Abstract
Initial administration of 60% nitrous oxide (N2O) at 21°C ambient temperature reduces core temperature (Tc) in rats, but tolerance develops to this hypothermic effect over several administrations. After additional N2O administrations, a hyperthermic overcompensation (sign-reversal) develops such that Tc exceeds control levels during N2O inhalation. This study investigated whether rats would employ behavioral thermoregulation to facilitate, or oppose, a previously acquired hyperthermic overcompensation during N2O administration. To establish a hyperthermic sign-reversal, male Long-Evans rats (N = 12) received 10 3-h administrations of 60% N2O while housed in a gas-tight, live-in, “inactive” thermal gradient (∼21°C). Following the tenth N2O exposure, the thermal gradient was activated (range of 10–37°C), and rats received both a control gas session and a 60% N2O test session in counterbalanced order. Mean Tc during N2O inhalation in the inactive gradient was reliably hypothermic during the first exposure but was reliably hyperthermic by the tenth exposure. When subsequently exposed to 60% N2O in the active gradient, rats selected a cooler Ta, which blunted the hyperthermic sign-reversal and lowered Tc throughout the remainder of the N2O exposure. Thus, autonomic heat production effectors mediating the hyperthermia were opposed by a behavioral effector that promoted increased heat loss via selection of a cooler ambient temperature. These data are compatible with an allostatic model of drug addiction that suggests that dysregulatory overcompensation in the drugged-state may motivate behaviors (e.g., drug taking) that oppose the overcompensation, thereby creating a vicious cycle of escalating drug consumption and recurring dysregulation.
Keywords: allostasis, drug tolerance, homeostasis, nitrous oxide, thermoregulation
Abbreviations
- N2
nitrogen
- N2O
nitrous oxide
- O2
oxygen
- Tc
core temperature
- Tsel
selected ambient temperature
Introduction
The homeostatic adaptation model of drug addiction asserts that drug-dependent individuals who stop taking their drug become increasingly motivated to re-administer it to prevent or ameliorate symptoms of drug withdrawal.1-6 Drug withdrawal involves “drug-opposite” effects that are thought to motivate drug-taking behavior to pharmacologically counter the withdrawal effects and return the motivationally relevant affective and physiological dependent measures to baseline levels. From this perspective, drug tolerance and dependence reflect a motivationally neutral balance between drug effects and acquired adaptive responses, while drug withdrawal reflects an imbalance that motivates drug-taking behavior. Successfully ameliorating or avoiding an aversive drug withdrawal state through drug taking increases the likelihood that drug-taking behaviors will occur in the future.2,7
In contrast to a homeostatic adaptation model of drug addiction, an allostatic model of drug addiction posits that drug-opposite responses, while initially adaptive, can eventually increase to such an extent that they overcompensate for a drug's effects, thereby causing the dependent measure to exceed fully tolerant levels in the opposite direction to the drug's effect (i.e., a sign-reversal). Because this overcompensation occurs in the continued presence of the drug, sign-reversals cannot be explained as drug withdrawal phenomena that result from waning drug concentrations.8 Some allostatic models of drug addiction suggest that the dysregulated overcompensation of the dependent measure can motivate behavior(e.g., increased drug consumption) that opposes the overcompensated state.9-11 This theme permeates several models of drug addiction (reviewed in8). For example,12 Colpaert et al. hypothesized that drug-opposite sign-reversal states have motivational properties that encourage further drug administration.13 Ossipov et al. proposed a vicious cycle of addiction hypothesis wherein allostatic over-corrections motivate increased drug taking as a means to correct the overcompensated state by increasing the pharmacological magnitude of the drug effect. Because excessive adaptive responses eventually develop to the increased drug effect, the allostatic-overcompensated state returns, and the individual is motivated to further increase drug taking.
In studies of chronic tolerance development to nitrous oxide (N2O)-induced hypothermia, we observed that when N2O administrations were continued after complete tolerance had developed, a robust hyperthermic sign-reversal effect emerged.14–16 In accordance with the allostatic concept of addiction, one would hypothesize that a hyperthermic sign-reversal state acquired by rats in response to breathing a particular concentration of N2O would motivate a further increase of N2O intake as a means to blunt the dysregulated warming response.16 However, a direct test of this concept awaits development of a suitable experimental method for N2O self-administration. In the interim, we chose an alternative strategy in which rats were provided with a non-pharmacological behavioral means of restoring normothermia. Specifically, rats that had already developed a hyperthermic sign-reversal during N2O administration at a fixed ambient temperature in an inactive thermal gradient were subsequently administered N2O in the activated thermal gradient to determine whether they would select lower ambient temperatures that would oppose the sign-reversal state.
