Abstract
Background
Depression has been found to predict the incidence of hypertension and other adverse cardiovascular events in prospective studies. Insomnia and short sleep duration, which are typical symptoms of depression, have also been shown to increase the risk for hypertension incidence. Insomnia is associated with increased activation of the hypothalamic–pituitary–adrenal axis, and short sleep duration raises average 24-h blood pressure, which over time could lead to structural adaptations that gradually reset the entire cardiovascular system to operate at an elevated pressure equilibrium. No previous published population studies have examined whether insomnia and sleep duration mediate the relationship between depression and hypertension incidence.
Methods
We conducted multivariate longitudinal (1982–1992) analyses stratified by age of the First National Health and Nutrition Examination Survey (NHANES I) (n = 4,913) using Cox proportional hazards models.
Results
Middle-aged subjects who suffered from depression at baseline were 44% more likely to be diagnosed with hypertension over the follow-up period after controlling for covariates (hazard ratio (HR) = 1.44, 95% confidence interval (CI) 1.15–1.80). Both short sleep duration and insomnia were also significantly associated with hypertension incidence. Consistent with insomnia and sleep duration acting as mediators of the relationship between depression and hypertension incidence, the inclusion of these variables in the multivariate models appreciably attenuated the association (HR = 1.27, 95% CI 1.00–1.61). Depression, sleep duration, and insomnia were not significantly associated with hypertension incidence in elderly subjects.
Conclusions
These results suggest the hypothesis that treatment of sleep problems in middle-aged individuals suffering from depression could reduce their risk for developing hypertension, and its vascular and cardiac complications.
Keywords: blood pressure, depression, essential hypertension, hypertension, insomnia, sleep
The World Health Organization predicts that depression will be the second leading cause of burden on society among all diseases worldwide by the year 2020 (ref. 1). Depression is also an established risk factor for the disease predicted to be the leading cause of societal burden, ischemic heart disease.1 Depression has been shown to increase the risk for the development of cardiovascular disease2 in healthy subjects, and for cardiac morbidity and mortality in subjects with established cardiovascular disease.3,4 One of the most important modifiable risk factors for cardiovascular disease is hypertension, and depression has been found to be associated cross-sectionally with the prevalence of hypertension5,6 and longitudinally with the incidence of hypertension.7,8 A number of mechanisms have been proposed by which depression could lead to hypertension, including increased hypothalamic–pituitary–adrenal axis reactivity,8 elevated sympathetic nervous system activity,9,10 shared genetic susceptibility,11 increased propensity for unhealthy lifestyles such as physical inactivity, smoking, and alcohol abuse,12 and comorbidity with obstructive sleep apnea13 and the metabolic syndrome.14
Short sleep duration and insomnia, which are typical symptoms of depression, could also be mechanisms by which depression plays a role in the etiology of hypertension. Short sleep duration and insomnia have been found to be associated with the prevalence of hypertension in cross-sectional studies15,16 and the incidence of hypertension in longitudinal studies.17,18 Sleep restriction has been shown to acutely increase blood pressure and sympathetic nervous system activity.19 Blood pressure dips by an average of 10–20% during sleep, so shorter sleep durations increase hemodynamic load by raising average 24-h blood pressure and heart rate, which over time can lead to structural adaptations that gradually reset the entire cardiovascular system to operate at an elevated pressure equilibrium.20 Sleep restriction has also been shown to compromise insulin sensitivity,21 and to increase appetite by decreasing leptin and increasing ghrelin.22 Short sleep duration is associated with diabetes incidence23 and obesity,24 potent risk factors for hypertension. Despite known connections between sleep parameters and hypertension, no previous epidemiological studies have explored whether sleep duration and insomnia act as mediators in the relationship between depression and hypertension.
Knowledge of whether there are mediating effects of sleep duration and insomnia on the relationship between depression and hypertension could lead to the development of interventions to decrease the excess cardiovascular morbidity and mortality associated with depression. In this study, we explored the relationship between depression and hypertension incidence between 1982 and 1992 among participants in the Epidemiologic Follow-up Studies of the First National Health and Nutrition Examination Survey (NHANES I).25
Methods
Participants
Subjects for this study were participants in the 1982–1984, 1986, 1987, and 1992 follow-up studies of the NHANES I.25 The baseline measures of depression, sleep duration, insomnia, and covariates were taken from the 1982–1984 survey, and then hypertension incidence was determined until 1992. The NHANES I survey was a probability sample of the civilian noninstitutionalized US population between 1971 and 1975. Approximately 85% of the subjects between the ages of 25 and 74 at baseline were successfully traced and interviewed in 1982–1984 (n = 12,220). Individuals who were deceased (n = 1,697), who had hypertension at or before the 1982–1984 survey (n = 5,192), who did not answer any of the depression questions (n = 237), and who did not answer all of the sleep questions (n = 181) were excluded from the analyses, yielding a final sample size of 4,913 subjects. All subjects gave informed consent. This study involved analyses of a publicly available dataset that did not include identifying information and, therefore, met federal guidelines for exemption from review by an institutional review board.
