic anemia, ↑ALP, ↓ ALB, ↓TP, ↑platelets | Chronic active colitis with loss or tortuous, branching crypts |
| Lewis et al. 19 | Poor weight gain, anorexia, alopecia, and transient diarrhea | Anemia | N/A |
| Barnard et al. 3 | 30% weight loss, alopecia, chronic diarrhea, and muscle atrophy | N/A | N/A |
| Shimwell et al. 28 | Failure to thrive and generalized weakness in weanlings | ↑ CK, ↑ AST, ↓ albumin | N/A |
| King 17 | Weight loss, unkempt hair coat, stiff movement in hindlimb, tail base alopecia, selective eating | N/A | N/A |
| Chalifoux et al. (1982) 10 | 25–50% weight loss | N/A | Within colon/cecum: decreased goblet cells, crypt abscesses, karyorrhexis and/or atypia of the epithelium, infiltration of lamina propria with mononuclear cells and/or neutrophils |
While multiple groups have proposed etiologies and targeted therapies, no consensus has emerged in the literature. Gluten sensitivity is one proposed cause, with provision of a gluten-free diet appearing to ameliorate gastrointestinal symptoms in some animals 18. Even within this study, however, only a fraction of the animals with clinical symptoms of MWS demonstrated gluten sensitivity. MWS has also been linked to infestation with Trichospirura leptostoma, but these marmosets showed hindlimb paresis without a decrease in albumin or total protein, all of which contrast to most other published descriptions of MWS. In our colony and others 5,20, marmoset wasting disease is associated with weight loss and low serum albumin. In fact, a serum albumin below 3.5 g/dl or a weight of <325 g has 92% sensitivity, 100% specificity, and 100% positive predictive value for postmortem lymphoplasmacytic inflammation in the small and/or large intestines in our animals 5. Thus, body weight and serum albumin may serve as useful markers of disease progression. Further, with no consensus as to etiology or specific therapeutic target for MWS, treatment might best be directed at the common clinical and pathological symptoms upon which most descriptions agree, namely GI inflammation and weight loss.
Glucocorticoids are arguably the most effective treatment for inducing remission in humans with inflammatory bowel disease (ulcerative colitis and Crohn's disease), with remission rates up to 89% 27. While prednisone is one example of an effective glucocorticoid, there are several adverse side effects associated with its use 11. In humans, one of the most common side effects associated with prednisone use is osteopenia 9. This is especially problematic in marmosets affected with MWS in our colony, as marmosets with MWS are over seven times more likely to be concurrently afflicted with metabolic bone disease and corresponding loss of bone density 5, a finding that has been mirrored elsewhere 15. Budesonide is a glucocorticoid with few reported side effects, which largely result from up to 90% of it being metabolized into inactive compounds by the liver before entering the systemic circulation 2. In addition, budesonide has a 15–200 times greater affinity for glucocorticoid receptors than prednisolone. Thus, budesonide primarily exerts its actions in the GI tract by altering the production of IL-6, IL-1, NF-κB, and TNF-α 4.
This study aimed to examine the efficacy of glucocorticoid therapy and specifically budesonide on MWS and predicted that the anti-inflammatory effects of such therapy would reverse the weight loss and decreases in serum albumin levels seen in our animals with MWS.
Materials and methods
Humane care guidelines
All experimental procedures were approved and overseen by the Institutional Animal Care and Use Committee of Johns Hopkins University. Strict adherence to the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health, the Animal Welfare Act by the United States Department of Agriculture, and the Weatherall report by the Medical Research Council was observed.