Materials and Methods
Subjects
Male Long-Evans rats (Charles River, N = 12; 6 squads of 2 each) arrived in the lab at 25–28 d of age. Both rats in a squad were housed together in a polycarbonate tub with free access to water and pelleted chow (5053 PicoLab Rodent Diet 20, Animal Specialties and Provisions, Quakertown, PA). The housing room and live-in thermal gradients had a 12-h:12-h light/dark cycle (lights on at 0700 h) at an ambient temperature of 22 ± 1°C. Following surgery and recovery, experimental testing began 12 d after the rats had arrived in the lab. Rats weighed 146.3 ± 38.9 g at the start of testing and 329.5 ± 32.7 g (Mean ± SD) at the end of the study. All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Thermal gradient
The thermal gradient system allows a rat to select its preferred ambient temperature as a function of its choice of location within an alleyway. In brief, a removable rectangular acrylic alleyway is suspended within an insulated copper shell that is cooled at one end and heated at the other end, thereby creating a temperature continuum along the length of the alleyway. Our lab's 2 thermal gradients are based on a previously published design17,18 that we modified to make the gradients gas-tight. Pelleted chow and water were freely available in the center of the alleyway. During the present study, the inactive thermal gradient condition provided a small range of ambient temperatures with a mean low temperature of 19.6°C (SD = 0.28) at either end of the alleyway and a mean high temperature of 21.8°C (SD = 0.49) in the center. The active thermal gradient provided a large range of ambient temperatures with a mean low temperature of 10.2°C (SD = 0.94) at one end of the alleyway and a mean high temperature of 36.9°C (SD = 0.67) at the other. The relationship between the temperature at each location along the length of the alleyway was similar to that described by Gordon and colleagues17. (A photograph of our thermal gradient system and additional details about its design and operation are available in Part I of the online supplement.)
One of 2 gas mixtures was delivered to each thermal gradient, i.e., either control gas consisting of room air, or 60% N2O. Specifically, the control gas was made from room air that was purified, dehumidified and compressed, and then delivered to the thermal gradient at a flow rate of 10 L/min. The N2O gas had the same flow rate and was composed of 60% N2O, 21% oxygen (O2), and 19% nitrogen (N2). [A 10 L/min blend of 79% N2O, 21% O2, and 0% control gas was delivered for the first 12 min of the 60% N2O gas condition to achieve the targeted 60% N2O gas concentration more quickly.] Concentrations of N2O, O2, and CO2 were measured using an infrared gas analyzer that sampled gas in the incurrent and excurrent gas lines connected to the gradient's copper shell.
Telemetric measurement of Tc, data acquisition and instrument control
Telemetric measurement of Tc was accomplished using a commercial system from Data Sciences International (Saint Paul, MN) that consists of a Data-Exchange Matrix, Physio-Tel Receiver (Model RPC-1), Dataquest ART 4.2 software, and an implantable battery-powered temperature sensor (model TA-F40) implanted in the rat's peritoneal cavity. The antenna wires surrounding the sides of the alleyway suspended inside the thermal gradient are exteriorized through a sealed port and connected to the Physio-Tel Receiver. All other instrument control and data acquisition were performed using custom programs written in LabVIEW 6.8 (National Instruments, Austin, Texas).
Surgical placement of the telemetric temperature sensor
At least one week prior to the start of testing, a telemetric temperature sensor was implanted surgically into each rat's peritoneal cavity using isoflurane anesthesia (3–5% for induction and 1–3% for maintenance) while the rat was on a 39°C heating pad. Meloxicam (an NSAID) was provided in the drinking water (0.02 mg/ml H2O) from 1 d before to 2 d after surgery.
Experimental design and procedures
Each rat received 10 3-h exposures to 60% N2O while in an “inactive” room-temperature thermal gradient. The inactive thermal gradient provided a small selection of ambient temperatures with a mean low temperature of 19.6°C (SD = 0.28) at either end of the alleyway and a mean high temperature of 21.8°C (SD = 0.49) in the center. The temperature-controlled water baths circulating water around either end of the gradient were both set at 18°C. During weekdays, rats lived in the inactive thermal gradient breathing control gas except when N2O administrations occurred. Specifically, during the first week each rat was placed in the inactive thermal gradient on Monday at 0900 h. At 1200 h, a 3-h steady-state 60% N2O administration occurred. At 1500 h, the gas delivery reverted to control gas. The rats continued to live in the thermal gradient with the subsequent N2O exposures occurring on Wednesday and then on Friday at the same time of day. Rats were briefly removed from the thermal gradient between 1600–1615 h on Wednesdays so that the waste trays could be cleaned, additional food provided as needed, and the alleyway inspected. At 1600 h on Fridays, rats were returned to the colony room for the weekend. The thermal gradient components were washed/sanitized prior to the rat entering the thermal gradient on Monday morning. Rats were weighed Monday morning prior to entering the gradient. This weekly schedule continued over consecutive weeks for a total of 10 N2O exposures.