Measures
The primary dependent variable for this study was the incidence of hypertension between 1982 and 1992. Individuals who had systolic blood pressure readings >140 mm Hg or diastolic readings >90 mm Hg at the time of the 1982–1984 survey or who had been diagnosed with hypertension at or before the 1982–1984 survey were excluded. Three blood pressure readings were taken at the same sitting, and readings were not taken if maximum inflation level was >160 mm Hg as specified by the Hypertension Detection and Follow-Up Program.26 Incident cases of hypertension were determined by self-report of physician diagnosis, by hospital diagnosis, or by cause of death at the times of the 1986, 1987, or 1992 follow-up surveys.
The primary independent variable for this study was depression. To measure the presence of depressive symptoms, we used the standard cutoff score of 16 out of a total possible score of 60 on the 20-question CES-D (Center for Epidemiologic Studies Depression Scale).27 Of the 89 subjects who reported taking antidepressants, 44 had scores on the CES-D in the nondepressed range. We included subjects taking antidepressants in the depressed category.
We tested the hypothesis that baseline sleep duration and insomnia would act as mediators of the relationship between the independent variable of baseline depression and the dependent variable of hypertension incidence. The subject’s average sleep duration in hours was determined by their responses to the question: “How many hours of sleep do you usually get a night (or when you usually sleep)?” We categorized the sleep duration variable (≤5, 6, 7–8, and ≥9 h) as opposed to retaining it as a continuous variable because each additional hour of sleep was not associated with the same change in the dependent variable. Subjects were asked three questions about how often they had trouble falling asleep, waking up during the night, and waking up too early and not being able to fall asleep again with responses including “1 = never,” “2 = rarely,” “3 = sometimes,” “4 = often,” and “5 = almost always.” We added the scores from the three questions to create an index of insomnia ranging from 3 to 15 with increasing scores being indicative of more severe insomnia.
Covariates in our multivariate models included age (5-year interval), sex, race/ethnicity (white or nonwhite including black, Hispanic, Asian, and others), education (high school graduate or <high school graduate), body weight (body mass index (kg/m2)—underweight <18.5, lean ≥18.5 and <25, overweight ≥25 and <30, and obese ≥30), history of diabetes (yes, no), alcohol consumption (0, >0 and <28, or ≥28 g per day), current smoking status (0, 1–5, 6–10, 11–20, or >20 cigarettes per day), physical activity (6 = high, 5, 4, 3, and 2 = low), and pulse rate (continuous). Missing values for covariates, which for all covariates represented <1% of the total sample size, were imputed using mean and mode substitution.
Statistical analyses
After preliminary univariate and bivariate analyses, we used Cox proportional hazards models to examine the effect of depression on the risk of being diagnosed with hypertension over follow-up. The time duration to diagnosis was determined from the baseline date to the first report of hypertension. The first adjusted model (Model 2) included multiple covariates. To test whether sleep duration and insomnia acted as mediators in the relationship between depression and hypertension incidence, we included these variables separately in Models 3 and 4, and then together in Model 5. The significance of individual coefficients was determined by the 95% confidence limits for hazard ratios (HRs).
We theorized that the mediating effects of sleep duration and insomnia on the relationship between depression and hypertension incidence would differ by age because age has been shown to act as an effect modifier in the relationship between sleep duration and hypertension incidence with associations in middle-aged subjects but not in elderly subjects.17 To test whether there would be differences between middle-aged and elderly adults in the mediating effects of sleep duration and insomnia, we divided the sample into two age-groups with subjects who, at the time of the 1982–1984 wave, were between the ages of 32 and 59 years in one group and subjects who were between the ages of 60 and 86 years in another group. We tested for interaction between depression and all covariates.