Study animals
As in 4,5, all marmosets in this study were between the ages of 2 and 6 years of age and housed in family units, in pairs, or singly as part of a large breeding and experimental colony at Johns Hopkins University, an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited institution. Animals were permitted ad libitum access to water and were fed a complete and balanced diet consisting of a custom homogenized blend of Teklad 8794N New World Primate Diet (Harlan Laboratories, Indianapolis, IN, USA), Zupreem 9920 CS canned marmoset diet (Shawnee, KS, USA), and Bio-Serv Newberne Hayes Vitamin Mix (San Diego, CA, USA), supplemented with various fruits and yogurt. Environmental enrichment in the form of visual and auditory contact with conspecifics, complex environments, and manipulanda were provided to all marmosets. Experienced animal care technicians observed animals at least once daily and more often if necessitated by experimental or medical need. The Johns Hopkins University Research Animal Resources veterinarians diagnosed, treated, and/or managed any medical illness or injury in the marmosets, and if euthanasia due to disease was warranted, marmosets were euthanized with an intravenous overdose of Euthasol (Virbac Corporation, Fort Worth, TX, USA) under deep ketamine anesthesia.
Marmosets presenting for progressive weight loss, adult body weight of <325 g, alopecia, or diarrhea received a physical exam, complete blood count, and serum chemistry, and fecal flotation and cultures were performed. The blood was collected from the femoral vein using 25-gauge needles, stored in lithium heparin tubes, and analyzed using a Hemavet 950 hematology analyzer (Drew Scientific Inc, Oxford, CT, USA) and vetACE chemical analyzer (Alfa Wassermann, West Caldwell, NJ, USA). The fecal flotation was performed in house using zinc sulfate solution to detect the presence of parasites such as Giardia, Cryptosporidium, Toxoplasmosis, and nematodes. Fecal cultures were submitted to detect the presence of Klebsiella spp, EPEC, Campylobacter spp, Yersinia spp, and Shigella spp. Although we did not specifically rule out Trichospirura infestation in individual cases, the vast majority of animals from this long-standing colony receive complete necropsies upon euthanasia or death, and this parasite has never been found in our facilities.
Animals were included in this study if their body weight was ≤325 g and their serum albumin was ≤3.5 g/dl 5. The body weight and albumin levels must have remained below their respective thresholds for at least two consecutive samples, drawn 2 weeks apart.
Budesonide therapy
Eleven animals met these inclusion criteria and were begun on glucocorticoid therapy. Prednisone was used as the initial treatment for four animals, starting at a once-daily oral dose of 1 mg/kg that was then adjusted according to response to therapy. All of these animals were subsequently switched to treatment with budesonide. The remaining seven animals received budesonide as their only therapy. Regardless of when budesonide therapy was begun, it was initiated at 0.5 mg per animal orally once per day for 8 weeks. If after the initial 8 weeks an animal had not responded to therapy (i.e., still met inclusion criteria), the dose was increased to 0.75 mg and the animal followed for an additional 8 weeks. The budesonide was compounded into a pina colada-flavored liquid suspension by Kaye's Pharmacy (Baltimore, MD, USA). Serum albumin and body weight were measured every 2 weeks during treatment.
Statistical analysis
All analyses were performed with a two-tailed repeated measures t-test using the stata statistical software (StataCorp LP, College Station, Texas USA) package. For each individual animal, a mean of all available weight and serum albumin values prior to treatment was used as the ‘baseline’ condition, and this was compared to a mean of all values collected during treatment, regardless of duration or drug sequence. A Bonferroni correction for multiple comparisons was applied to base criteria for significance of P < 0.05. An ABAB single-subject research design and graph were used to depict the results from one of the study marmosets that happened to relapse after the cessation of the first 8-week course of glucocorticoid therapy. These data do not lend itself to statistical analysis and instead are presented in graphical form for visual inspection. However, this design allows the replication of both control and treatment conditions and encourages multiple data points to be collected and interpreted within each condition without being obscured via summary statistics.
Results
The results of a repeated measures t-test revealed that marmosets in our colony had a higher mean weight (M = 318.03 g) during treatment with glucocorticoids than they did prior to treatment [M = 287.75 g, t(10) = 2.75, P = 0.021]. Concordantly, a repeated measures t-test substantiated that marmosets had a high albumin during glucocorticoid therapy (M = 3.86 g/dl) than they did before treatment was initiated [M = 2.80 g/dl, t(10) = 6.23, P = 0.0001]. These results are depicted in Table2.