The final (10th) N2O exposure in the inactive gradient occurred on a Monday and was completed at 1500 h. At 1700 h, the thermal gradient was activated and the rat continued to live in the active thermal gradient while breathing control gas. The active thermal gradient provided a large range of ambient temperatures with a mean low temperature of 10.2°C (SD = 0.94) at one end of the alleyway and a mean high temperature of 36.9°C (SD = 0.67) at the other. The temperature-controlled recirculating water bath at one end was set at 1°C and the one at the other end was set at 42°C. Rats were briefly removed from the thermal gradient between 1600–1615 h on Tuesday so that the waste trays could be cleaned, additional food provided as needed, and the alleyway inspected. Each rat subsequently received both a 3-h 60% N2O administration and a control gas administration in the active thermal gradient between 1200 and 1500 h on Wednesday and Thursday in counter-balanced order.
Data reduction
The rat's position in the alleyway was recorded at 7-s intervals via infrared beam breaks from 24 locations, spaced 7.62 cm apart. Position was computed as the average value of the location numbers of the interrupted infrared signals. Distance traveled (Dist.) was computed as the absolute value of the difference between successive time-stamped rat-position values multiplied by 7.62 cm. Distance was summed during each 6-min time bin. Ambient temperature at the rat's position within the gradient (Tsel) was logged at the time the rat's position was recorded. Tsel was calculated as the mean temperature of the thermistor(s) corresponding with the interrupted infrared beam location(s). Tc data were recorded at 30-s intervals. Median Tc and mean Tsel values were computed within each 6-min bin.
Statistical analyses
The correlated within-subjects longitudinal data were analyzed using the linear mixed-model program in SPSS Statistics 20 (IBM, Somers, NY). Session and condition were treated as fixed effects. Unless otherwise specified, unstructured covariance matrices were employed for statistical comparisons because variances for thermal outcomes differed between N2O and control-gas conditions. For comparisons between N2O and control-gas conditions, means and 95% confidence intervals were adjusted for baseline values.
Statistical analyses refer to 5 temporal periods: baseline (−60 to 0 min), early-experimental (0 to 90 min), late-experimental (90 to 180), entire-experimental (0 to 180 min) and post-experimental (180 to 240 min).
Normally distributed data (Tc, Tsel and Δdistance) were summarized as means with 95% confidence intervals (CI) to convey the magnitude and uncertainty range of each outcome. Distance magnitudes were summarized in terms of medians ±05th percentile (p05) and 95th percentile (p95). Baseline values were defined as the means or medians over the 60-min baseline. The null hypothesis was that N2O = control. Accordingly, 95% confidence intervals for N2O compared to control conditions that exclude zero are significant at P < 0.05, 2 tailed. We did not adjust for multiple comparisons due to the conundrums and misplaced emphasis that accompany this class of procedures when implemented in the context of basic preclinical research.19-21 [see Part II of the online supplement for additional details]. Readers are urged to judge our results on the basis of the 95% confidence intervals and their coherence across sessions.
Results
Patterns of Tc, Tsel and distance traveled during N2O administrations
Figure 1 provides an overview of the experimental design and summary of findings based on the mean difference (Δ) scores (with 95% confidence intervals) for Tc and Tsel that were calculated as the mean 1-h baseline value subtracted from the mean value of the entire 3-h experimental period. ΔTc is depicted during each session; i.e., when the thermal gradient was both inactive (off) and active (on), whereas ΔTsel is only provided during sessions when the thermal gradient was active (on). The hypothermic effect of 60% N2O on ΔTc was obvious during the initial N2O administration in the inactive gradient.
Figure 1.