The NHANES I included weights to account for the complex sampling design and to allow approximations of the US population. We conducted both nonweighted analyses using SAS software (SAS System for Windows, version 9.1; SAS Institute, Cary, NC) and weighted analyses using SUDAAN software (release 10.0; Research Triangle Institute, Research Triangle Park, NC). We chose to present only the unweighted results for four reasons. First, the unweighted results were generally consistent with minimal effects of the complex survey design on the main conclusions derived from the weighted estimates. Second, our objective was not to provide national estimates, but to look at the potential mediating effects of sleep duration and insomnia on the relationship between depression and hypertension incidence. Third, our study’s baseline measures were taken from the 1982–1984 follow-up to the NHANES I, so the weights created for baseline measures taken from the 1971–1975 NHANES I did not account for subjects who were lost to follow-up between the two waves. Fourth, there have been differences of opinion regarding the appropriateness of using the sample weights with the NHANES.28
Results
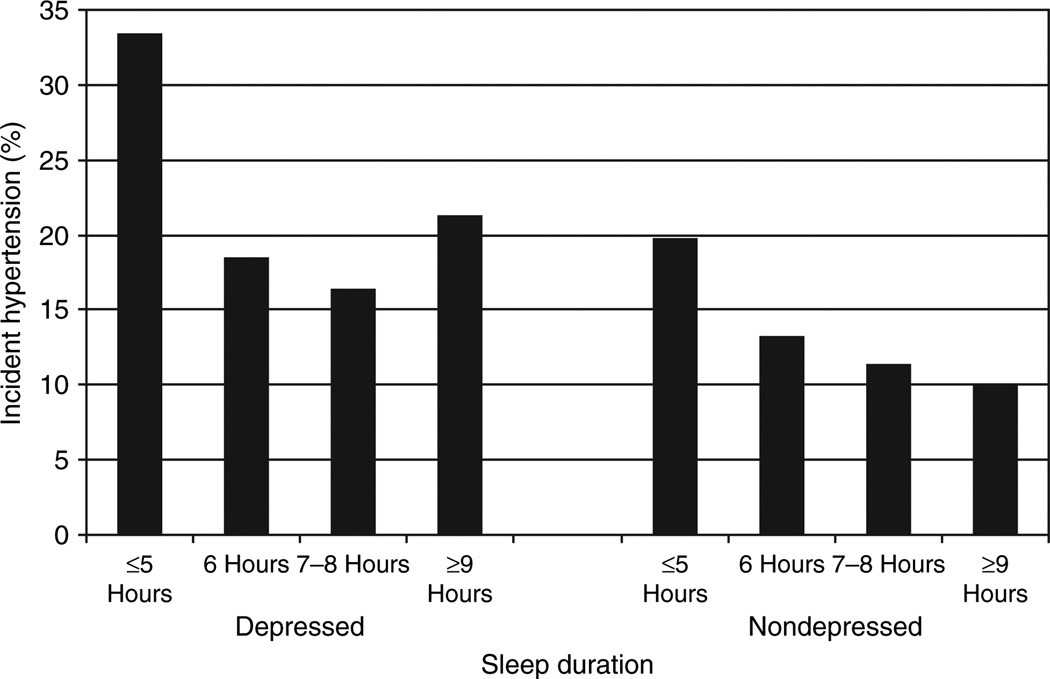
We found sleep duration to act as an effect modifier in the relationship between depression and hypertension incidence in middle-aged subjects (P = 0.05). In middle-aged subjects, the presence of depression, and either short or long sleep duration was associated with a higher incidence of hypertension (Figure 1). None of the interaction terms between depression and the other covariates were significant. Tables 1 and 2 show the results from bivariate analyses. Depression and hypertension incidence were both associated with short and long sleep durations, non-Caucasian race/ethnicity, low education, obesity, diabetes, alcohol abstinence, physical inactivity, higher insomnia scores, and higher pulse rate. Depression was also associated with hypertension incidence, female sex, age ≥70, underweight obese body weights, and increased cigarette smoking, whereas hypertension incidence was also associated with age ≥80. Both short and long sleep durations were associated with older age, non-Caucasian ethnicity, low education, alcohol abstinence, and low physical activity. Short sleep duration was associated with overweight and obese body weights, diabetes, and increased cigarette smoking. Higher insomnia scores were associated with increasing age, female sex, Caucasian ethnicity, low education, diabetes, alcohol abstinence, decreased cigarette smoking, and lower physical activity.
Figure 1.
Relationship between sleep duration and hypertension incidence in both depressed and nondepressed middle-aged subjects.
Table 1.