Table 2.
Mean weight and albumin levels for each of the 11 marmosets included in our study both before and during glucocorticoid therapy
| Animal | Mean weight (g) | Mean albumin (mg/dl) | ||
|---|---|---|---|---|
| Pre-treatment | During treatment | Pre-treatment | During treatment | |
| A | 255 | 346 | 2.35 | 4.53 |
| B | 293 | 321 | 2.95 | 3.81 |
| C | 253 | 310 | 2.50 | 3.85 |
| D | 249 | 304 | 2.93 | 4.22 |
| E | 288 | 308 | 3.33 | 4.15 |
| F | 318 | 277 | 3.17 | 3.48 |
| G | 335 | 340 | 2.83 | 3.58 |
| H | 300 | 324 | 2.85 | 4.23 |
| I | 290 | 281 | 3.30 | 3.52 |
| J | 273 | 317 | 2.43 | 3.35 |
| K | 313 | 372 | 2.15 | 3.78 |
| Group means | 288 | 318 | 2.80 | 3.86 |
| SE | 8.50 | 8.31 | 0.12 | 0.11 |
All weights are in grams and albumin is in milligrams per deciliter. SE, standard error.
The results from one marmoset using a single-subject (ABAB) design are depicted in Figs1 and 2. The graphs reveal stable and substantial increases in albumin (Fig.1) and body weight (Fig.2) in response to glucocorticoid therapy and decreases in both parameters after the cessation of therapy.
Figure 1.
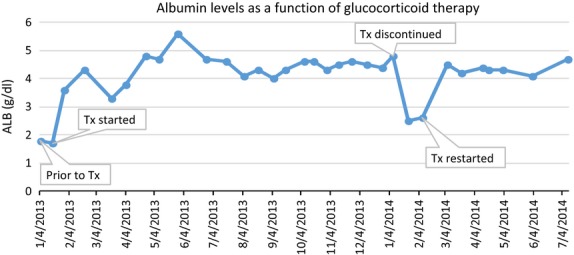
Change in albumin levels in one marmoset depending on its treatment status. Prior to therapy, albumin was below 2.0 g/dl. It steadily increased while being administered glucocorticoids and then sharply declined after the cessation of treatment. After treatment was re-initiated, albumin levels returned to the same level they were during the initial treatment period.
Figure 2.
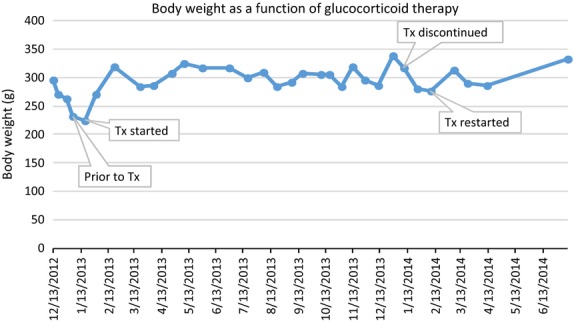
Body weight changes in one marmoset varied with treatment status. This marmoset's weight declined before glucocorticoid therapy was initiated and increased during the treatment. After discontinuing the first round of treatment, the marmoset's weight declined again and was ameliorated when glucocorticoids were readministered.