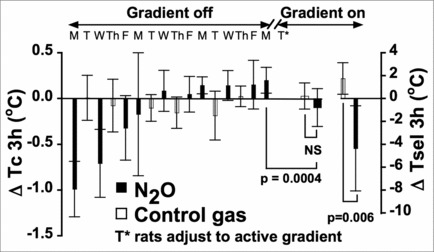
Overview of experimental design and summary of findings. Individual rats were housed continuously in the thermal gradient Monday through Friday and returned to their home cages on Saturday and Sunday. The 3-h N2O sessions (or continued control gas administrations) occurred between 1200 and 1500 h. The range of ambient temperatures within the gradient was maintained at 21 ± 1°C during the initial 16 d (i.e., “Gradient off”). The gradient was then activated (i.e., “Gradient on”) to provide a temperature range of approximately 10–37°C with the first Tuesday provided as a day to learn to use the active gradient. While in the active thermal gradient, each rat received both a 3-h 60% N2O administration and a control gas administration on Wednesday and Thursday in counter-balanced order. Tc and Tsel (for the active gradient phase) values depict mean change from 1-h baseline during the 3-h administration period ±95% confidence intervals. Major findings: Rats acquired a hyperthermic core temperature (Tc) change during 60% N2O inhalation in the inactive gradient over trials, but exhibited a relatively normothermic Tc during N2O administration in the active gradient while selecting cooler ambient temperatures (Tsel). Interpretation: Access to a behavioral thermoregulatory response can offset an acquired hyperthermic change of Tc.
Chronic tolerance followed by a hyperthermic sign-reversal developed for ΔTc over the subsequent 9 N2O administrations (Fig. 1). For the 6 interspersed control sessions, ΔTc did not change. Following these 16 inactive gradient sessions, the thermal gradient was activated and rats received both a 60%-N2O session and a control-gas session. The elevated ΔTc observed during the tenth N2O administration in the inactive gradient was significantly greater than the ΔTc when N2O was administered in the active gradient (0.20 ± 0.14°C in inactive gradient versus −0.10 ± 0.21 in the active gradient; P = 0.0004; see Fig. 1).
A direct, within-subjects comparison of the N2O session and the control gas session in the active gradient revealed that: 1) ΔTc did not differ between sessions (0.03 ± 0.14°C control session vs. −0.10 ± 0.21°C in N2O session; P > 0.05), and 2) ΔTsel was significantly reduced during the N2O session compared to the control session (1.75 ± 1.38°C control session vs. −4.35 ± 3.72°C in N2O session; P = 0.006; see Fig. 1). Subsequent analyses (below) add a nuanced understanding of these observations.
Figure 2 presents Tc profiles across sessions in the inactive thermal gradient. As we observed previously,15 there was a prompt reduction of Tc during the initial N2O administration, but by the fourth and fifth N2O administrations, tolerance had developed fully. Subsequent N2O administrations revealed the gradual development of a transient hyperthermic sign-reversal; i.e., Tc began to rise at the onset of N2O, peaked within the first hour and then tapered off such that the sign-reversal of Tc occurred primarily during the first 90-min of N2O delivery. When Tc was averaged across the 6 control gas sessions in the inactive thermal gradient (i.e., control sessions 2, 4, 7, 9, 12, 14), Tc was 37.2 ± 0.20°C (mean ± SD) during the 3-h experimental period (last panel in Fig. 2). Tc did not differ by control session number (P = 0.49). Mean Tc during the experimental period in control sessions was slightly lower than during the 1-h baseline period (by 0.08 ± 0.037 (SE) °C; P = 0.03) as analyzed using a linear mixed model with session and interval as fixed effects.
Figure 2.
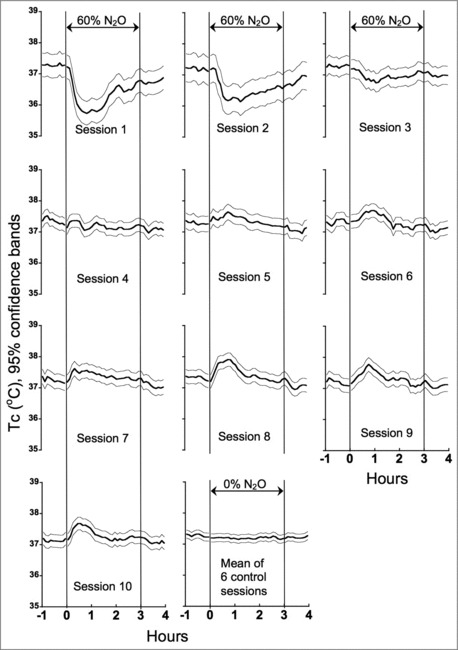
Temporal profiles of Tc across sessions in the inactive thermal gradient that provided a restricted range of ambient temperatures (∼21 ± 1°C). The mean ambient temperature selected by the rats (Tsel) in the inactive thermal gradient was 21.4°C ± 0.58 (SD).