Relationships between baseline characteristics, depression, and hypertension incidence over follow-up
| Baseline characteristics | n | Depression at baseline | P value | Hypertension incidence over follow-up | P value | ||
|---|---|---|---|---|---|---|---|
| Yes (%) | No (%) | Yes (%) | No (%) | ||||
| n (%) | 743 (15.1) | 4,170 (84.9) | 663 (13.5) | 4,250 (86.5) | |||
| Depression at baseline | |||||||
| Yes | 743 | 19 | 81 | <0.0001 | |||
| No | 4,170 | 12 | 88 | ||||
| Sleep duration | |||||||
| ≤5 h | 363 | 31 | 69 | <0.0001 | 20 | 80 | 0.0003 |
| 6 h | 946 | 16 | 84 | 14 | 86 | ||
| 7–8 h | 3,240 | 13 | 87 | 12 | 88 | ||
| ≥9 h | 364 | 18 | 82 | 15 | 85 | ||
| Age in years | |||||||
| 32–39 | 1,067 | 15 | 85 | 0.0102 | 11 | 89 | 0.0227 |
| 40–49 | 1,574 | 14 | 86 | 14 | 86 | ||
| 50–59 | 1,052 | 15 | 85 | 14 | 86 | ||
| 60–69 | 595 | 14 | 86 | 14 | 86 | ||
| 70–79 | 453 | 19 | 81 | 14 | 86 | ||
| 80–86 | 172 | 23 | 77 | 18 | 82 | ||
| Sex | |||||||
| Women | 3,127 | 18 | 82 | <0.0001 | 14 | 86 | 0.1396 |
| Men | 1,786 | 11 | 89 | 13 | 87 | ||
| Race/ethnicity | |||||||
| Caucasian | 4,510 | 15 | 85 | 0.0289 | 13 | 87 | 0.0175 |
| Non-Caucasian | 403 | 19 | 81 | 17 | 83 | ||
| Education | |||||||
| >High school graduate | 1,436 | 21 | 79 | <0.0001 | 16 | 84 | 0.0002 |
| High school graduate | 3,477 | 13 | 87 | 12 | 88 | ||
| Body weight | |||||||
| Underweight (BMI ≤18.5) | 177 | 22 | 78 | 0.0216 | 8 | 92 | <0.0001 |
| Normal weight (BMI >18.5 and <25) | 2,485 | 15 | 85 | 9 | 91 | ||
| Overweight (BMI ≥25 and <30) | 1,628 | 14 | 86 | 16 | 84 | ||
| Obese (BMI ≥30) | 623 | 17 | 83 | 26 | 74 | ||
| Diabetes at baseline | |||||||
| Yes | 168 | 29 | 71 | <0.0001 | 21 | 79 | 0.0046 |
| No | 4,745 | 15 | 85 | 13 | 87 | ||
| Alcohol abstinent | |||||||
| Yes | 1,683 | 19 | 81 | <0.0001 | 16 | 84 | 0.0009 |
| No | 3,230 | 13 | 87 | 12 | 88 | ||
| Cigarettes per day | |||||||
| = 0 | 3,412 | 14 | 86 | 0.0029 | 13 | 87 | 0.0905 |
| >0 and <20 | 507 | 17 | 83 | 11 | 89 | ||
| ≥20 | 994 | 18 | 82 | 15 | 85 | ||
| Physical activity | |||||||
| 2-Low | 437 | 33 | 67 | <0.0001 | 18 | 82 | 0.0039 |
| 3 | 887 | 17 | 83 | 15 | 85 | ||
| 4 | 2,094 | 13 | 87 | 13 | 87 | ||
| 5 | 962 | 12 | 88 | 12 | 88 | ||
| 6-High | 533 | 11 | 89 | 11 | 89 | ||
| Average insomnia score | 4,913 | 8.4 (2.9)a | 6.5 (2.3)a | <0.0001 | 7.1 (2.6)a | 6.7 (2.5)a | <0.0001 |
| Pulse rate | 4,913 | 72.1 (10.6)a | 70.0 (9.7)a | <0.0001 | 71.2 (10.1)a | 70.2 (9.9)a | 0.0108 |
BMI, body mass index.
Mean (s.d.).
Table 2.