Discussion
The results of this study suggest that oral glucocorticoid therapy ameliorates decreases in the two diagnostic parameters most closely associated with MWS, body weight, and serum albumin. The lymphoplasmacytic inflammation found in the intestines of our marmosets with MWS shares histological characteristics with inflammatory bowel disease of dogs, cats, and humans, where oral glucocorticoids, most often in the form of prednisone, are the first-line therapy in mild to moderate cases. Although some research suggests that prednisone is more effective for inducing remission than budesonide 27, the side effects of prednisone administration make this drug less ideal and can include polyuria, polydipsia, polyphagia, redistribution of fat, thinning of skin, osteopenia, increased susceptibility to infections, and hyperglycemia 11. There is evidence that budesonide, a drug with fewer systemic affects due to high ‘first pass’ metabolism in the liver, is effective at inducing remission in humans with mild to moderate ulcerative colitis 16, microscopic colitis 22, and Crohn's disease 27. Further, budesonide has been observed to reduce disease activity indices or induce clinical improvement and has been observed in dogs with signs of gastrointestinal inflammation 13,24, in murine experimental models of colitis 1, and anecdotally in cats with inflammatory bowel disease 30.
Unlike prednisone, which has fairly well-established dosing guidelines, there is no established dosing guide for budesonide therapy in any animal species, and dose-dependent efficacy varies greatly. For instance, humans with IBD have needed anywhere from 3 to 18 mg per day 2, while dogs have been given doses from 1 to 9 mg per day 13,24, and mice have been administered doses ranging from 0.168 to 0.5 mg/kg 1,26. While feline doses have not been published in the literature, clinical guidelines suggest doses of 1–2 mg per animal daily 30. It is worth noting that in mice, it has been demonstrated that higher doses are not necessarily more effective, but are more likely to cause adverse side effects 1,26. Moreover, humans are more likely to respond to treatment with a higher concentration once per day than they are to the same dose split and given every 12 hours 8. Thus, it is plausible that marmosets would also follow these trends. While our dosing of 0.5 mg per animal was somewhat arbitrary, this dose was based upon per animal clinical dosing guidelines used in cats and dogs.
While budesonide is a relatively effective drug for inducing remission in humans with IBD, neither it nor prednisone is very effective at maintaining remission 2,27,29. Azathioprine, 6-MP, and methotrexate are used to maintain remission in Crohn's disease, and any of these drugs or aminosalicylates are effective at maintaining remission in ulcerative colitis 29. The relapse rate for patients treated with budesonide therapy has been reported to be as high as 67% 14. Thus far we have had one marmoset relapse approximately 2 months after the cessation of budesonide therapy. This animal responded well to a second 8-week course and is currently being maintained on 0.125 mg per day with no adverse consequences (Figs1 and 2). Of 11 marmosets that have been treated, six have ceased therapy (some for as long as 6 months) and continue to thrive.
Although this study demonstrates the usefulness of budesonide as therapy for animals with chronic MWS, our clinical experience using budesonide for animals with more acute forms of GI illness has failed to produce similar results. In three cases, we initiated budesonide therapy to animals showing acute signs of gastrointestinal illness but not meeting the criteria for inclusion in this experiment. These three animals all died within a week of presentation despite budesonide therapy. However, remission rates and/or clinical improvement resulting from budesonide treatment can be as low as 18% in humans while still being more effective than placebo or other drugs. Moreover, budesonide is typically only indicated for mild to moderate forms of IBD in humans and thus is unlikely to be successful in the acute management of end stage disease 2,25. Other forms of therapy, which include TNF-α inhibitors, intravenous methylprednisolone, and cyclosporine, are more appropriate for refractory or severe IBD in humans and might be useful in such acute marmoset cases 12,29. However, TNF-α inhibitors may be cost prohibitive, limiting their availability in most vivaria.
It is possible that budesonide treatment increased weight and albumin via mechanisms other than by reducing gastrointestinal inflammation. For instance, it is possible that the budesonide worked by simply increasing appetite as is seen with prednisone administration. While we cannot definitively rule this out, this is not a common side effect of budesonide in humans or dogs 11,13. Another possibility is that the animals included in this study would have improved regardless of budesonide therapy. However, this is not likely because MWS is known as a uniformly progressive disease without treatment, and these animals met previously established diagnostic criteria for MWS 5. Additionally, in the one animal that was observed to relapse after the cessation of budesonide therapy, reinstitution of budesonide treatment again reversed this animal's decline.