Figure 3 (top and bottom row) presents averaged values for Tc and distance traveled (Dist.) stratified by within-session measurement interval. Baseline-adjusted ΔTc (Fig. 3, second row) was below control levels in both the early (first 4 sessions) and late (first 3 sessions) N2O periods, and ΔTc remained below control levels in the post-N2O period for the first 2 N2O sessions.
Figure 3.
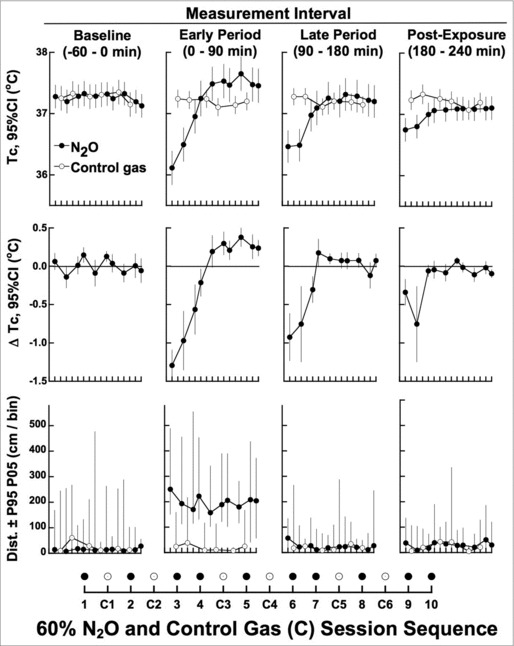
Mean Tc and distance measurements with statistical comparisons stratified by measurement interval in the inactive thermal gradient. First row depicts mean Tc within each measurement interval across all 16 N2O and control-gas sessions. Second row depicts statistical assessments involving linear mixed model repeated measures analysis of Tc differences between each N2O session and the average of all control sessions; values are adjusted for baseline Tc in the early, late and post N2O intervals. 95% confidence intervals that do not contain zero are significantly different from control at P < 0.05, 2-tailed. Third row depicts median distance traveled per 6-min bin with 5th and 95th percentile limits. Distance is expressed as change from baseline and adjusted for baseline during N2O and control administrations and differed reliably from control in every N2O session during the early measurement interval only (P < 0.001).
Rats developed an unambiguously reliable hyperthermic Tc sign-reversal in the early measurement interval in the 6th N2O session (see 95% confidence intervals for ΔTc). The hyperthermic Tc was largely confined to the early measurement period. Note the modest but reliable and relatively consistent increase of locomotion in the early measurement interval. This is important in part because physical activity could contribute to an increase in heat production, which could be one mechanism for increased Tc. However, the increases of locomotion were sufficiently modest (2–3 m/6 min) to be expected to have little impact on overall heat production.
Figure 4 provides more detail regarding the effect of providing rats with a powerful behavioral effector (i.e., the active thermal gradient) to influence Tc following establishment of a N2O-induced hyperthermic sign-reversal in the inactive gradient. Figure 4 also provides a more nuanced portrait of the behavioral thermoregulatory responses than suggested by averaging the data over the entire 3-h experimental period (Fig. 1). Note in particular that when N2O was administered in the active thermal gradient (Fig. 4, middle row), Tsel (mean ± SE) averaged across the entire 3-h N2O test session was 21.7 ± 1.14°C, which was significantly lower (P = 0.005) compared to the 1-h baseline of 26.03 ± 0.81°C, but did not differ significantly from the mean Tsel of 21.4 ± 0.17°C observed when N2O was administered during the final inactive gradient session. Despite the similarity in mean Tsel during N2O administration in the active and inactive gradients, rats in the active gradient intermittently selected ambient temperatures well below 21°C. When rats had access to the active thermal gradient during N2O administration, Tc was significantly lower than that observed during N2O administration in the final inactive gradient session [by −0.17 ± 0.077°C (P = 0.049) in the early measurement interval; by −0.27 ± 0.079°C (P = 0.005) in the late measurement interval].
Figure 4.