Relationships between baseline characteristics, sleep duration, and insomnia
| Baseline characteristics | Sleep duration in hours | P value | Mean insomnia score (mean (s.d.)) |
P value | |||
|---|---|---|---|---|---|---|---|
| ≤5 | 6 | 7–8 | ≥9 | ||||
| n (%) | 363 (7.4) | 946 (19.3) | 3,240 (66.0) | 364 (7.4) | |||
| Age in years | |||||||
| 32–39 | 16 | 22 | 23 | 19 | <0.0001 | 6.06 (2.3) | <0.0001 |
| 40–49 | 28 | 35 | 33 | 22 | 6.54 (2.4) | ||
| 50–59 | 25 | 22 | 22 | 15 | 7.15 (2.5) | ||
| 60–69 | 14 | 12 | 12 | 14 | 7.23 (2.7) | ||
| 70–79 | 13 | 7 | 8 | 20 | 7.43 (2.8) | ||
| 80–86 | 4 | 3 | 3 | 11 | 7.62 (2.8) | ||
| Sex | |||||||
| Women | 64 | 62 | 64 | 63 | 0.5409 | 6.98 (2.6) | <0.0001 |
| Men | 36 | 38 | 36 | 37 | 6.39 (2.4) | ||
| Race/ethnicity | |||||||
| Caucasian | 87 | 90 | 93 | 88 | <0.0001 | 6.84 (2.5) | <0.0001 |
| Non-Caucasian | 13 | 10 | 7 | 12 | 6.05 (2.7) | ||
| Education | |||||||
| >High school graduate | 43 | 29 | 26 | 44 | <0.0001 | 7.08 (2.8) | <0.0001 |
| High school graduate | 57 | 71 | 74 | 56 | 6.64 (2.4) | ||
| Body weight | |||||||
| Underweight (BMI ≤18.5) | 4 | 3 | 3 | 7 | <0.0001 | 7.06 (2.6) | 0.3701 |
| Normal weight (BMI >18.5 and <25) | 42 | 47 | 53 | 48 | 6.79 (2.5) | ||
| Overweight (BMI ≥25 and <30) | 37 | 35 | 32 | 34 | 6.72 (2.5) | ||
| Obese (BMI ≥30) | 17 | 15 | 12 | 11 | 6.75 (2.6) | ||
| Diabetes at baseline | |||||||
| Yes | 6 | 3 | 3 | 4 | 0.0086 | 7.79 (3.1) | <0.0001 |
| No | 94 | 97 | 97 | 96 | 6.74 (2.5) | ||
| Alcohol abstinent | |||||||
| Yes | 42 | 32 | 33 | 47 | <0.0001 | 6.88 (2.8) | 0.0320 |
| No | 58 | 68 | 67 | 53 | 6.72 (2.4) | ||
| Cigarettes per day | |||||||
| = 0 | 65 | 66 | 70 | 74 | 0.0141 | 6.87 (2.5) | 0.0001 |
| >0 and <20 | 12 | 10 | 11 | 8 | 6.56 (2.6) | ||
| ≥20 | 2 | 24 | 19 | 18 | 6.53 (2.5) | ||
| Physical activity | |||||||
| 2-Low | 15 | 7 | 8 | 16 | <0.0001 | 7.57 (2.9) | <0.0001 |
| 3 | 19 | 17 | 18 | 19 | 7.03 (2.5) | ||
| 4 | 34 | 43 | 44 | 41 | 6.78 (2.4) | ||
| 5 | 21 | 20 | 20 | 18 | 6.45 (2.4) | ||
| 6-High | 11 | 13 | 11 | 6 | 6.22 (2.5) | ||
BMI, body mass index.
Table 3 shows the hazards ratios of being diagnosed with hypertension over follow-up. There were 663 total incident cases of hypertension over this period. The relationships between depression, sleep duration, insomnia, and hypertension incidence differed between middle-aged and elderly subjects. Among middle-aged subjects, those who suffered from depression were significantly more likely (Model 1, HR = 1.65, 95% confidence interval (CI) 1.33–2.05) to have been diagnosed with hypertension over the follow-up period, and these results were attenuated with the inclusion of the covariates in Model 2 (HR = 1.44, 95% CI 1.15–1.80). Consistent with our hypothesis that sleep duration and insomnia would act as mediators of the relationship between depression and hypertension incidence, the inclusion of sleep duration and insomnia together in Model 5 appreciably attenuated the association (Model 5, HR = 1.27, 95% CI 1.00–1.61) with the preponderance of the change attributable to insomnia. In fully adjusted models, subjects who reported sleeping ≤5 h per night were 50% more likely (HR = 1.50, 95% CI 1.11–2.02) to have been diagnosed with hypertension over the follow-up period than subjects who reported sleeping 7–8 h per night, and for each one unit increase in the insomnia score, the odds of being diagnosed with hypertension over the follow-up period increased by 5% (HR = 1.05, 95% CI 1.01–1.09). Depression, sleep duration, and insomnia were not significantly associated with hypertension incidence in elderly subjects.
Table 3.