In conclusion, glucocorticoid therapy has demonstrated substantial potential to ameliorate clinical signs of MWS in our colony. Further, budesonide is an effective choice of glucocorticoid and is preferable to prednisone because of its decreased risk of side effects. Future experiments could be aimed at refining dosage requirements or comparing budesonide to other anti-inflammatory treatments such as TNF-α inhibitors or mesalamine.
Acknowledgments
We are indebted to the following individuals for their assistance with data collection and monitoring of the animals: Caroline Garrett, Theresa Meade, Rachael Cohen, Meghan Vermillion, Jessica Izzi, and Cassie Moats. Thanks to Xiaoqin Wang and his laboratory for permitting us to use his marmoset colony for this project.
References
- 1.Ali H, Weigmann B, Neurath MF, Collnot EM, Windbergs M, Lehr CM. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J Control Release. 2014;183:167–77. doi: 10.1016/j.jconrel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Angelucci E, Malesci A, Danese S. Budesonide: teaching an old dog new tricks for inflammatory bowel disease treatment. Curr Med Chem. 2008;15:2527–35. doi: 10.2174/092986708785909049. [DOI] [PubMed] [Google Scholar]
- 3.Barnard D, Knapka J, Renquist D. The apparent reversal of a wasting syndrome by nutritional intervention in Saguinus-Mystax. Lab Anim Sci. 1988;38:282–8. [PubMed] [Google Scholar]
- 4.Barnes PJ. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25:451–68. doi: 10.1016/j.iac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Baxter VK, Shaw GC, Sotuyo NP, Carlson CS, Olson EJ, Zink MC, Mankowski JL, Adams RJ, Hutchinson EK, Pate KAM. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One. 2013;8:e82747. doi: 10.1371/journal.pone.0082747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beglinger R, Illgen B, Pfister R, Heider K. The parasite Trichospirura leptostoma associated with wasting disease in a colony of common marmosets, Callithrix jacchus. Folia Primatol (Basel) 1988;51:45–51. doi: 10.1159/000156355. [DOI] [PubMed] [Google Scholar]
- 7.Brack M, Rothe H. Chronic tubulointerstitial nephritis and wasting disease in marmosets (Callithrix-jacchus) Vet Pathol. 1981;18:45–54. doi: 10.1177/0300985881018s0605. [DOI] [PubMed] [Google Scholar]
- 8.Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG, Malchow H, Prantera C, Mani V, OMorain C, Selby W, Pallone F, diPietralata MM, Sjodahl R, Florin T, Smith P, Bianchi P, Lofberg R, Rutgeerts P, Smallwood R, Lamers HW, TasmanJones C, Hunter JO, Hodgson H, Danielsson A, Lee FI, Piacitelli G, Giovanni S, Ellis A, Weir DG. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. Gut. 1997;41:209–14. doi: 10.1136/gut.41.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 10.Chalifoux LV, Bronson RT, Escajadillo A, McKenna S. An analysis of the association of gastroenteric lesions with chronic wasting syndrome of marmosets. Vet Pathol. 1982;19:141–162. [PubMed] [Google Scholar]
- 11.Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis. 2013;31:368–73. doi: 10.1159/000354699. [DOI] [PubMed] [Google Scholar]
- 12.Danese S, Peyrin-Biroulet L. IBD IN 2013 enriching the therapeutic armamentarium for IBD. Nat Rev Gastroenterol Hepatol. 2014;11:84–5. doi: 10.1038/nrgastro.2013.246. [DOI] [PubMed] [Google Scholar]
- 13.Dye TL, Diehl KJ, Wheeler SL, Westfall DS. Randomized, controlled trial of budesonide and prednisone for the treatment of idiopathic inflammatory bowel disease in dogs. J Vet Intern Med. 2013;27:1385–91. doi: 10.1111/jvim.12195. [DOI] [PubMed] [Google Scholar]