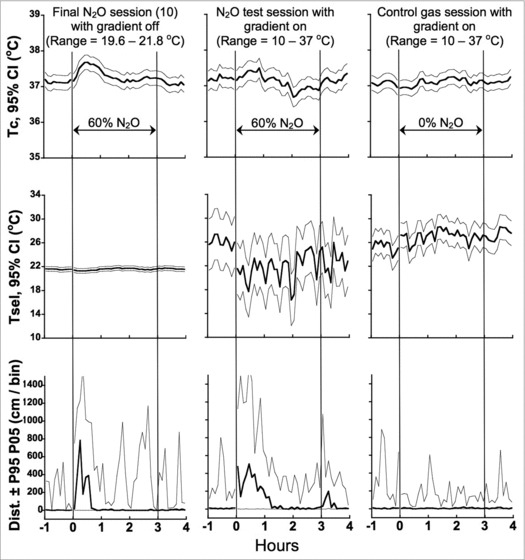
Temporal profiles of mean Tc and selected ambient temperature (Tsel) (95% confidence intervals) and median distance (5th and 95th percentile bands) outcomes in the final inactive-thermal-gradient session, and in the subsequent test and control sessions in the active thermal gradient.
For the 2 sessions in the active gradient, N2O was compared to the control gas for both ΔTc and ΔTsel measures during the baseline, early and late experimental periods, and the post-experimental period (Fig. 4). The change (Δ) scores were calculated for both measures as the N2O test session minus control test session and analyzed using linear mixed model with session as a fixed effect (mean ± SE). ΔTc did not differ during baseline nor the post-experimental period, while access to the active gradient blunted the hyperthermic sign-reversal in the early experimental period and caused hypothermia to occur in the late experimental period (Baseline ΔTc: 0.13 ± 0.11, P = 0.25; early experimental period ΔTc: 0.25 ± 0.11, P = 0.050; late experimental period ΔTc: −0.24 ± 0.072; P = 0.007; post-experimental period ΔTc: 0.062 ± 0.085; P = 0.49). ΔTsel did not differ during baseline but was then significantly reduced during both experimental periods as well as the post-experimental period (Baseline ΔTsel: 0.73 ± 1.10, P = 0.52; early experimental period ΔTsel: −5.74 ± 1.20, P = 0.001; late experimental period ΔTsel: −5.01 ± 1.34, P = 0.003; post-experimental period ΔTsel: −4.70 ± 1.38, P = 0.006).
Discussion
Consistent with previous findings in a calorimeter environment,14 mean Tc during serial 3-h 60% N2O administrations in the inactive gradient inverted from a significant hypothermia on the first exposure to a significant hyperthermia by the tenth exposure (P < 0.0001 for within-subjects change). However, when 60% N2O was subsequently administered in the now active gradient, rats voluntarily selected a significantly cooler ambient temperature (Tsel) and exhibited relatively normal Tc (Fig. 1).
These findings are consistent with the hypothesis that individuals are motivated to obviate an allostatic overcompensation during a drug administration if provided with the behavioral means to do so. The acquired overcompensation of Tc when N2O was administered in the “clamped” ambient temperature environment of the inactive thermal gradient was mitigated when rats were allowed to select cooler ambient temperatures in the active thermal gradient during N2O administration. This effect likely reflects the intermittent selection of ambient temperatures below 21°C.
During the active-gradient N2O test, ΔTc in the early measurement interval tended to be higher compared to the active-gradient control gas session (0.25 ± 0.113°C; P = 0.050). Previous total calorimetric work in our lab15,22 firmly implicates an acquired increase of metabolic heat production at N2O onset as the primary mechanism mediating the hyperthermic Tc sign-reversal phenomenon. Thus, it seems likely that the hyperthermia observed in the thermal gradient during N2O administration reflects increased metabolic heat production. Nevertheless, the effectiveness of the behavioral thermoregulatory response is revealed by the finding that during the active-gradient N2O test, Tc in both the early and late measurement intervals was significantly lower than when N2O was last administered in the inactive gradient [i.e., reduced by −0.17 ± 0.077°C (P = 0.049) in the early measurement interval; by −0.27 ± 0.079°C (P = 0.005) in the late measurement interval]. As depicted in Figure 1, the overall mean Tcs during the N2O and the control gas sessions in the active-gradient were not statistically different. The selection of cooler ambient temperatures during the active gradient N2O test blunted the expression of hyperthermia relative to what was present during the tenth N2O administration in the inactive gradient.