Hazards ratios (95% CI) as computed with Cox proportional hazards models for hypertension incidence over the follow-up period by depression, sleep duration, and insomnia at baseline
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |
|---|---|---|---|---|---|
| Ages 32–86 | |||||
| Depression | 1.61 (1.34–1.94) | 1.41 (1.16–1.71) | 1.37 (1.13–1.67) | 1.32 (1.08–1.62) | 1.31 (1.07–1.60) |
| Sleep duration | |||||
| ≤5 h | 1.34 (1.04–1.73) | 1.25 (0.96–1.63) | |||
| 6 h | 1.02 (0.84–1.24) | 0.99 (0.81–1.21) | |||
| 7–8 h | 1.00 | 1.00 | |||
| ≥9 h | 1.15 (0.87–1.53) | 1.17 (0.88–1.56) | |||
| Insomnia | 1.04 (1.01–1.07) | 1.03 (1.00–1.07) | |||
| Ages 32–59 | |||||
| Depression | 1.65 (1.33–2.05) | 1.44 (1.15–1.80) | 1.39 (1.10–1.74) | 1.28 (1.01–1.62) | 1.27 (1.00–1.61) |
| Sleep duration | |||||
| ≤5 h | 1.66 (1.25–2.22) | 1.50 (1.11–2.02) | |||
| 6 h | 1.07 (0.86–1.34) | 1.03 (0.82–1.30) | |||
| 7–8 h | 1.00 | 1.00 | |||
| ≥9 h | 0.97 (0.64–1.47) | 0.99 (0.65–1.50) | |||
| Insomnia | 1.07 (1.03–1.11) | 1.05 (1.01–1.09) | |||
| Ages 60–86 | |||||
| Depression | 1.45 (1.02–2.07) | 1.29 (0.89–1.87) | 1.32 (0.91–1.93) | 1.34 (0.92–1.97) | 1.33 (0.91–1.96) |
| Sleep duration | |||||
| ≤5 h | 0.80 (0.46–1.38) | 0.81 (0.46–1.44) | |||
| 6 h | 0.83 (0.54–1.29) | 0.84 (0.54–1.31) | |||
| 7–8 h | 1.00 | 1.00 | |||
| ≥ 9 h | 1.29 (0.85–1.94) | 1.28 (0.85–1.94) | |||
| Insomnia | 0.98 (0.92–1.03) | 0.99 (0.93–1.06) |
CI, confidence interval.
Model 1, unadjusted.
Model 2, adjusted for age, sex, race/ethnicity, education, body weight, diabetes, alcohol consumption, cigarette smoking, physical activity, and pulse rate.
Model 3, adjusted for variables in Model 2 plus sleep duration.
Model 4, adjusted for variables in Model 2 plus insomnia.
Model 5, adjusted for variables in Model 2 plus sleep duration and insomnia.
Discussion
We found depression to increase the risk for hypertension incidence, and our results are consistent with insomnia, and to a lesser extent, sleep duration acting as mediators of this relationship in middle-aged subjects. We found significant associations between the exposures of depression, insomnia, and sleep duration, and the outcome of hypertension incidence only in middle-aged subjects and not in elderly subjects. There are a number of potential explanations for the different relationships found in middle-aged and elderly subjects. First, short sleep duration has been found to be more strongly associated with hypertension incidence17 and obesity24 in middle-aged subjects than in elderly subjects. Second, advanced age is associated with changes in sleep architecture with increased difficulties initiating and maintaining sleep,29 so differences in sleep parameters between depressed and nondepressed elderly subjects would be less marked than for middle-aged subjects. Third, depression has been found in elderly subjects to be associated with systolic hypotension30 and a lowering of systolic blood pressure over time.31 One recent cross-sectional study with adult subjects (ages 18–65) found depression to be associated with lower systolic blood pressure and a lower prevalence of isolated systolic hypertension,32 but the authors did not test for interaction by age or conduct analyses stratified by age, so it is likely that the associations they found were weighted by lower systolic blood pressures in elderly subjects suffering from depression. Combined systolic and diastolic hypertension is common in middle-aged hypertensive patients, whereas isolated systolic hypertension accounts for over 60% of hypertension in elderly populations,33 so the lack of an association between depression and hypertension in elderly subjects in our study could be due to the specific influences of depression on systolic blood pressure in elderly subjects.