- 14.Gross V, Andus T, Ecker KW, Raedler A, Loeschke K, Plauth M, Rasenack J, Weber A, Gierend M, Ewe K, Scholmerich J, Grp BS. Low dose oral pH modified release budesonide for maintenance of steroid induced remission in Crohn's disease. Gut. 1998;42:493–6. doi: 10.1136/gut.42.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarcho MR, Power ML, Layne-Colon DG, Tardif SD. Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus. Am J Primatol. 2013;75:153–60. doi: 10.1002/ajp.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller R, Stoll R, Foerster EC, Gutsche N, Domschke W. Oral budesonide therapy for steroid-dependent ulcerative colitis: a pilot trial. Aliment Pharmacol Ther. 1997;11:1047–52. doi: 10.1046/j.1365-2036.1997.00263.x. [DOI] [PubMed] [Google Scholar]
- 17.King G. An investigation into Wasting Marmoset Syndrome at Jersey Zoo. Ann Rp, Jersey Wildl Presev Trust. 1976:97–107. [Google Scholar]
- 18.Kuehnel F, Mietsch M, Buettner T, Vervuert I, Ababneh R, Einspanier A. The influence of gluten on clinical and immunological status of common marmosets (Callithrix jacchus. J Med Primatol. 2013;42:300–9. doi: 10.1111/jmp.12055. [DOI] [PubMed] [Google Scholar]
- 19.Lewis DH, Stein FJ, Sis RF, Mcmurray DN. Fecal microflora of marmosets with wasting marmoset syndrome. Lab Anim Sci. 1987;37:103–5. [PubMed] [Google Scholar]
- 20.Logan AC, Khan KNM. Clinical pathologic changes in two marmosets with wasting syndrome. Toxicol Pathol. 1996;24:707–9. doi: 10.1177/019262339602400605. [DOI] [PubMed] [Google Scholar]
- 21.Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus. Comp Med. 2003;53:369–82. [PubMed] [Google Scholar]
- 22.Miehlke S, Madisch A, Kupcinskas L, Petrauskas D, Bohm G, Marks HJ, Neumeyer M, Nathan T, Fernandez-Banares F, Greinwald R, Mohrbacher R, Vieth M, Bonderup OK, Grp B-CS. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology. 2014;146:1222–30. doi: 10.1053/j.gastro.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima E, Okano Y, Niimi K, Takahashi E. Detection of calprotectin and apoptotic activity in the colon of marmosets with chronic diarrhea. J Vet Med Sci. 2013;75:1633–6. doi: 10.1292/jvms.13-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietra M, Fracassi F, Diana A, Gazzotti T, Bettini G, Peli A, Morini M, Pagliuca G, Roncada P. Plasma concentrations and therapeutic effects of budesonide in dogs with inflammatory bowel disease. Am J Vet Res. 2013;74:78–83. doi: 10.2460/ajvr.74.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, Huang M, Yeung P, Ballard ED. Once-daily budesonide MMX (R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143:1218–26. doi: 10.1053/j.gastro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci. 2013;92:708–18. doi: 10.1016/j.lfs.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Seow CH, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH. Budesonide for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;8:CD002913. doi: 10.1002/14651858.CD000296.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Shimwell M, Warrington BF, Fowler JSL. Dietary habits relating to wasting marmoset syndrome (Wms) Lab Anim. 1979;13:139–42. doi: 10.1258/002367779780943369. [DOI] [PubMed] [Google Scholar]
- 29.Silverman J, Otley A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2011;7:419–28. doi: 10.1586/eci.11.34. [DOI] [PubMed] [Google Scholar]
- 30.Willard M. Canine and Feline Diarrheas: Diagnosis/Management of Infiltrative Disorders. Montreal, Quebec, Canada: ACVIM forum proceedings; , June 3-6, 2009, [Google Scholar]