Recent research22 demonstrated that when rats receive 12 3-h administrations of 60% N2O in an active thermal gradient, hypothermia occurs initially, and this is followed by tolerance development and then by a transient hyperthermic sign-reversal of Tc. Despite these dramatic changes in Tc across repeated N2O administrations, rats consistently select a cooler ambient temperature while inhaling N2O. The concurrent competition between the effectors that increase heat production, and the behavioral effectors that facilitate heat loss (moving to a cool Tsel), is also consistent with allostatic regulation.8 Interestingly, those rats could have selected an even cooler Tsel to oppose the development of the hyperthermic sign-reversal, but this did not occur.22 Yet, in the present study, the rats did offset the sign-reversal when the thermal gradient initially became available during the eleventh N2O administration. However, based on the finding that serial N2O administrations in the active gradient did allow the hyperthermic sign-reversal to develop,22 the presumption is that the hyperthermic sign-reversal of Tc would be reestablished in the present study as the heat production effectors eventually overcompensate for the cooler selected ambient temperatures. This is compatible with a vicious cycle allostatic model of addiction. A next step would be to determine whether rats would administer greater concentrations of N2O in an attempt to increase the hypothermic pharmacological effect of N2O, as an alternative method of reducing the hyperthermic sign-reversal.
The results of this experiment have important implications for understanding physiological regulation in the context of drug use. A common interpretation of these data would conceive of Tc as a regulated variable that is defended around a set-point value by centrally coordinated effector action. Thus, it may seem incongruous to have effectors that are supposed to act in the defense of Tc that are simultaneously increasing HP while facilitating HL via selection of cooler ambient temperature. Because of that limitation, views of regulation that do not invoke an integrated and centrally controlled set-point of a putative regulated variable are better able to accommodate these observations. A putative regulated variable may be better conceived as a balance point that is influenced by, rather than defended by, the effects of relatively independent sensor-effector loops. Thus, challenges to regulatory systems by stimuli that did not occur in the animal's evolutionary history (e.g., N2O, or other drugs of abuse) may be especially likely to trigger individual sensor-effector loops in ways that appear puzzling or paradoxical.8
From this perspective, the Tc values observed in the present study reflect a balance point rather than a defended set-point. While the selection of a cooler Tc did blunt the sign-reversal in the current study, this does not necessarily imply that the rat was motivated to move to a cooler ambient temperature by effectors triggered by the sign-reversal of Tc. The identities of the stimuli that trigger each of the pertinent sensor-effector loops operating during the current study are not known. Additionally, the location of the sensors and / or the stimuli that trigger the different sensor-effectors responsible for increasing HP and motivating cool seeking behavior may differ, and the triggering stimuli need not be limited to temperature. For example,18 describes how cool-seeking behavior can be triggered by toxic insults from drug or chemical exposures as well as from other pathological insults (e.g., hypoxia, hemorrhage). A balance point model involving relatively independent sensor-effector loops allows for inefficient dyscoordination (e.g., concurrent competition between opposing effectors) of effector activity, especially when the regulatory system is confronted with non-naturalistic challenges (e.g., drugs of abuse).
Cabanac23 addressed the conundrum of trying to explain apparently contradictory findings such as this. He pointed out that while it is possible to dissociate experimentally the various thermoregulatory defense responses, thus demonstrating that they are relatively independent from one another, this only occurs in artificial experimental conditions; i.e., he emphasized that when the organism operates under normal (i.e., not artificial) environmental conditions, all available evidence suggests that all thermoregulatory responses normally take place synchronously.