Middle-aged subjects who suffered from depression were 65% more likely to be diagnosed with hypertension over the follow-up period. Controlling for covariates partially attenuated the relationship. The relationship was then further attenuated after controlling for sleep duration, so the influence of depression on hypertension incidence is likely to be related in part to the effects of sleep duration on blood pressure. Our findings that both short and long sleep durations are associated with depression are consistent with the fact that insomnia and hypersomnia are symptoms of depression that can directly affect sleep duration. We found short, but not long, sleep duration to be associated with hypertension incidence. Chronic short sleep durations could result in prolonged exposure to raised 24-h blood pressure and heart rate that could entrain the cardiovascular system to operate at a higher pressure equilibrium through structural adaptations, such as arterial and left ventricular hypertrophic remodeling.20 The relationship between depression and hypertension incidence was also attenuated with the inclusion of insomnia in the multivariate models, so insomnia could also be on the causal pathway between depression and hypertension. Insomnia could contribute toward hypertension through increased activation of the hypothalamic–pituitary–adrenal axis34 and through less pronounced nocturnal fall in systolic and diastolic blood pressure.35
Short sleep duration and insomnia are different, yet related entities. Insomnia entails dissatisfaction with the quality of sleep and an inability to sleep given adequate opportunity. Insomnia can result in short sleep duration, but individuals with short sleep duration do not necessarily suffer from insomnia. They may sleep less because they choose to do so or because they lack the opportunity to sleep. In the final multivariate model that included both sleep duration and insomnia, both short sleep duration and insomnia were significantly associated with hypertension incidence. The hazards ratios for both short sleep duration and insomnia were attenuated when they were included together in the final multivariate model, indicating their influence upon one another’s ability to affect hypertension incidence.
Adjustment for sleep duration and insomnia reduced the strength of the association between depression and hypertension incidence by 33% (Model 2 β = 0.36, Model 5 β = 0.24). The ability of covariates, such as body weight, diabetes, and physical activity, to reduce the strength of the association between depression and hypertension incidence could also be partially attributable to the effects of sleep duration and insomnia on these covariates. Sleep duration has been linked to diabetes and obesity in both experimental21,22 and epidemiologic studies.23,24 Feeling tired from inadequate sleep could lessen one’s desire to engage in physical activity.
Although the results from this study are consistent with the hypothesis that insomnia, and to a lesser extent sleep duration, act as mediators of the relationship between depression and hypertension incidence, a number of limitations to these analyses must be considered as well. Statistical analyses alone cannot distinguish between mediation and confounding, and although strong arguments can be made that sleep disturbances represent a causal mechanism by which depression increases the risk for hypertension incidence, it is possible that sleep disturbances are associated with some other unmeasured mechanisms. Sleep disorders, such as sleep apnea, have been shown to be associated with both depression and hypertension incidence, so the presence of these disorders could have played a part in our results. The NHANES I follow-up survey did not include questions on sleep disorders, but we would expect that individuals with sleep apnea would be more likely to self-report longer sleep durations because they are often unaware of their disrupted sleep patterns and require more sleep to compensate for poor sleep quality. The prevalence of sleep apnea in patients with insomnia has been found to be only 6% (ref. 36), so it is unlikely that sleep apnea played a large role in our results. Another limitation of the study is the lack of repeated measures of depression, sleep duration, and insomnia over the follow-up period. Changes in these variables over follow-up could have affected the results. Hypertension also frequently goes undiagnosed, and we have no way of knowing whether subjects suffering from depression, short sleep duration, and insomnia were more or less likely than subjects without these conditions to seek or receive treatment, and therefore to be diagnosed with hypertension. Other limitations include possible bias arising from lost to follow-up and missing data on baseline risk variables.
The results from this study are consistent with the hypothesis that insomnia and sleep duration play roles in the etiology of hypertension in middle-aged individuals suffering from depression, suggesting that interventions that increase the amount and improve the quality of sleep could potentially serve as treatments and as primary preventative measures for hypertension in these individuals. Behavioral interventions could include assistance with implementing sleep hygiene practices and with modifying maladaptive sleep habits.
Acknowledgments
Financial support for this study was provided by R24 HL76857 from the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University’s Behavioral Cardiovascular Health and Hypertension Program.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Murray CJ, Lopez AD. Evidence-based health policy—lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 3.Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 4.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66:814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawara M, Witzke W, Matussek N. Hypertension in depression. Psychiatry Res. 1987;21:85–86. doi: 10.1016/0165-1781(87)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Adamis D, Ball C. Physical morbidity in elderly psychiatric inpatients: prevalence and possible relations between the major mental disorders and physical illness. Int J Geriatr Psychiatry. 2000;15:248–253. doi: 10.1002/(sici)1099-1166(200003)15:3<248::aid-gps102>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 1997;6:43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- 8.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 9.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 10.Townsend MH, Bologna NB, Barbee JG. Heart rate and blood pressure in panic disorder, major depression, and comorbid panic disorder with major depression. Psychiatry Res. 1998;79:187–190. doi: 10.1016/s0165-1781(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 11.Grewen KM, Girdler SS, Hinderliter A, Light KC. Depressive symptoms are related to higher ambulatory blood pressure in people with a family history of hypertension. Psychosom Med. 2004;66:9–16. doi: 10.1097/01.psy.0000106881.60228.16. [DOI] [PubMed] [Google Scholar]
- 12.Scalco AZ, Scalco MZ, Azul JB, Lotufo Neto F. Hypertension and depression. Clinics (Sao Paulo) 2005;60:241–250. doi: 10.1590/s1807-59322005000300010. [DOI] [PubMed] [Google Scholar]
- 13.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–121. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Skilton MR, Moulin P, Terra JL, Bonnet F. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry. 2007;62:1251–1257. doi: 10.1016/j.biopsych.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 16.Gislason T, Almqvist M. Somatic diseases and sleep complaints. An epidemiological study of 3,201 Swedish men. Acta Med Scand. 1987;221:475–481. [PubMed] [Google Scholar]
- 17.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 18.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–350. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 19.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–1324. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 20.Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 23.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services, National Center for Health Statistics. National Health and Nutrition Examination Survey I: Epidemiologic Followup Study, 1982–1984, 1986, 1987, 1992 (Computer Files) Hyattsville, MD: U.S. Department of Health and Human Services, National Center for Health Services (producer); 1992, 1996. Inter-university Consortium for Political and Social Research (distributor):Ann Arbor, MI, 1992, 1997. [Google Scholar]
- 26.Cohen BB, Barbano HE, Cox CS, Feldman JJ, Finucane FF, Kleinman JC, Madans JH. Plan and operation of the NHANES I Epidemiologic Followup Study: 1982–84. Vital Health Stat 1. 1987;22:1–142. [PubMed] [Google Scholar]
- 27.Radloff LS, Locke BZ. The community mental health assessment survey and the CES-D scale. In: Weissman MM, Myers JK, Ross CE, editors. Community Surveys of Psychiatric Disorders. New Brunswick, NJ: Rutgers University Press; 1986. pp. 177–189. [Google Scholar]
- 28.Ingram DD, Makuc DM. Statistical issues in analyzing the NHANES I Epidemiologic Followup Study. Series 2: data evaluation and methods research. Vital Health Stat 2. 1994;121:1–30. [PubMed] [Google Scholar]
- 29.Prinz PN. Age impairments in sleep, metabolic and immune functions. Exp Gerontol. 2004;39:1739–1743. doi: 10.1016/j.exger.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Stroup-Benham CA, Markides KS, Black SA, Goodwin JS. Relationship between low blood pressure and depressive symptomatology in older people. J Am Geriatr Soc. 2000;48:250–255. doi: 10.1111/j.1532-5415.2000.tb02642.x. [DOI] [PubMed] [Google Scholar]
- 31.Hildrum B, Mykletun A, Holmen J, Dahl AA. Effect of anxiety and depression on blood pressure: 11-year longitudinal population study. Br J Psychiatry. 2008;193:108–113. doi: 10.1192/bjp.bp.107.045013. [DOI] [PubMed] [Google Scholar]
- 32.Licht CM, de Geus EJ, Seldenrijk A, van Hout HP, Zitman FG, van Dyck R, Penninx BW. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631–638. doi: 10.1161/HYPERTENSIONAHA.108.126698. [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 34.Rodenbeck A, Hajak G. Neuroendocrine dysregulation in primary insomnia. Rev Neurol (Paris) 2001;157:S57–S61. [PubMed] [Google Scholar]
- 35.Prejbisz A, Kabat M, Januszewicz A, Szelenberger W, Piotrowska AJ, Piotrowski W, Piwonski J, Makowiecka-Ciesla M, Widecka K, Patera B, Bieniaszewski L, Narkiewicz K, Tykarski A, Piejko A, Grodzicki T, Czerwienska B, Wiecek A. Characterization of insomnia in patients with essential hypertension. Blood Press. 2006;15:213–219. doi: 10.1080/08037050600963040. [DOI] [PubMed] [Google Scholar]
- 36.Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn MA, Karacan I, Kupfer DJ, Lemmi H, Miles LE, Orr WC, Phillips ER, Roth T, Sassin JF, Schmidt HS, Weitzman ED, Dement WC. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA. 1982;247:997–1003. [PubMed] [Google Scholar]