Regardless of how repeated N2O administrations cause the observed effects, the present findings support the possibility of a vicious-cycle allostatic model of drug addiction. The observed blunting of the hyperthermic sign-reversal that is mediated by acquired HP responses but is opposed by the opposing action of a behavioral response is compatible with an allostatic model of drug addiction. If the sign-reversal state does participate in eliciting behaviors that support addiction (e.g., escalation of drug taking), then discovering ways to eliminate or diminish sign-reversals may be a therapeutically important goal.24
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge Christopher W. Prall and Hoang Yen Ho for their technical contributions to this study. The Helen Riaboff Whiteley Center, located at the University of Washington's Friday Harbor Laboratories, provided an ideal environment for completing this manuscript. Supplemental data for this article can be accessed on the publisher's website
References
- 1. Baker TB, Japuntich SJ, Hogle JM, McCarthy DE, Curtin JJ. Pharmacologic and behavioral withdrawal from addictive drugs. Curr Dir Psychol Sci 2006; 15:232–36; http://dx.doi.org/ 10.1111/j.1467-8721.2006.00442.x [DOI] [Google Scholar]
- 2. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 2004; 111:33–51; PMID: 14756584; http://dx.doi.org/ 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- 3. Himmelsbach CK. Clinical studies of addiction. Arch Intern Med 1942; 69:766–72; http://dx.doi.org/ 10.1001/archinte.1942.00200170048004 [DOI] [Google Scholar]
- 4. Himmelsbach CK. Symposium: can the euphoric, analgesic and physical dependence effects of drugs be separated? IV. With reference to physical dependence. Fed Proc 1943; 2:201–8. [Google Scholar]
- 5. Newlin DB, Regalia PA, Seidman TI, Bobashev G. Control theory and addictive behavior. In: Computational neuroscience of drug addiction. Gutkin B. and Ahmed SH. (eds). Springer series in computational neuroscience, v.10 New York: Springer Science and Business Media; 2012: 57-108. [Google Scholar]
- 6. Peper A. Intermittent adaptation: a mathematical model of drug tolerance, dependence and addiction. In: Computational neuroscience of drug addiction. Gutkin B. and Ahmed SH. (eds) Springer series in computational neuroscience, v.10 New York: Springer Science and Business Media; 2012:19–56. [Google Scholar]
- 7. Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol 2013; 23:559–63; PMID: 23628232; http://dx.doi.org/ 10.1016/j.conb.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 2014; 121:225–47; PMID: 24730599; http://dx.doi.org/ 10.1037/a0035942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001; 24:97–129; PMID: 11120394; http://dx.doi.org/ 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- 10. Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 2005; 8:1442–4; PMID: 16251985; http://dx.doi.org/ 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- 11. Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008; 59:29–53; PMID: 18154498; http://dx.doi.org/ 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- 12. Colpaert FC, Deseure K, Stinus L, Adriaensen H. High-efficacy 5-hydroxytryptamine 1A receptor activation counteracts opioid hyperallodynia and affective conditioning. J Pharmacol Exp Ther 2006; 316:892–9; PMID: 16254131; http://dx.doi.org/ 10.1124/jpet.105.095109 [DOI] [PubMed] [Google Scholar]
- 13. Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr., Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol 2004; 61:126–48; PMID: 15362157; http://dx.doi.org/ 10.1002/neu.20091 [DOI] [PubMed] [Google Scholar]
- 14. Kaiyala KJ, Butt S, Ramsay DS. Systems-level adaptations explain chronic tolerance development to nitrous oxide hypothermia in young and mature rats. Psychopharmacology (Berl) 2007; 191:233–42; PMID: 17216156; http://dx.doi.org/ 10.1007/s00213-006-0655-1 [DOI] [PubMed] [Google Scholar]
- 15. Kaiyala KJ, Chan B, Ramsay DS. Robust thermoregulatory overcompensation, rather than tolerance, develops with serial administrations of 70% nitrous oxide to rats. J Therm Biol 2012; 37:30–40; PMID: 22247586; http://dx.doi.org/ 10.1016/j.jtherbio.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramsay DS, Kaiyala KJ, Leroux BG, Woods SC. Individual differences in initial sensitivity and acute tolerance predict patterns of chronic drug tolerance to nitrous-oxide-induced hypothermia in rats. Psychopharmacology (Berl) 2005; 181:48–59; PMID: 15778887; http://dx.doi.org/ 10.1007/s00213-005-2219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon CJ, Lee KL, Chen TL, Killough P, Ali JS. Dynamics of behavioral thermoregulation in the rat. Am J Physiol: 1991; 261:R705-11 [DOI] [PubMed] [Google Scholar]
- 18. Gordon CJ. Temperature and toxicology: an integrative, comparative and environmental approach. Boca Raton: CRC Press; 2005. [Google Scholar]
- 19. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002; 2:8; PMID: 12069695; http://dx.doi.org/ 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perneger TV. What's wrong with bonferroni adjustments. BMJ 1998; 316:1236–8; PMID: 9553006; http://dx.doi.org/ 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–46; PMID: 2081237; http://dx.doi.org/ 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 22. Ramsay DS, Woods SC, Kaiyala KJ. Repeated nitrous oxide exposure in rats causes a thermoregulatory sign-reversal with concurrent activation of opposing thermoregulatory effectors. Temperature; 2014; 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabanac M. Adjustable set point: to honor Harold T. Hammel. J Appl Physiol 2006; 100:1338–46; PMID: 16540712; http://dx.doi.org/ 10.1152/japplphysiol.01021.2005 [DOI] [PubMed] [Google Scholar]
- 24. Kaiyala KJ, Woods SC, Ramsay DS. Persistence of a hyperthermic sign reversal during nitrous oxide inhalation despite cue-exposure treatment with and without a drug-onset cue. Temperature; 2014; 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


